Introduction
Cytochromes P450 (CYPs) belong to a superfamily of
proteins that contain a heme cofactor. They are primarily located
in the inner membrane of mitochondria or the endoplasmic reticulum
of cells (1). CYPs, as the oxidase
enzymes in electron transfer chains, catalyze a number of enzymatic
reactions involving small molecules. The CYPs are classified as
endogenous CYPs or xenobiotic CYPs. The endogenous CYPs are
involved with the biosynthesis or catabolism of steroids, sterols,
retinoids, prostaglandins and fatty acids, while the xenobiotic
CYPs function to defend against environmental toxins and
carcinogens (2). The human CYP family
1–4 includes the major enzymes involved in drug metabolism,
accounting for ~75% of the total CYPs (3).
Recently, CYP studies have increasingly focused on
drug metabolism (3). However, the
systematic association between endogenous CYPs and cancer remains
unclear. Thus, the aim of the present study was to investigate the
association between endogenous CYPs and cancer. Using data obtained
from The Cancer Gene Atlas (TCGA), the gene expression profiles and
somatic mutations of endogenous CYPs were analyzed in six cancer
types in order to determine whether any common features may
exist.
In the human body, the CYP11 gene family, including
CYP11A1, CYP11B1 and CYP11B2, is one of the families of CYP genes
involved in steroid biosynthesis (2).
Previously, almost no research has been conducted with regards to
the CYP11 family and cancer; therefore, the present study aimed to
investigate the associations between them. The results of the
present study may be important for expanding the global view of
cancer research.
Materials and methods
TCGA
Data was obtained from TCGA (http://cancergenome.nih.gov/) (4). Data regarding six diverse cancer types,
including colon adenocarcinoma (COAD), kidney renal clear cell
carcinoma (KIRC), liver hepatocellular carcinoma (LIHC), lung
squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD) and
uterine corpus endometrial carcinoma (UCEC), were selected for
analysis, to cover cancer types belonging to digestive system,
respiratory system and reproductive system. The data set included
2,754 gene expression samples and 1,461 somatic mutation samples
(Table I). The gene expression
samples were further divided into two groups, which included 2,450
carcinoma and 304 pericarcinoma tissue samples.
 | Table I.Expression and mutation of 56 genes in
six cancer types. |
Table I.
Expression and mutation of 56 genes in
six cancer types.
| Cancer type | Cancer samples,
n | Normal samples,
n | Mutated samples,
n | Endogenous CYP
mutations, n |
|---|
| COAD | 433 | 42 | 154 | 30 |
| KIRC | 516 | 73 | 417 | 70 |
| LIHC | 148 | 51 | 203 | 25 |
| LUSC | 490 | 51 | 178 | 123 |
| PRAD | 334 | 51 | 261 | 26 |
| UCEC | 529 | 36 | 248 | 275 |
| Total | 2450 | 304 | 1461 | 549 |
Gene expression analysis
Endogenous CYPs were selected from the gene
expression profile. The Wilcoxon signed-rank test was performed to
analyze the differential expression of CYPs between carcinoma and
pericarcinoma tissue for each cancer type.
Somatic mutation analysis
Data regarding the somatic mutations in endogenous
CYPs were obtained (Table I).
Subsequently, PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) (5) was used to evaluate the severity of these
mutations. PolyPhen-2 is a tool that predicts the possible impact
of an amino acid substitution on the structure and function of a
human protein using physical and comparative considerations.
PolyPhen-2 generates a score for every mutation, which ranges
between 0 and 1, whereby a higher score indicates a more severe
mutation. Thus, the severity of mutations may be divided into three
categories: Probably damaging, possibly damaging and benign. PyMOL
(http://www.pymol.org/) was used to create the
protein secondary structure. PyMOL is a molecular visualization
system on an open-source foundation. The protein structure data of
CYP11B2 was downloaded from the Research Collaboratory for
Structural Bioinformatics Protein Data Bank (RCSB PDB; http://www.rcsb.org/pdb/home/home.do).
Pathway analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG)
PATHWAY database (6) (http://www.kegg.jp/kegg/pathway.html)
was used to identify the metabolic pathway of CYPs. KEGG presents a
collection of databases that contain information regarding genomes,
biological pathways, diseases, drugs and chemical substances. KEGG
is utilized for bioinformatics studies, including data analysis in
genomics, metagenomics and metabolomics, modeling and simulation in
systems biology and translational research in drug development.
