Introduction
Tissue factor (TF), the primary initiator of the
coagulation cascade, is a 47-kDa cell-associated transmembrane
protein that acts as a high-affinity receptor for factor VIIa
(FVIIa). Formation of the TF/FVIIa complex (extrinsic tenase
complex) on cellular surfaces triggers the generation of active
coagulation proteases, followed by deposition of activated
platelets and fibrin (1). TF is
constitutively expressed in specific cell types and has been shown
to be upregulated in a number of pathological processes (2,3).
A strong correlation between TF expression and
malignancy grade has been reported for several tumor types, and may
result in vascular occlusion and hypoxia, therefore influencing
various aspects of tumor progression, including angiogenesis,
metastasis and other cellular responses (4–8). Increased
TF expression has been also associated with cancer-associated
thrombosis, a highly relevant systemic response (8,9). In fact,
several studies suggest that circulating extracellular vesicles
containing TF may be important in a patient's prothrombotic state
(10,11).
The majority of the pro-tumoral responses induced by
TF are associated with the functions of activated clotting factors
[FVIIa, factor Xa (FXa) and thrombin] as well as other proteases,
such as matrix metalloproteinase-1 (MMP1), in cleaving and
activating G protein-coupled protease-activated receptors (PARs)
(7,9,12).
Activation of PAR1 by thrombin or MMP1 triggers a number of tumor
cell responses, while the TF/FVIIa binary complex predominantly
functions through activation of PAR2. In fact, it is proposed that
PAR1 and PAR2 induce several redundant responses in tumor cells
(7,13).
Glioblastoma multiforme (GBM), the most common type
of adult brain tumor, infiltrates the normal brain area, rendering
complete surgical resection impossible. The disease has a poor
median survival time of 15 months after diagnosis, despite the
application of aggressive multimodality treatments following
surgery, such as radiation and chemotherapy (14). The aggressiveness of GBM has been
correlated with severe hypoxia, which generates large areas of
necrosis and an extensive, hyperpermeable vasculature (15). Hypoxia activation is a hallmark of
growing tumors that occurs as a result of inadequate oxygen supply.
Increased angiogenesis serves to restore the influx of nutrients
and oxygen to the tumor environment (16). It has been proposed that the TF
signaling pathway has a relevant role in GBM progression (17,18).
Therefore, oncogenic signaling pathways, such as the expression of
epidermal growth factor receptor (EGFR) and its mutant, EGFRvIII,
as well as inactivation of the tumor suppressor phosphatase and
tensin homolog (PTEN), correlate with the elevated expression of TF
in GBM cell lines and patient samples (19–22).
To determine whether hypoxia can modulate the
hypercoagulative activity of glioma cells, as well as TF
signaling-associated elements, such as PAR receptors, the present
study examined two cell lines derived from C6 rat glioma cells that
differently respond to glucocorticoid treatment. Non-tumorigenic
ST1 cells undergo a dramatic tumoral-to-normal phenotypic
reversion, characterized by growth inhibition in monolayer cultures
(with a blockage at the G0/G1 cell cycle phase), loss of the
ability to form colonies in semi-solid medium, loss of ability to
induce tumor formation in nude mice, and dramatic morphological
changes. By contrast, P7 cells are highly aggressive and unaffected
by glucocorticoid treatment (23,24).
The results of the present study show that hypoxia
upregulates TF expression, as well as PAR1 and PAR2 expression, in
GBM cell lines. In addition, hypoxic stress increases the shedding
of procoagulant TF-bearing microvesicles (MVs) by tumor cells.
Together, the results provide a better understanding of the
processes involved in the deregulation of the coagulation system in
GBM, which appears to be critical for glioma progression. Thus,
modulating these molecular interactions may offer additional
insights for the development of more efficient therapeutic
strategies against aggressive glioma.
Materials and methods
Cell culture
ST1 and P7 rat glioma cell lines were originally
established by Armelin et al (23,24) from
rat C6 glial cells (American Type Culture Collection, Rockville,
MD, USA) by subjecting cultures to successive passages of
serum-free medium. Both cell lines were grown at 37°C in a
humidified, 5% CO2 atmosphere in culture flasks by
subconfluent passages in Dulbecco's modified Eagle medium/F12
(DMEM/F12; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 2 g/l HEPES, 60 mg/l streptomycin and 1.2 g/l
sodium bicarbonate. For hypoxia experiments, cells were cultured in
fresh medium containing 250 or 500 µM CoCl2
(Sigma-Aldrich, St. Louis, MO, USA) for 4, 12 or 24 h.
MV purification from cell culture
supernatants
Cell culture supernatants were consecutively
centrifuged at 800 × g for 10 min and at 20,000 × g for 20 min,
both at 4°C. The final pellet was then washed once in
phosphate-buffered saline (PBS), resuspended in PBS and stored at
−80°C until utilization. MVs were quantified by counting in a
FACSCalibur Flow Cytometer (BD Biosciences).
In vitro activation of plasma
coagulation
The procoagulant activity of cells and MVs was
measured by performing a clotting assay employing platelet-poor
plasma (PPP) from rats. Cells or MVs (50 µl) resuspended in PBS at
different concentrations were added to 50 µM PPP containing 3.8%
sodium citrate (1:9 v/v dilution). After 1 min incubation at 37°C,
100 µM of 6.25 mM CaCl2 was added and the clotting times
were recorded using a KC-4 coagulometer (Sigma Amelung, Lemgo,
Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated from P7 or ST1 cells
(2.5×105) using the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and reverse transcribed into cDNA using
SuperScript III Reverse Transcriptase (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
mRNA expression levels were quantified by qPCR on a 7300 Real-Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.)
using SYBR Green Master Mix. Sequence-specific primers were
designed using Primer Express software (version 3; Applied
Biosystems; Thermo Fisher Scientific, Inc.). The qPCR primers were
as follows: Forward, 5′-CAGAGCAGGACAGAAAAGGAAGAA-3 and reverse,
5′-GCGTCAGCCTCCTCGTCTAT-3′ for rat TF; forward,
5′-AACTGCTAGCCTCTGGATTTGATG and reverse,
5′-AAAGACAAGGCAACCGATACTTC-3′ for rat PTEN; forward
5′-TGTGCGGGCTGCTGCAATGAT-3′ and reverse
5′-TGTGCTGGCTTTGGTGAGGTTTGA-3′ for rat vascular endothelial growth
factor (VEGF); forward, 5′-CCTGTGCGGTCCTTTGCT-3′ and reverse,
5′-CATCCTCTCAGATTCTGGCTGTCT-3′ for rat PAR1; forward,
5′-AGAGGTATTGGGTCATGTG-3′ and reverse, 5′-GCAGGAATGAACATGGTCTG-3′
for rat PAR2; forward, 5′-GCTGAAGATTTGGAAAGGTGT-3′ and reverse,
5′-GCTGAAGATTTGGAAAGGTGT-3′ for the control, rat HPRT. Gene
expression levels were analyzed using the software provided by the
PCR system's manufacturer. The ΔΔCq method (25) was used to quantify the
amplification-fold difference between P7 and ST1 cells with the Cq
value of the target genes being adjusted to the housekeeping gene
(HPRT). Assays were performed in triplicate with variability
<0.5 Ct.
Flow cytometric analysis
For surface phosphatidylserine (PS) detection, tumor
cells (100 µl at 1×106 cells/ml) were incubated for 15
min at room temperature with fluorescein isothiocyanate-conjugated
Annexin V (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
diluted to 1:100 in binding buffer (10 mM HEPES, 150 mM NaCl, 2.5
mM CaCl2). Tumor cells (100 µl at 1×106
cells/ml) were then resuspended in 100 µl PBS containing 1% BSA and
incubated for 30 min at 4°C with a goat polyclonal antibody against
rat TF (200 µg/ml; cat no. 4501; Sekisui Diagnostics, LLC,
Lexington, MA, USA). After washing with 1% BSA in PBS to remove
unbound antibody, cells were incubated with a
fluorescein-conjugated anti-goat IgG (1:200, sc-2777, Santa Cruz
Biotechnology Inc.). Cells were then washed again with 1% BSA in
PBS and analyzed using a FACSCalibur Flow Cytometer (BD
Biosciences, San Jose, CA, USA). In all cases, data were analyzed
using CellQuest Pro software version 5.1.1 (BD Biosciences). Data
were expressed as global geometric mean fluorescence intensity
(ΔMFI), which represents the difference between the MFI of stained
and the MFI of unstained samples compared with the P7 cell line
FX activation via hydrolysis of
chromogenic substrates
Activation of FX by the extrinsic tenase complex
(TF/FVII)awas performed in HEPES-BSA buffer [50 mM HEPES, 100 mM
NaCl, 5 mM CaCl2, 1 mg/ml BSA (pH 7.5)], as follows:
FVIIa (final concentration, 1 nM) was incubated with cells
(5×105/ml) or MVs (5×103/ml) for 10 min at
37°C in HEPES-BSA buffer. The reaction was initiated by the
addition of FX (final concentration, 100 nM) and aliquots of 25 µl
were removed at different times into microplate wells containing 25
µl Tris-EDTA buffer [50 mM Tris-HCl, 150 mM NaCl, 20 mM EDTA, 1
mg/ml polyethyleneglycol-6000 (pH 7.5)]. Following the addition of
50 µl of 200 µM S-2765 (chromogenic substrate; Diapharma, West
Chester, OH, USA) prepared in Tris-EDTA buffer at 37°C for 30 min,
absorbance was recorded at 405 nm in 6-sec intervals using a
ThermoMax Microplate Reader (Molecular Devices, LLC, Menlo Park CA,
USA). Velocities [mean optical density (mOD)/min] obtained in the
first minute of the reaction were used to calculate the amount of
FXa formed, as compared with a standard curve using known enzyme
concentrations. In order to ensure that FX activation was strictly
dependent on extrinsic tenase complex assembly, negative controls
were performed in the absence of FVIIa and showed no significant
formation of FXa.
Prothrombin activation via hydrolysis
of chromogenic substrates
Activation of prothrombin by the prothrombinase
complex [FXa/factor Va (FVa)] was performed in HEPES-BSA buffer
using a discontinuous assay. FXa (final concentration, 10 pM) was
incubated with FVa (final concentration, 1 nM; Haematological
Technologies Inc., Essex Junction, VT, USA) in the presence of
cells (5×105/ml) for 2 min at 37°C. The reaction was
initiated by addition of prothrombin (final concentration, 500 nM;
Haematological Technologies Inc.) and aliquots of 10 µl were
removed every 1 min into microplate wells containing 40 µl
Tris-EDTA buffer. Following the addition of 50 µl of 200 µM S-2238
(Diapharma) prepared in Tris-EDTA buffer at 37°C for 20 min,
absorbance was recorded at 405 nm in 6-sec intervals using a
ThermoMax Microplate Reader (Molecular Devices, LLC). Velocities
(mOD/min) obtained in the first minute of the reaction were used to
calculate the amount of thrombin formed, as compared with a
standard curve using known enzyme concentrations. In order to
ensure that prothrombin activation was strictly dependent on
prothrombinase complex assembly, negative controls were performed
in the absence of FXa/FVa and showed no significant formation of
thrombin.
Statistical analysis
One-way analysis of variance followed by
Bonferroni's multiple comparison test or unpaired t-test were
performed using GraphPad Prism software (version 5; GraphPad
Software, Inc., San Diego, CA, USA). Data are presented as the mean
± standard deviation of three independent experiments. The
differences were considered to be statistically significant at
P<0.05.
Results
Differential expression of TF in rat
GBM cell lines
Previous studies have demonstrated that TF
expression is correlated with the histological grade of
malignancies in glioma (26,27). Using a one-stage clotting assay, the
present study observed that P7 cells display potent procoagulant
activity when compared with ST1 cells (Fig. 1A). As TF significantly contributes to
the activation of plasma coagulation, the levels of TF expression
were also determined in both GBM cell lines. Analysis by RT-qPCR
demonstrated that P7 cells express significantly higher levels of
TF mRNA than ST1 cells (Fig. 1B).
In vitro studies previously suggested that upregulation of
TF in high-grade glioma is associated with loss of tumor suppressor
PTEN (19,21,22).
Consistent with this hypothesis, Fig.
1B shows that the levels of PTEN mRNA are inversely correlated
with TF expression in both P7 and ST1 cells. In addition, flow
cytometric analysis showed less pronounced levels of TF antigen on
the surface of ST1 cells compared with P7 cells. The ST1 cell line
presented an ~3.5-fold increase in ΔMFI (Fig. 1C). Finally, TF activity was analyzed
in the P7 and ST1 cells. Notably, P7 cells supported the assembly
of extrinsic tenase complex with significant FX activation by FVIIa
compared with the control cells, whereas no significant FXa
generation was observed for ST1 cells under the same conditions
(Fig. 1D).