Results
CYP gene expression profile in six
cancer types
To identify differences in CYP expression patterns
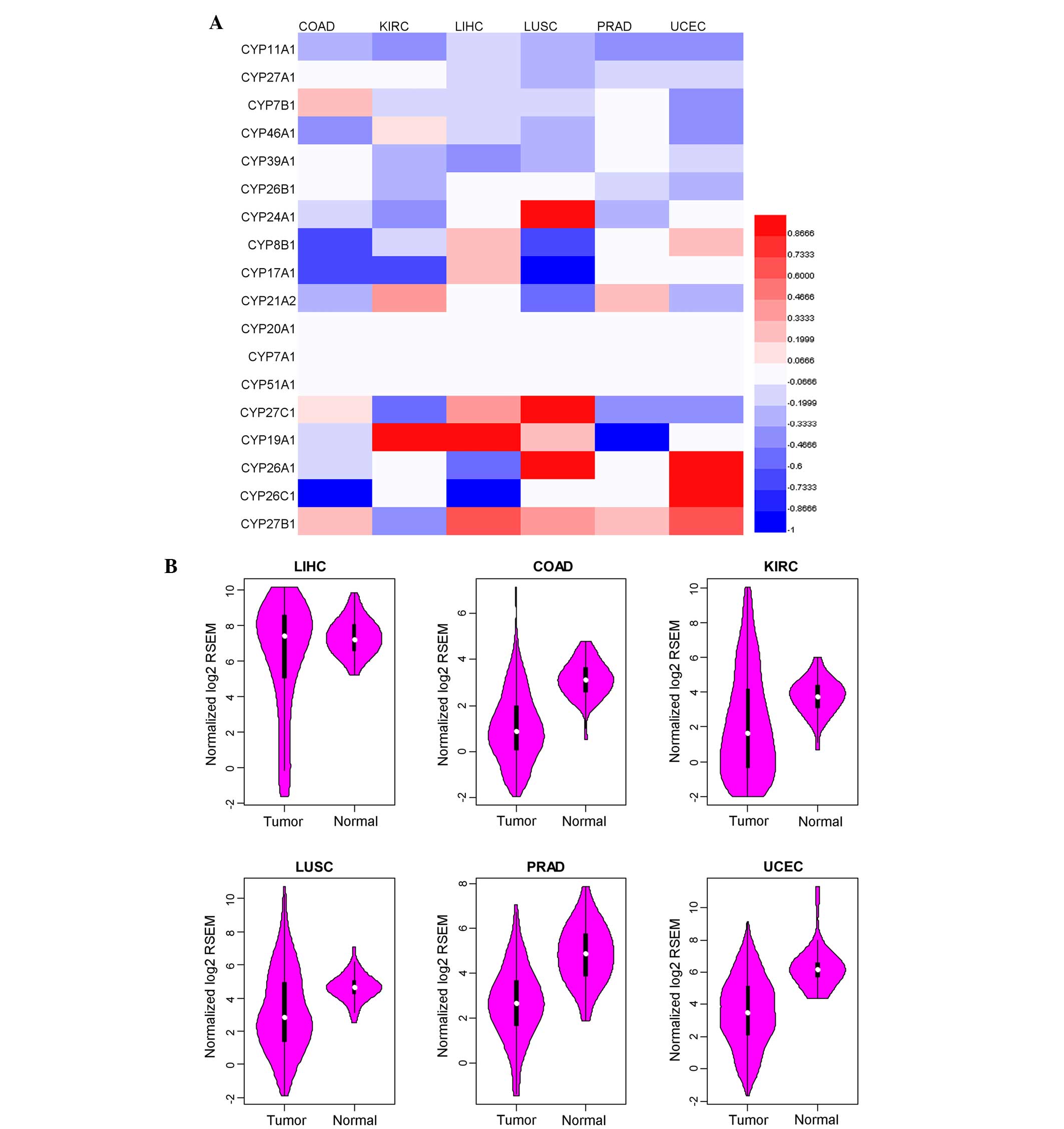
in various cancers, the expression of various genes was analyzed.
The expression of CYP11A1 was significantly downregulated in all
cancer types (P<0.001), while the expression of CYP27B1 was
significantly upregulated (P<0.001) (Fig. 1A).