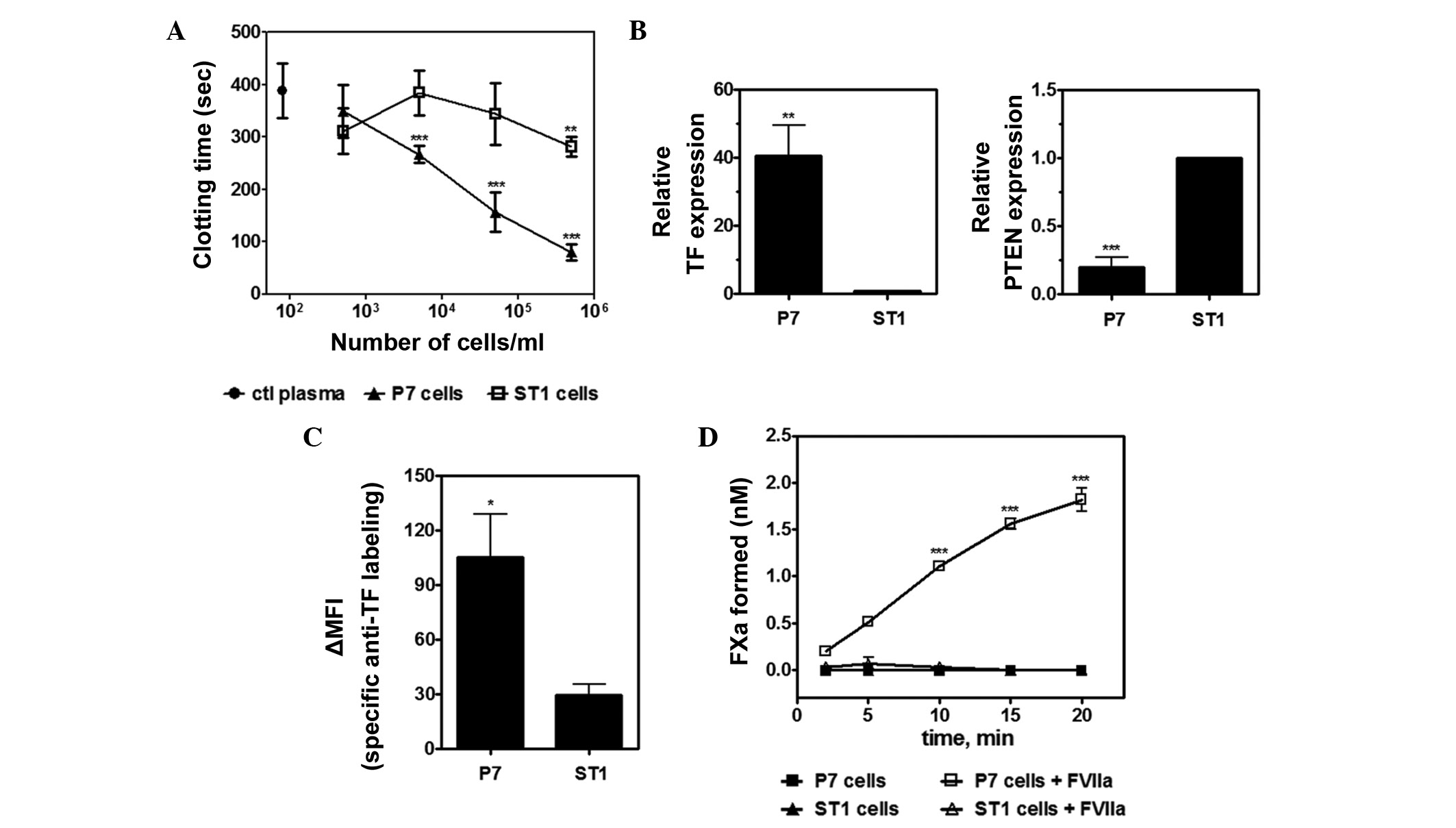 | Figure 1.Analysis of the procoagulant
properties of P7 and ST1 glioma cells. (A) Clotting time of P7 and
ST1 cells. The black circle represents coagulation time of rat
platelet-poor plasma in the absence of tumor cells. **P<0.01 or
***P<0.001 vs. ctl plasma, one-way analysis of variance (ANOVA)
followed by Bonferroni's post-hoc test. (B) Reverse
transcription-quantitative polymerase chain reaction analysis of TF
and PTEN gene expression in P7 and ST1 cells. **P=0.0048 and
***P=0.00041, respectively, unpaired t-test. (C) Flow cytometric
analysis of TF expression in P7 and ST1 cells. Relative specific
labelling of cells by an anti-rat TF polyclonal antibody ∆MFI
represents the geometric MFI difference between stained and
unstained samples. *P=0.0361, unpaired t-test. (D) Assembly of the
extrinsic tenase complex on ST1 and P7 cells. Kinetics for the
activation of FX (100 nM) in the presence of FVIIa (1 nM) in P7 or
ST1 cells (5×105/ml). Controls were performed in the
absence of FVIIa. ***P<0.001 vs. control, one-way ANOVA followed
by Bonferroni's post-hoc test. Data are presented as the mean±
standard deviation of three independent experiments. ctl, control;
MFI, mean fluorescence intensity; TF, tissue factor; PTEN,
phosphatase and tensin homolog; FXa, factor Xa. |
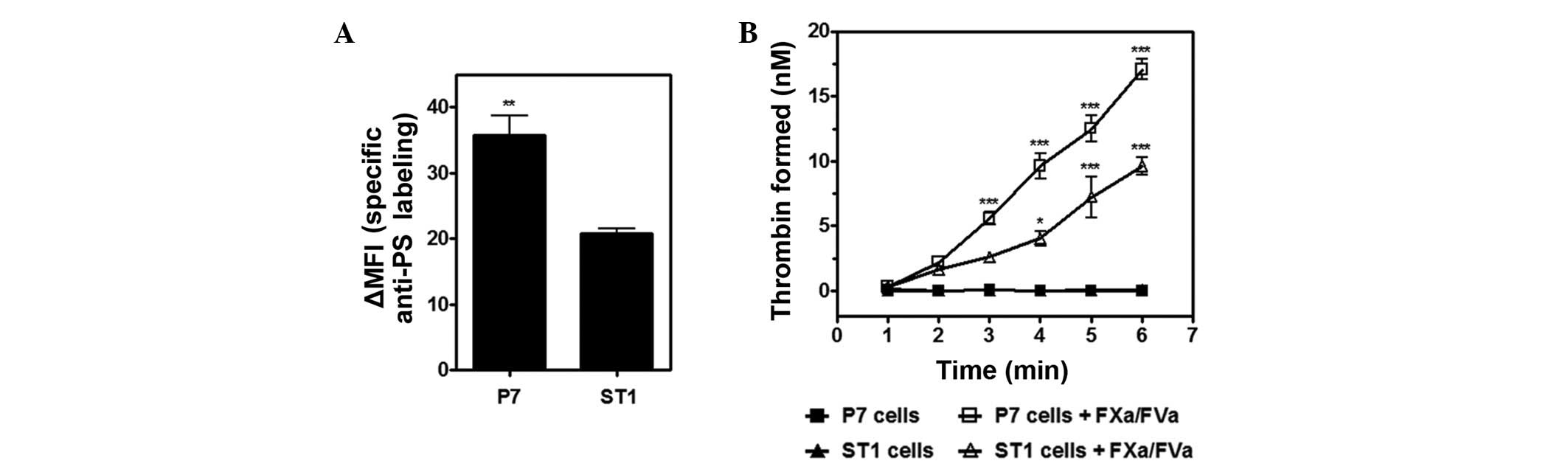
Our previous studies have demonstrated that viable
tumor cells may expose the anionic phospholipid PS on the outer
leaflet of the cell membrane (28,29). This
exposure is required for the assembly of membrane-dependent
procoagulant complexes. Therefore, the present study investigated
whether differential PS exposure could contribute to the variable
procoagulant properties of P7 and ST1 cells. Flow cytometric
analysis employing Annexin V indicated that P7 cells presented an
~1.7-fold increase in global geometric ΔMFI compared with ST1 cells
(Fig. 2A). Furthermore, the assembly
of the prothrombinase complex (FVa/FXa/prothrombin), a process that
is critically dependent on the presence of PS-rich anionic
membranes, was investigated. Consistently, P7 cells supported
prothrombin activation more efficiently than ST1 cells (Fig. 2B). Controls performed in the absence