CYP11A1 is a mitochondrial enzyme that catalyzes the
conversion of cholesterol to pregnenolone. This represents the
first reaction in the process of steroidogenesis in all steroid
hormone-producing mammalian tissues (Fig.
1B) (7). Low expression of
CYP11A1 may cause steroid biosynthesis disorders (Fig. 2).
As shown in Fig. 1A,
CYP27B1 was upregulated in 5/6 of the cancer types analyzed, with
the exception of KIRC. CYP27B1 is most commonly identified in the
proximal tubule of the kidney and a variety of other tissues,
including skin, immune cells and bone. The enzyme catalyzes the
hydroxylation of calcifediol to calcitriol (the bioactive form of
vitamin D) (8).
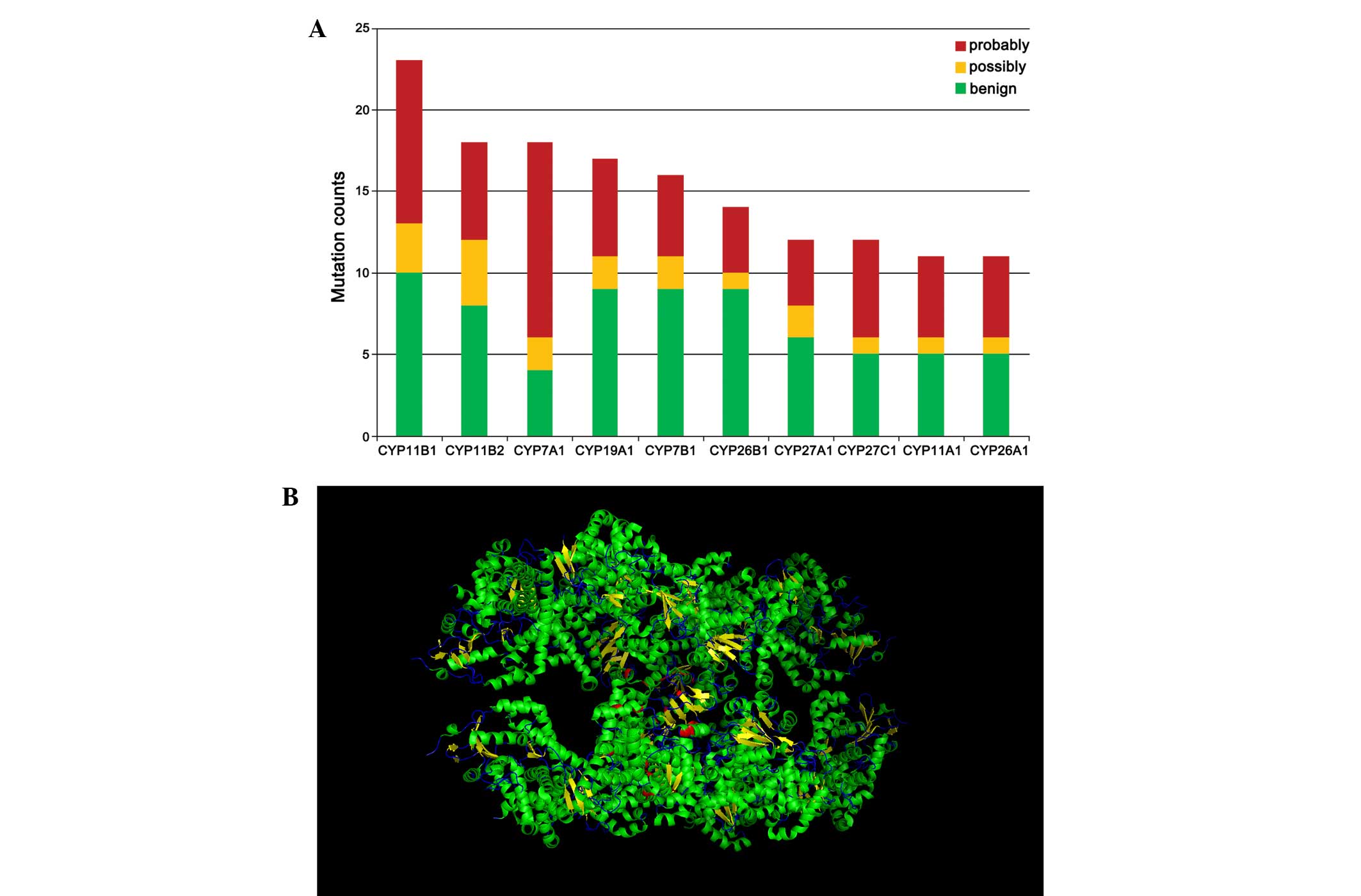
Somatic mutations in the six cancer
types
In the present study, the 10 most commonly mutated
genes were identified and their functional effect was predicted
using PolyPhen-2. The results revealed that all members of the
CYP11 family, including CYP11A1, CYP11B1 and CYP11B2, were among
the 10 most commonly mutated genes. In particular, CYP11B1 and
CYP11B2 exhibited the most mutations among all genes analyzed. The
major function of the CYP11 family is to promote steroid
biosynthesis (9). CYP11B1 is a
steroid hydroxylase present in the zona glomerulosa and zona
fasciculate (10,11) that generates cortisol from
11-deoxycortisol and corticosterone from 11-deoxycorticosterone.
CYP11B2 is a steroid hydroxylase enzyme involved in the
biosynthesis of the mineralocorticoid aldosterone. The CYP11B2
protein is only expressed in the zona glomerulosa (12) of the adrenal cortex and is primarily
regulated by the renin-angiotensin system (11). CYP11B2 is the only enzyme capable of
synthesizing aldosterone in humans and is important for electrolyte
balance and blood pressure regulation (13). CYP7A1 exhibited the second highest
number of mutations among the genes studied. It also exhibited the
highest number of ‘probably damaging’ mutations (Fig. 3A). CYP7A1 is the rate-limiting enzyme
for the synthesis of bile acid from cholesterol via the classical
pathway, catalyzing the formation of 7-alpha-hydroxycholesterol
(14).
As the structure of CYP11B2 may be indicated in RCSB
PDB, PyMOL was used to visualize somatic mutations in the structure
of CYP11B2 (Fig. 3B). Approximately
80% of mutations occurred in the α-helix region. These mutations
may alter the protein structure and subsequently affect its
function.
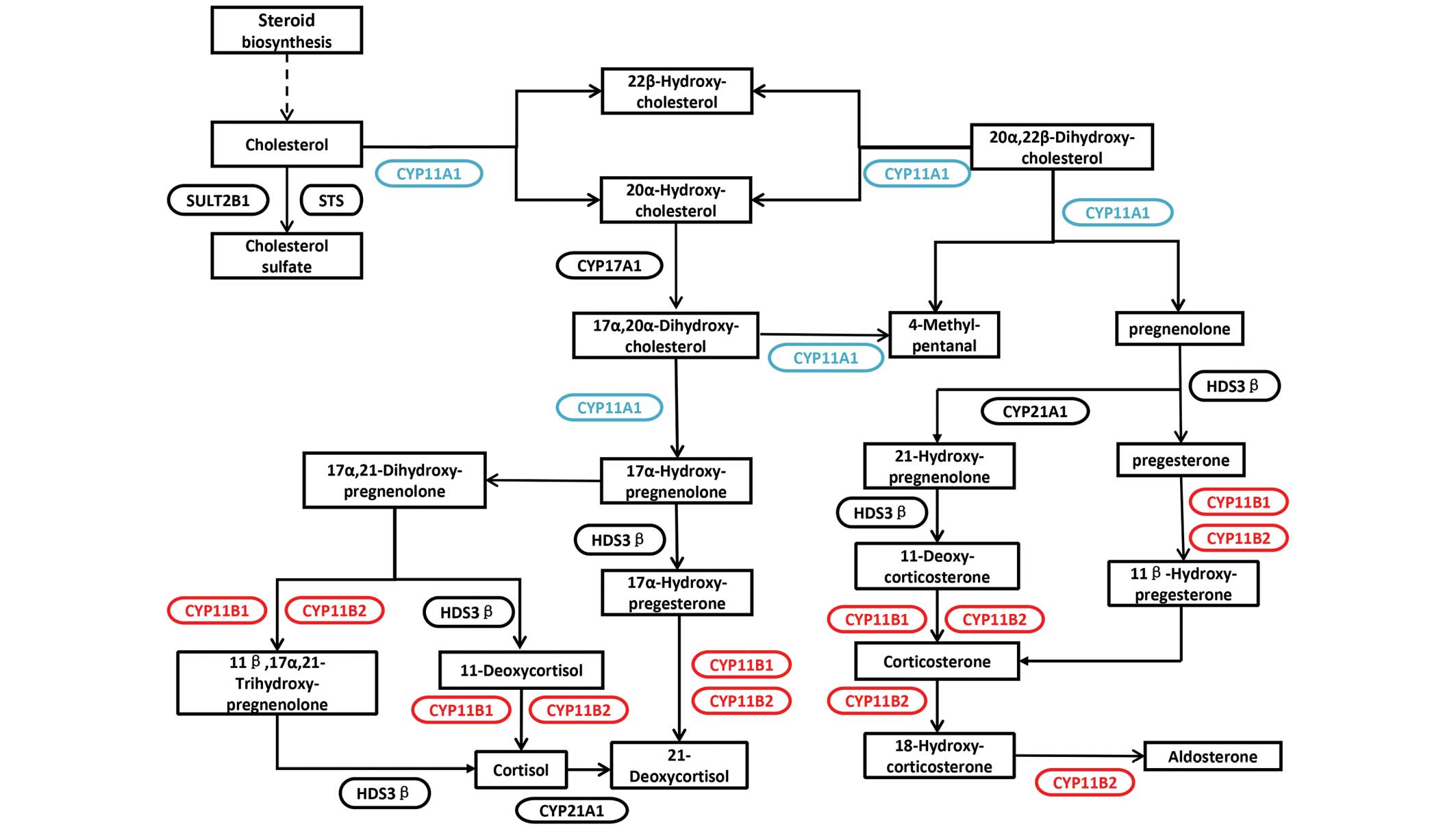
Steroid biosynthesis pathway
analysis
For the expression and mutation analysis of crucial
members of the CYP11 family, the KEGG pathway database was used to
investigate the role of the CYP11 family in metabolic pathways.
Members of the CYP11 family are involved in steroid biosynthesis.
The low expression of CYP11A1 may lead to decreased expression of
pregnenlone and 17α-hydroxy pregnenolone. Furthermore, mutations in
CYP11B1 and CYP11B2 may affect cortisol and aldosterone levels.
Pregnenolone, cortisol and aldosterone are crucial components
involved in steroid biosynthesis (Fig.
2).
Pregnenolone is a type of endogenous steroid, and is
the forerunner of several steroids, including glucocorticoids,