of FXa and FVa showed negligible thrombin formation.
The results indicate that the higher procoagulant
activity of P7 cells relative to ST1 appears to be particularly
associated with their expression of the primary clotting initiator,
TF. However, distinct patterns of PS exposure may also augment
these discrepancies.
Hypoxic stress upregulates TF
expression and TF-FVIIa activity
In GBM, intratumoral activation of the coagulation
system is directly associated with vascular occlusions, triggering
interlinked processes, such as hypoxia, necrosis and angiogenesis
(17,30). It is well established that hypoxia
induces the expression of angiogenic regulators, such as VEGF, in
GBM cells, promoting the formation of new vessels and driving rapid
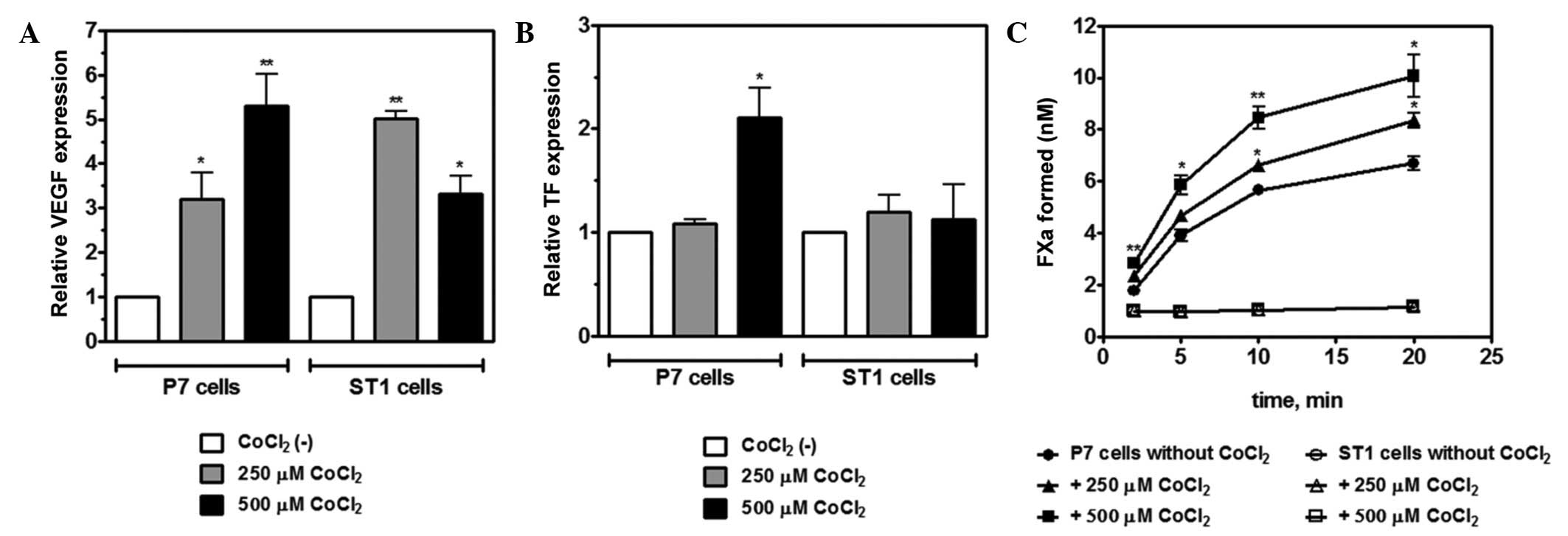
tumor growth. Fig. 3A reveals
upregulation of VEGF mRNA expression in P7 and ST1 cells upon
exposure to CoCl2 (250 or 500 µM CoCl2 for 4
h), which is known to induce hypoxic-like stress. Next, the present
study investigated whether treatment with CoCl2 could
upregulate TF expression in cancer cells. P7 and ST1 cells were
exposed to CoCl2, and TF mRNA and protein levels were
determined by RT-qPCR and flow cytometry, respectively. In
comparison to normoxic conditions, the cultivation of P7 cells in
500 µM CoCl2 for 4 h significantly increased TF mRNA
levels; however, this change was not observed in ST1 cells
(Fig. 3B). Accordingly, flow
cytometry showed a time-dependent upregulation of TF levels in P7
cells upon treatment with CoCl2 (250 or 500 μM), with a peak at 12
h (data not shown). To evaluate whether hypoxia-induced
upregulation of TF expression is accompanied by an increase in TF
procoagulant activity, an FXa generation assay by the extrinsic
tenase complex was performed. P7 and ST1 cells were cultured under
normoxic and hypoxic conditions (250 or 500 µM CoCl2)
for 12 h. The FX activation supported by P7 cells was enhanced at
both CoCl2 concentrations (Fig. 3C). However, no increase in TF activity
was observed for stimulated ST1 cells. The results of the present
study indicate that hypoxia upregulates functionally active TF
antigen on the surface of P7 cells, but not on ST1 cells.