mineralocorticoids, progestogens, estrogens and androgens (15). Furthermore, pregnenolone is a
biologically active neurosteroid (15). Pregnenolone is synthesized from
cholesterol, a transversion that requires hydroxylation at the C20
and C22 positions of the side-chain and is performed by the enzyme
CYP11A1, which is located in the mitochondria and is controlled by
anterior pituitary tropic hormones. Cortisol (a glucocorticoid
steroid hormone) is composed by the zona fasciculata of the adrenal
cortex. During stress or hypoglycemia, cortisol will be released to
suppress the immune system, to increase blood glucose, to decrease
bone formation and to support the metabolism of carbohydrates, fat
and protein (16,17). Aldosterone (a mineralocorticoid
steroid hormone) is formed by the zona glomerulosa of the adrenal
cortex, and is important for blood pressure regulation (18). Blood pressure is managed by processes
that occur in the distal convoluted tubules and collecting ducts of
the nephron, which encourage the reabsorption of ions and water,
the secretion of potassium, the conservation of sodium, and the
increase in water retention, blood volume and blood pressure
(18).
Discussion
The present study revealed that low CYP11A1
expression is common in several cancers, and CYP11B1 and CYP11B2
exhibit the highest number of mutations in these cancers.
Approximately 80% of these mutations may alter the function of the
CYP11B1 and CYP11B2 proteins. The findings of the present study
indicate that the CYP11 family is commonly involved with a wide
variety of cancers.
Decreased expression and mutations of the CYP11
family may influence the biosynthesis of steroid hormones. In
recent years, the majority of studies of steroid hormones have been
performed in breast cancer patients (19–22).