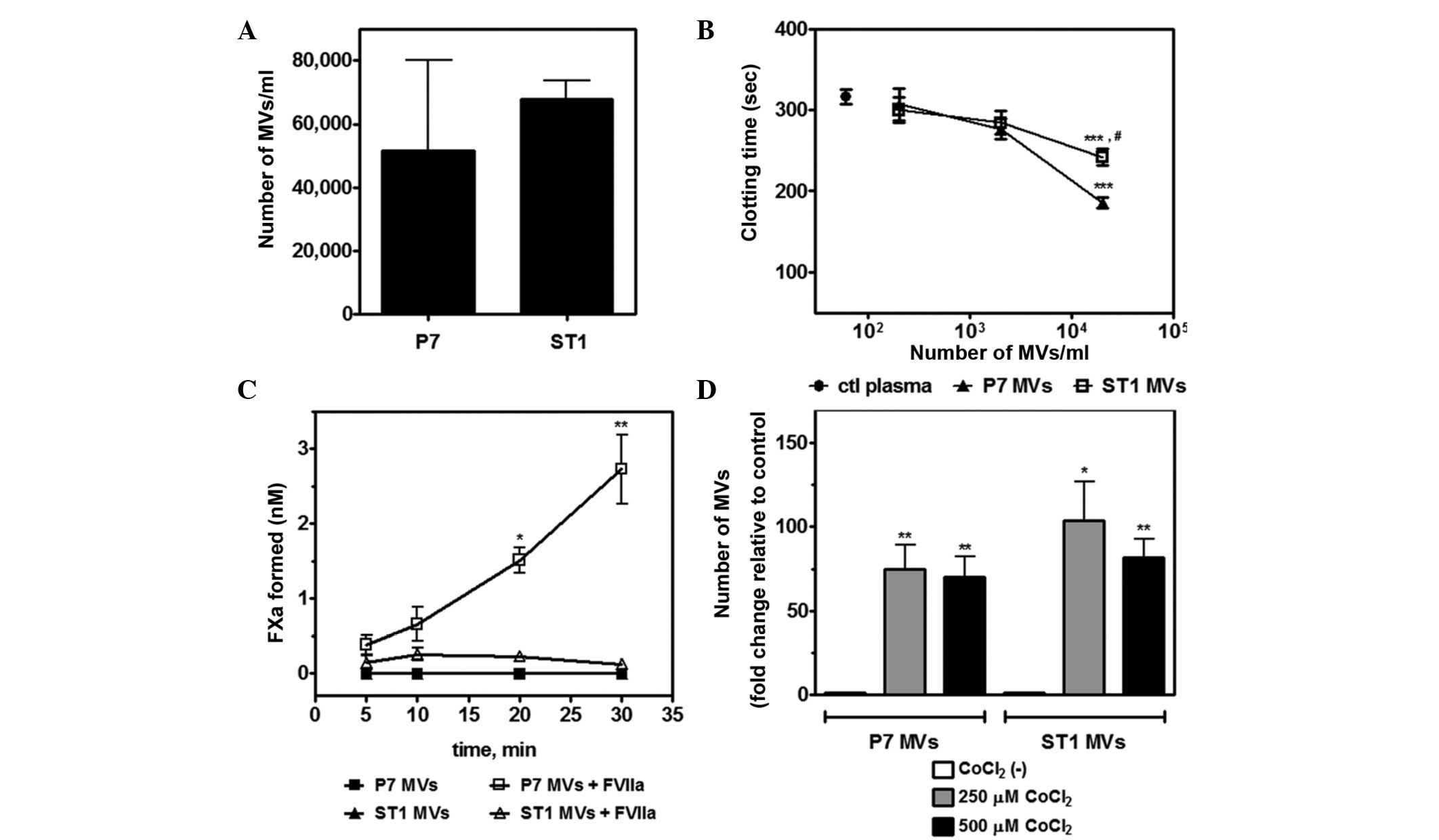
Shedding of procoagulant MVs by P7 and
ST1 cells
TF has been shown to circulate in the blood as a
component of cell-derived MVs. Increased secretion of TF-positive
MVs has been observed in several types of cancer and has been
associated with the activation of the hemostatic system (7,31).
Therefore, the present study analyzed the spontaneous shedding of
MVs by P7 and ST1 cell lines in vitro. Differential
centrifugation was performed to isolate MVs from the supernatants
of P7 and ST1 cell cultures. Flow cytometric analysis revealed a
similar rate of MV production for both cell lines (Fig. 4A). Using a one-stage clotting assay,
it was observed that MVs derived from P7 and ST1 cells shortened
the coagulation time of rat plasma at similar concentration ranges
(Fig. 4B). TF activity was also
investigated in these MVs. Comparably to their parental cells, P7
MVs supported FX activation by the extrinsic tenase complex
(TF/FVIIa), while ST1 MVs showed no TF activity (Fig. 4C). No significant FXa generation was
observed in the absence of FVIIa.
Hypoxia has been shown to markedly increase
TF-containing extracellular vesicle secretion in glioma cells
(32). Therefore, the present study
investigated whether hypoxia increases the secretion of MVs into
the conditioned media of P7 and ST1 cell cultures. The incubation
of both cell lines with 250 or 500 µM CoCl2 for 24 h
promoted a significant enhancement in MV secretion when compared
with normoxic conditions (Fig.
4D).
Taken together, these results demonstrate that P7
cells and TF-bearing MVs exhibit higher TF activity than ST1 cells,
a process that is significantly enhanced upon exposure to
hypoxia.