However, studies have investigated the role of steroid hormones in
prostate (23), lung (24), endometrial (25), colon (26) and liver cancer (27). Steroid hormones have been demonstrated
to activate focal adhesion kinase, which regulates early actin
reorganization in colon cancer cells (28). Steroid hormones were not previously
considered to be involved with lung function (29); however, numerous studies have reported
that steroid hormones are important in normal lung development and
function (30) and in the
pathogenesis of pulmonary diseases, including lung cancer (31–33). A
study of prostate cancer validated the hypothesis that the
biosynthesis of steroid hormones downstream of CYPs contributes to
the progression of castration-resistant prostate cancer (34). Steroid hormones may also be utilized
for the treatment of endometrial cancer. For example, progestin
therapy has been demonstrated as a viable treatment option for type
1 endometrial cancer (35). These
studies support the results of the present study, which indicated
that steroid hormones are extremely important in numerous cancer
types. In conclusion, the CYP11 family, which may affect steroid
biosynthesis, is commonly involved in various types of cancer. The
present study provides novel ideas that indicate that, with the
development of technology, the CYP11 family could used as biomarker
or drug target in the future research of cancers. Additional data
and experiments will help to determine whether the genes of CYP11
family may be used as biomarkers in the diagnosis of various types
of cancer. The method of computer-aided drug design may be used to
simulate the interaction between the CYP11 family and chemical
molecules, which will verify whether the CYP11 family could become
drug targets.
Acknowledgements
The present study was supported by the National
Basic Research Program of China (grant nos. 2011CB910204,
2011CB510102 and 2010CB529200) and the National Key Scientific
Instrument and Equipment Development Project (grant no.
2012YQ03026108), the National Key Technology Support Program (grant
no. 2013BAI101B09), and the Strategic Priority Research Program of
the Chinese Academy of Sciences (grant no. XDA12000000).
References
|
1
|
Berka K, Hendrychová T, Anzenbacher P and
Otyepka M: Membrane position of ibuprofen agrees with suggested
access path entrance to cytochrome P450 2C9 active site. J Phys
Chem A. 115:11248–11255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas JH: Rapid birth-death evolution
specific to xenobiotic cytochrome P450 genes in vertebrates. PLoS
Genet. 3:e672007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guengerich FP: Cytochrome p450 and
chemical toxicology. Chem Res Toxicol. 21:70–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network.
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, Benz CC, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adzhubei I, Jordan DM and Sunyaev SR:
Predicting functional effect of human missense mutations using
PolyPhen-2. Curr Protoc Hum Genet Chapter. 7:Unit7.202013.
|
|
6
|
Kanehisa M, Golo S, Hattori M,
Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M and
Hirakawa M: From genomics to chemical genomics: New developments in
KEGG. Nucleic Acids Res. 34:D354–D357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanukoglu I: Steroidogenic enzymes:
Structure, function, and role in regulation of steroid hormone
biosynthesis. J Steroid Biochem Mol Biol. 43:779–804. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon JY, Moon MH, Kim KT, Jeong DH, Kim
YN, Chung BC and Choi MH: Cytochrome P450-mediated metabolic
alterations in preeclampsia evaluated by quantitative steroid
signatures. J Steroid Biochem Mol Biol. 139:182–191. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helmberg A: Twin genes and endocrine
disease: CYP21 and CYP11B genes. Acta Endocrinol (Copenh).
129:97–108. 1993.PubMed/NCBI
|
|
11
|
Stowasser M, Gunasekera TG and Gordon RD:
Familial varieties of primary aldosteronism. Clin Exp Pharmacol
Physiol. 28:1087–1090. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alvarez-Madrazo S, Mackenzie SM, Davies E,
et al: Common polymorphisms in the CYP11B1 and CYP11B2 genes:
Evidence for a digenic influence on hypertension. Hypertension.
61:232–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Padmanabhan N, Padmanabhan S and Connell
JM: Genetic basis of cardiovascular disease - the
renin-angiotensin-aldosterone system as a paradigm. J Renin
Angiotensin Aldosterone Syst. 1:316–324. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davis RA, Miyake JH, Hui TY and Spann NJ:
Regulation of cholesterol-7alpha-hydroxylase: BAREly missing a SHP.
J Lipid Res. 43:533–543. 2002.PubMed/NCBI
|
|
15
|
Marx CE, Bradford DW, Hamer RM, Naylor JC,
Allen TB, Lieberman JA, Strauss JL and Kilts JD: Pregnenolone as a
novel therapeutic candidate in schizophrenia: Emerging preclinical
and clinical evidence. Neuroscience. 191:78–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hoehn K and Marieb EN: The endocrine
system. Human Anatomy & Physiology (8th). Benjamin Cummings.