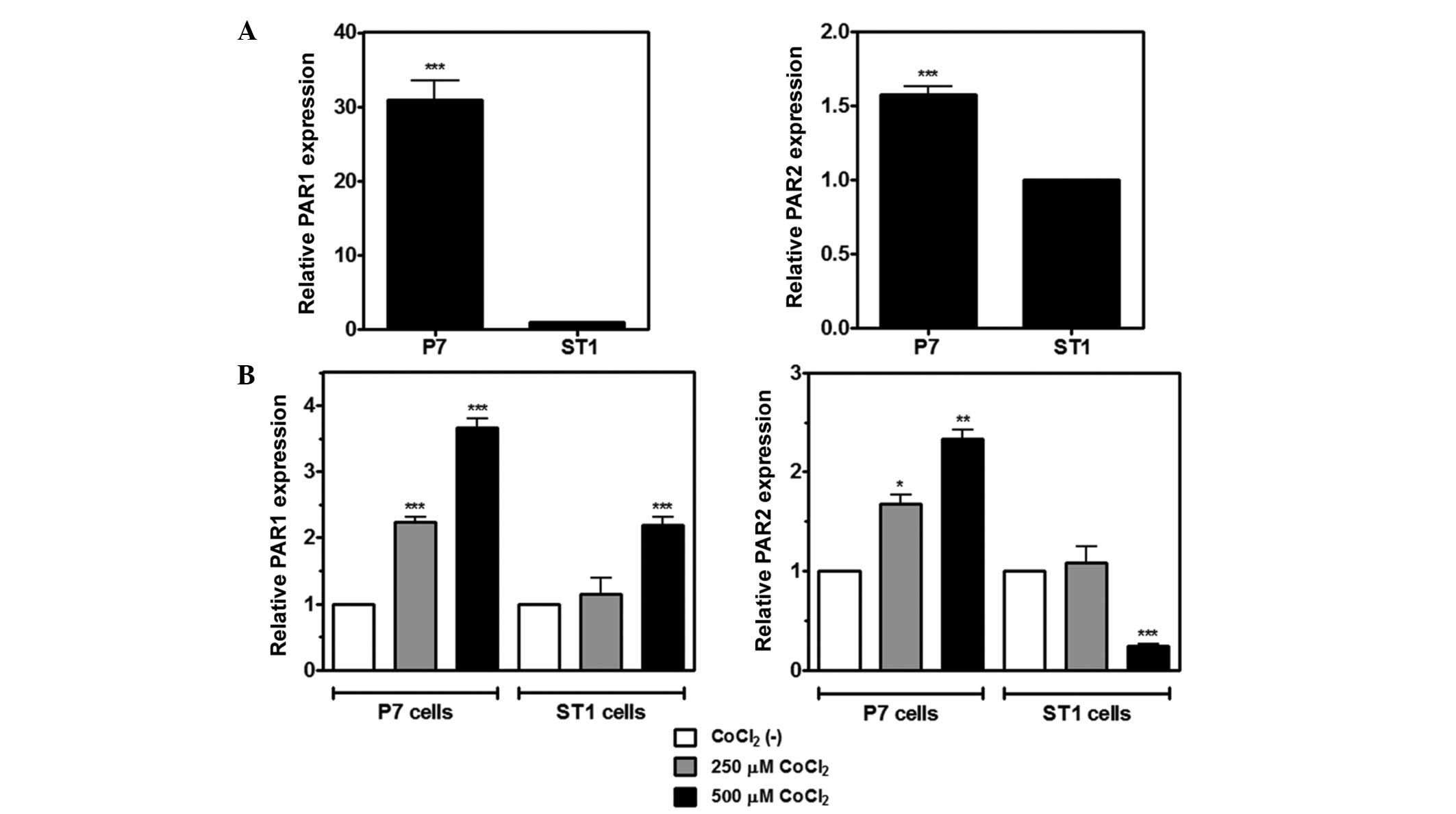
Hypoxic induction of PAR expression in
P7 and ST1 cells
In addition to TF, hypoxic stress has been shown to
modulate PAR1 expression in tumor cell lines (32,33).
Furthermore, our recent study observed increased PAR1 and PAR2
staining around necrotic areas in samples from patients with GBM
(22). In this context, the present
study analyzed the effect of CoCl2 treatment on PAR
expression in rat glioma cells. RT-qPCR showed that PAR1 and PAR2
mRNA are expressed in P7 and ST1 cells, and that P7 cells express
higher mRNA levels of both receptors when compared with ST1 cells
(Fig. 5A). Following treatment with
250 or 500 µM CoCl2 for 4 h, mRNA expression of PAR1 was
significantly upregulated in both cell lines, whereas an increase
in PAR2 expression was observed only in P7 cells (Fig. 5B). Conversely, ST1 cells exhibited
significantly reduced levels of PAR2 mRNA when treated with 500 µM
CoCl2.
Discussion
A dramatic shift in biological behavior occurs
following the transition from astrocytoma to GBM. These changes
include accelerated growth and rapid progression to mortality. The
mechanisms responsible for this abrupt onset of rapid growth are
still being defined but are likely associated with the development
of necrosis and intense angiogenesis, which are two defining
features of GBM histology and powerful predictors of poor prognosis
(15,34). Almost all GBM specimens show
microscopic intravascular thrombosis within tissue specimens and
this event has been documented as an additional distinguishing
pathological feature of GBM compared with lower grade astrocytoma
(35). Furthermore, necrosis and
subsequent hypoxia-induced angiogenesis may be initiated or
propagated by intravascular thrombosis.
In the present study, it was demonstrated that
hypoxia, a key event in GBM, induces increased expression of the
potent procoagulant TF in GBM cells and promotes TF/FVIIa activity.
This phenomenon markedly differs between the GBM cell lines P7, a
highly aggressive, glucocorticoid-resistant cell line, and ST1, a
non-tumorigenic, glucocorticoid-sensitive cell line. Under
normoxia, P7 exhibits ~3.5-fold higher expression of TF as compared
with ST1, with strong significant inverse correlation of PTEN
expression. This reflects the ability of these cell lines to
promote plasma clotting and FX activation. Notably, hypoxic stress
promoted by treatment with CoCl2 significantly enhanced
TF expression and activity in the P7 cell line.
In addition to TF, the present study also
demonstrated the presence of the procoagulant phospholipid PS on
the outer leaflet of P7 and ST1 cells, which supports FX activation
through the assembly of the prothrombinase complex, leading to
rapid thrombin generation. Both cell lines exhibit PS exposure,
with the lipid exposure more prominent in the aggressive cell line,
P7. Notably, Blanco et al recently demonstrated that
increased PS exposure occurs in vivo in a xenograft mice
model (36). Therefore, PS is
proposed as a target for the delivery of nanovesicles in the
treatment and imaging of GBM.
Previous studies have demonstrated that hypoxia
elevates the release of MVs by tumor cells (31,37).