(San Francisco, CA). 606–612. 2010.
|
|
17
|
Chyun YS, Kream BE and Raisz LG: Cortisol
decreases bone formation by inhibiting periosteal cell
proliferation. Endocrinology. 114:477–480. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu C, Rusin CG, Tan Z, Guagliardo NA and
Barrett PQ: Zona glomerulosa cells of the mouse adrenal cortex are
intrinsic electrical oscillators. J Clin Invest. 122:2046–2053.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Claus EB, Risch N and Thompson WD: Genetic
analysis of breast cancer in the cancer and steroid hormone study.
Am J Hum Genet. 48:232–242. 1991.PubMed/NCBI
|
|
20
|
He Q, Liang CH and Lippard SJ: Steroid
hormones induce HMG1 overexpression and sensitize breast cancer
cells to cisplatin and carboplatin. Proc Natl Acad Sci USA.
97:5768–5772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Colleoni M, Viale G, Zahrieh D, et al:
Chemotherapy is more effective in patients with breast cancer not
expressing steroid hormone receptors: A study of preoperative
treatment. Clin Cancer Res. 10:6622–6628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eliassen AH, Missmer SA, Tworoger SS,
Spiegelman D, Barbieri RL, Dowsett M and Hankinson SE: Endogenous
steroid hormone concentrations and risk of breast cancer among
premenopausal women. J Natl Cancer Inst. 98:1406–1415. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Platz EA, Leitzmann MF, Rifai N, Kantoff
PW, Chen YC, Stampfer MJ, Willett WC and Giovannucci E: Sex steroid
hormones and the androgen receptor gene CAG repeat and subsequent
risk of prostate cancer in the prostate-specific antigen era.
Cancer Epidemiol Biomarkers Prev. 14:1262–1269. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaiser U, Hofmann J, Schilli M, Wegmann B,
Klotz U, Wedel S, Virmani AK, Wollmer E, Branscheid D, Gazdar AF
and Havemann K: Steroid-hormone receptors in cell lines and tumor
biopsies of human lung cancer. Int J Cancer. 67:357–364. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Potischman N, Hoover RN, Brinton LA,
Siiteri P, Dorgan JF, Swanson CA, Berman ML, Mortel R, Twiggs LB,
Barrett RJ, et al: Case-control study of endogenous steroid
hormones and endometrial cancer. J Natl Cancer Inst. 88:1127–1135.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lointier P, Wildrick DM and Boman BM: The
effects of steroid hormones on a human colon cancer cell line in
vitro. Anticancer Res. 12:1327–1330. 1992.PubMed/NCBI
|
|
27
|
De Maria N, Manno M and Villa E: Sex
hormones and liver cancer. Mol Cell Endocrinol. 193:59–63. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu S, Kounenidakis M, Schmidt EM,
Deshpande D, Alkahtani S, Alarifi S, Föller M, Alevizopoulos K,
Lang F and Stournaras C: Rapid activation of
FAK/mTOR/p70S6K/PAK1-signaling controls the early
testosterone-induced actin reorganization in colon cancer cells.
Cell Signal. 25:66–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koehler KF, Helguero LA, Haldosén LA,
Warner M and Gustafsson JA: Reflections on the discovery and
significance of estrogen receptor beta. Endocr Rev. 26:465–478.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Patrone C, Cassel TN, Pettersson K, Piao
YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA and Nord M:
Regulation of postnatal lung development and homeostasis by
estrogen receptor beta. Mol Cell Biol. 23:8542–8552. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bardin A, Boulle N, Lazennec G, Vignon F
and Pujol P: Loss of ERbeta expression as a common step in
estrogen-dependent tumor progression. Endocr Relat Cancer.
11:537–551. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bouchardy C, Benhamou S, Schaffar R,
Verkooijen HM, Fioretta G, Schubert H, Vinh-Hung V, Soria JC,
Vlastos G and Rapiti E: Lung cancer mortality risk among breast
cancer patients treated with anti-estrogens. Cancer. 117:1288–1295.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kazmi N, Márquez-Garbán DC, Aivazyan L,
Hamilton N, Garon EB, Goodglick L and Pietras RJ: The role of
estrogen, progesterone and aromatase in human non-small-cell lung
cancer. Lung Cancer Manag. 1:259–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased
survival in metastatic prostate cancer. N Engl J Med.
364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim JJ, Kurita T and Bulun SE:
Progesterone action in endometrial cancer, endometriosis, uterine
fibroids, and breast cancer. Endocr Rev. 34:130–162. 2013.
View Article : Google Scholar : PubMed/NCBI
|