Herein, it was demonstrated that hypoxic stress significantly
increases the production of MVs from P7 or ST1 cells. The increased
incidence of venous thrombosis throughout the course of GBM has
been well documented (38). In fact,
the elevated levels of circulating MVs in cancer patients has been
correlated with thrombosis in a number of cancer types (9,11,30). However, it has previously been
demonstrated that elevated levels of TF-positive MVs do not
correlate with thrombosis occurrence in patients with GBM (39). Additionally, intratumoral expression
of TF has not been correlated with thrombosis in patients with GBM
(40). However, increased MV
production upon the induction of hypoxic stress has been associated
with TF transfer from tumor to endothelial cells in a GBM model.
This process triggers a proangiogenic cascade dependent on PAR2
signaling in endothelial cells that in turn results in an increase
in PAR2 expression upon hypoxia (32). In addition, TF-containing MVs may
promote TF transfer between tumor cell lines with different
aggressive behaviors, thus, modulating their procoagulant
properties (41).
Several lines of evidence suggest that PAR1 has a
significant role in GBM progression. For example, Zhang et
al observed elevated PAR1 expression in GBM and identified a
correlation with patient survival (42). In addition, our previous study
demonstrated that PAR1 expression was significantly enhanced in
patients with GBM compared with those exhibiting lower grade
gliomas, and PAR1 expression was positively correlated with VEGF
expression (22). PAR1 expression has
also been documented in a rat glioma model (43). Herein, it was demonstrated that
hypoxic stress upregulates PAR1 expression in P7 and ST1 cell
lines. Notably, the aggressive cell line, P7, exhibited a
significantly higher degree of PAR1 expression under normoxia than
the ST1 cell line. Therefore, PAR1 may contribute to GBM
progression and may have an important role in hypoxia-driven
events.
In addition to PAR1, PAR2 has been associated with
GBM progression. In vitro assays demonstrate that PAR2
activation mediates several pro-tumoral responses in GBM cell lines
(20–24,26–46).
Therefore, blocking the TF/PAR2 signaling axis has been identified
as a feasible target for the treatment of GBM (45,47,48). As
observed with PAR1, the P7 cell line expressed higher levels of
PAR2 than ST1 under normoxia in the present study. Notably, hypoxic
stress significantly enhanced PAR2 expression in P7 but not ST1
cells. Thus, PAR2 signaling may be involved in hypoxia-driven
progression of patients with GBM, resulting in a more aggressive
pattern.
In conclusion, the present study demonstrates that
hypoxia enhances TF expression and MVs generation in rat GBM cell
lines. In addition, hypoxic stress upregulates PAR1 and PAR2
expression, a process that was highly evident in a glucocorticoid
resistant cell line. The results indicate that therapies targeted
against TF-PAR signaling axis may be relevant in GBM, particularly
those presenting with more aggressive behavior.
References
|
1
|
Williams JC and Mackman N: Tissue factor
in health and disease. Front Biosci. 1:358–372. 2012. View Article : Google Scholar
|
|
2
|
Østerud B and Bjørklid E: Sources of
tissue factor. Semin Thromb Hemost. 32:11–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Francischetti IM, Seydel KB and Monteiro
RQ: Blood coagulation, inflammation, and malaria. Microcirculation.
15:81–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brat DJ and Van Meir EG: Vaso-occlusive
and prothrombotic mechanisms associated with tumor hypoxia,
necrosis, and accelerated growth in glioblastoma. Lab Invest.
84:397–405. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rak J, Milsom C, Magnus N and Yu J: Tissue
factor in tumour progression. Best Pract Res Clin Haematol.
22:71–83. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ruf W, Disse J, Carneiro-Lobo TC, Yokota N
and Schaffner F: Tissue factor and cell signalling in cancer
progression and thrombosis. J Thromb Haemost. 9(Suppl 1):
S306–S315. 2011. View Article : Google Scholar
|
|
8
|
van den Berg YW, Osanto S, Reitsma PH and
Versteeg HH: The relationship between tissue factor and cancer
progression: Insights from bench and bedside. Blood. 119:924–932.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lima LG and Monteiro RQ: Activation of
blood coagulation in cancer: Implications for tumour progression.
Biosci Rep. 33:e000642013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lima LG, Oliveira AS, Campos LC, Bonamino
M, Chammas R, Werneck C, Vicente CP, Barcinski MA, Petersen LC and
Monteiro RQ: Malignant transformation in melanocytes is associated
with increased production of procoagulant microvesicles. Thromb
Haemost. 106:712–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou L, Qi XL, Xu MX, Mao Y, Liu ML and
Song HM: Microparticles: New light shed on the understanding of
venous thromboembolism. Acta Pharmacol Sin. 35:1103–1110. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boire A, Covic L, Agarwal A, Jacques S,
Sherifi S and Kuliopulos A: PAR1 is a matrix metalloprotease-1
receptor that promotes invasion and tumorigenesis of breast cancer
cells. Cell. 120:303–313. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albrektsen T, Sørensen BB, Hjortø GM,
Fleckner J, Rao LV and Petersen LC: Transcriptional program induced
by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J
Thromb Haemost. 5:1588–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen PY and Kerasi S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans SM, Judy KD, Dunphy I, Jenkins WT,
Nelson PT, Lustig RA, Jenkins K, Magarelli DP, Hahn SM, Collins RA,
et al: Hypoxia is important in the biology and aggression of human
glial brain tumors. Clin Cancer Res. 10:8177–8184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anand M and Brat DJ: Oncogenic regulation
of tissue factor and thrombosis in cancer. Thromb Res. 129(Suppl
1): S46–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magnus N, D'Asti E, Meehan B, Garnier D
and Rak J: Oncogenes and the coagulation system-forces that
modulate dormant and aggressive states in cancer. Thromb Res.
133(Suppl 2): S1–S9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rong Y, Post DE, Pieper RO, Durden DL, Van
Meir EG and Brat DJ: PTEN and hypoxia regulate tissue factor
expression and plasma coagulation by glioblastoma. Cancer Res.
65:1406–1413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magnus N, Garnier D and Rak J: Oncogenic
epidermal growth factor receptor up-regulates multiple elements of
the tissue factor signaling pathway in human glioma cells. Blood.
116:815–818. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
D'Asti E, Fang Y and Rak J: Brain
neoplasms and coagulation-lessons from heterogeneity. Rambam
Maimonides Med J. 5:e00302014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carneiro-Lobo TC, Lima MT,
Mariano-Oliveira A, Dutra-Oliveira A, Oba-Shinjo SM, Marie SK,
Sogayar MC and Monteiro RQ: Expression of tissue factor signaling
pathway elements correlates with the production of vascular
endothelial growyh factor and interleukin-8 in human astrocytoma
patients. Oncol Rep. 31:679–686. 2014.PubMed/NCBI
|
|
23
|
Armelin MC and Armelin HÀ: Glucocorticoid
hormone modulation of both cell surface and cytoskeleton related to
growth control of rat glioma cells. J Cell Biol. 97:459–465. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armelin MC, Stocco RC and Armelin HA:
Control of rat C6 glioma cell proliferation: Uncoupling of the
inhibitory effects of hydrocortisone hormone in suspension and
monolayer cultures. J Cell Biol. 97:455–458. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hamada K, Kuratsu J, Saitoh Y, Takeshima
H, Nishi T and Ushio Y: Expression of tissue factor correlates with
grade of malignancy in human glioma. Cancer. 77:1877–1883. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guan M, Jin J, Su B, Liu WW and Lu Y:
Tissue factor expression and angiogenesis in human glioma. Clin
Biochem. 35:321–325. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fernandes RS, Kirszberg C, Rumjanek VM and
Monteiro RQ: On the molecular mechanisms for the highly
procoagulant pattern of C6 glioma cells. J Thromb Haemost.
4:1546–1552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kirszberg C, Lima LG, Da Silva de Oliveira
A, Pickering W, Gray E, Barrowcliffe TW, Rumjanek VM and Monteiro
RQ: Simultaneous tissue factor expression and phosphatidylserine
exposure account for the highly procoagulant pattern of melanoma
cell lines. Melanoma Res. 19:301–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brat DJ, Castellano-Sanchez AA, Hunter SB,
Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B and Van Meir EG:
Pseudopalisades in glioblastoma are hypoxic, express extracellular
matrix proteases and are formed by an actively migrating cell
population. Cancer Res. 64:920–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rautou PE and Mackman N: Microvesicles as
risk markers for venous thrombosis. Expert Rev Hematol. 6:91–101.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Svensson KJ, Kucharzewska P, Christianson
HC, Sköld S, Löfstedt T, Johansson MC, Mörgelin M, Bengzon J, Ruf W
and Belting M: Hypoxia triggers a proangiogenic pathway involving
cancer cell microvesicles and PAR-2-mediated heparin-binding EGF
signaling in endothelial cells. Proc Natl Acad Sci USA.
108:13147–13152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naldini A, Filippi I, Ardinghi C, Silini
A, Giavazzi R and Carraro F: Identification of a functional role
for the protease-activated receptor-1 in hypoxic breast cancer
cells. Eur J Cancer. 45:454–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammoud MA, Sawaya R, Shi W, Thall PF and
Leeds NE: Prognostic significance of preoperative MRI scans in
glioblastoma multiforme. J Neurooncol. 27:65–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tehrani M, Friedman TM, Olson JJ and Brat
DJ: Intravascular thrombosis in central nervous system
malignancies: A potential role in astrocytoma progression to
glioblastoma. Brain Pathol. 18:164–171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blanco VM, Chu Z, Vallabhapurapu SD,
Sulaiman MK, Kendler A, Rixe O, Warnick RE, Franco RS and Qi X:
Phosphatidylserine-selective targeting and anticancer effects of
SapC-DOPS nanovesicles on brain tumors. Oncotarget. 5:7105–7118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang T, Gilkes DM, Takano N, Xiang L, Luo
W, Bishop CJ, Chaturvedi P, Green JJ and Semenza GL:
Hypoxia-inducible factors and RAB22A mediate formation of
microvesicles that stimulate breast cancer invasion and metastasis.
Proc Natl Acad Sci USA. 111:E3234–E3242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marras LC, Geerts WH and Perry JR: The
risk of venous thromboembolism is increased throughout the course
of malignant glioma: An evidence-based review. Cancer. 89:640–664.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thaler J, Ay C, Mackman N, Bertina RM,
Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, et
al: Microparticle-associated tissue factor activity, venous
thromboembolism and mortality in pancreatic, gastric, colorectal
and brain cancer patients. J Thromb Haemost. 10:1363–1370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thaler J, Preusser M, Ay C, Kaider A,
Marosi C, Zielinski C, Pabinger I and Hainfellner JA: Intratumoral
tissue factor expression and risk of venous thromboembolism in
brain tumor patients. Thromb Res. 131:162–165. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lima LG, Leal AC, Vargas G, Porto-Carreiro
I and Monteiro RQ: Intercellular transfer of tissue factor via the
uptake of tumor-derived microvesicles. Thromb Res. 132:450–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Zhan H, Xu W, Yuan Z, Lu P, Zhan
L and Li Q: Upregulation of matrix metalloproteinase-1 and
proteinase-activated receptor-1 promotes the progression of human
gliomas. Pathol Res Pract. 207:24–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Itsekson-Hayosh Z, Shavit-Stein E, Last D,
Goez D, Daniels D, Bushi D, Gera O, Zibly Z, Mardor Y, Chapman J
and Harnof S: Thrombin activity and thrombin receptor in rat
glioblastoma model: Possible markers and targets for intervention?
J Mol Neurosci. 56:644–651. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dutra-Oliveira A, Monteiro RQ and
Mariano-Oliveira A: Protease-activated receptor-2 (PAR2) mediates
VEGF production through the ERK1/2 pathway in human glioblastoma
cell lines. Biochem Biophys Res Commun. 421:221–227. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harter PN, Dützmann S, Drott U, Zachskorn
C, Hattingen E, Capper D, Gessler F, Senft C, Seifert V, Plate KH,
et al: Anti-tissue factor (TF9-10H10) treatment reduces tumor cell
invasiveness in a novel migratory glioma model. Neuropathology.
33:515–525. 2013.PubMed/NCBI
|
|
46
|
Luo R, Wang X, Dong Y, Wang L and Tian C:
Activation of protease-activated receptor 2 reduces glioblastoma
cell apoptosis. J Biomed Sci. 21:252014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carneiro-Lobo TC, Konig S, Machado DE,
Nasciutti LE, Forni MF, Francischetti IM, Sogayar MC and Monteiro
RQ: Ixolaris, a tissue factor inhibitor, blocks primary tumor
growth and angiogenesis in a glioblastoma model. J Thromb Haemost.
7:1855–1864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Carneiro-Lobo TC, Schaffner F, Disse J,
Ostergaard H, Francischetti IM, Monteiro RQ and Ruf W: The
tick-derived inhibitor Ixolaris prevents tissue factor signaling on
tumor cells. J Thromb Haemost. 10:1849–1858. 2012. View Article : Google Scholar : PubMed/NCBI
|