Introduction
Cutaneous squamous cell carcinoma (cSCC) is one of
the most common types of skin cancer, and is responsible for ~20%
of skin cancer-associated mortalities yearly (1,2). Although
the risk factors of cSCC are well characterized, the molecular
pathogenesis of this particular tumor type is still not well
understood. As increasing numbers of patients succumb to cSCC, it
is an urgent requirement to clarify the molecular mechanisms of
this cancer and to develop novel and more effective treatment
strategies against the malignancy.
microRNA (miRNA), a class of naturally occurring,
17–25 nucleotide small noncoding small RNA, regulates the
expression of genes through binding to the 3′ untranslated regions
of target mRNAs. miRNAs have emerged as key factors involved in
several biological processes, including development,
differentiation, cell proliferation and tumorigenesis (3,4). Several
studies have demonstrated that alterations in miRNA genes lead to
tumor formation, and several miRNAs that regulate either tumor
suppression or tumor formation have been identified (5–7). In
previous studies, a number of dysregulated miRNAs were observed in
cSCC (8,9). Zhou et al demonstrated that
miR-365 was overexpressed in cells and clinical specimens of cSCC
(10). miR-31 is overexpressed in
cSCC and regulates cancer-associated phenotypes of cSCC (11). The decreased expression of the
miR-193b/365a cluster during tumor progression suggests a tumor
suppressor role of cSCC (12).
Previous studies have demonstrated that miR-199a-5p
expression is deregulated in various cancer types (7,13). For
example, downregulated miR-199a-5p expression was observed in
small-cell carcinoma and hepatocellular carcinoma (14,15).
Decreased expression of miR-199a-5p contributes to increased cell
invasion by functional deregulation of discoidin domain receptor 1
activity (15). Forced expression of
miR-199a-5p promoted cisplatin-induced inhibition of cell
proliferation by targeting autophagy-associated gene 7 in
hepatocellular carcinoma (16). In
addition, the increased expression of miR-199a-5p was observed in
gastric and colorectal cancer (17,18).
However, the roles and underlying molecular mechanisms of
miR-199a-5p in cSCC are not completely understood.
This study revealed that miR-199a-5p is an upstream
regulator of CDH1 (E-cadherin) which suppresses the expression of
E-cadherin in cSCC cells. To further analyze the oncogenic role of
miR-199a-5p in cSCC progression, we analyzed the effect of
miR-199a-5p on cell invasion and the activity of matrix
metalloproteinase (MMP)2 and MMP9 in cSCC cells.
Materials and methods
Cell culture and treatment
A-431 SCC cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultivated in
RPMI-1640 medium with a final concentration of 10% fetal bovine
serum. Cells were cultured in conditions of 95% air and 5% carbon
dioxide at 37°C.
Ectopic expression of miR-199a-5p was achieved in
the cells by transfection with miR-199a-5p mimics or inhibitors
using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad,
CA, USA). Cells were plated in 24-well plates and transfected for
24 h or 48 h. Total RNA or protein was extracted from the indicated
cells for analysis.
Quantitative polymerase chain reaction
(qPCR) assay
Total RNA was extracted from the indicated cells
according to the manufacturer's instructions using the Qiagen
RNeasy kit (Qiagen Nordic, Solna, Sweden). The expression of CD44
and Ezrin mRNA was assessed by SYBR-Green qPCR assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). β-actin was used as an
endogenous control. The specific primers were as follows:
E-cadherin: F, cgacccaacccaagaatcta; R, aggctgtgccttcctacaga; and
β-actin: F, cattaaggagaagctgtgct; R, gttgaaggtagtttcgtgga. A
miScript reverse transcription kit was used to reverse transcribe
RNA into cDNA and a miScript SYBR-Green PCR kit (both Qiagen
Nordic) was used for qPCR to detect the expression of miR-199a-5p.
The specific primers were as follows: miRNA-199a: F,
tcccagtgttcagactacc; R, tttggcactagcacatt; U6: F,
ctcgcttcggcagcaca; R, aacgcttcacgaatttgcgt. The expression of U6
was used as an endogenous control. Data were analyzed using the
2-ΔΔCT method.
Western blot analysis
Total protein (60 µg) extracted from the indicated
cells was separated on SDS-polyacrylamide gels for E-cadherin and
GAPDH detection. GAPDH was used as a loading control. The protein
in gels was transferred to nitrocellulose membranes, and then
incubated with the indicated antibodies at the recommended
dilutions overnight at 4°C. Next, the membranes were washed with
0.1 M phosphate-buffered saline with Tween-20 and incubated with
horseradish peroxidase-conjugated secondary antibody. Signals were
visualized using enhanced chemiluminescence substrates and
quantified using Optiquant 3.0 software (PerkinElmer, Inc.,
Waltham, MA, USA).
Invasion assay
Cells were cultivated to 80% confluence on 12-well
plates. Then, cellular growth procedures were observed at 24 h. All
the experiments were repeated in triplicate. Transwell invasion
chambers were used to evaluate cell invasion. Then cells invading
across the membrane were counted under a light microscope (YS100;
Nikon Corporation, Tokyo, Japan).
Gelatin zymography
An MMP zymography assay kit (Applygen Technologies,
Inc., Beijing, China) was used to assess the activity of MMP2 and
MMP9. Protein extracts and positive mixture were mixed with an
equal volume of 2X sodium dodecyl sulphate (SDS)-polyacrylamide gel
electrophoresis non-reducing buffer, and electrophoresed on 8% SDS
polyacrylamide gels containing 2 mg/ml gelatin. Gels were then
washed twice for 30 min in buffer A at room temperature, and
incubated for 4 h at room temperature in incubation buffer B. Gels
were then stained for 2 h with 0.25% Coomassie brilliant blue and
then destained in destaining buffer (10% acetic acid and 20%
methanol) for 60 min.
Statistical analysis
Data were expressed as the means ± standard
deviation and analyzed by Student's t-test. P<0.05
compared with respective controls was considered to indicate a
statistically significant difference.
Results
miR-199a-5p regulates the expression
levels of E-cadherin in cSCC
E-cadherin is downregulated in cSCC tumors (19). To investigate whether the
downregulation of E-cadherin is regulated by miR-199a-5p,
miR-199a-5p mimics and inhibitors were transfected into A-431 cells
and E-cadherin expression was assessed by qPCR and western blot
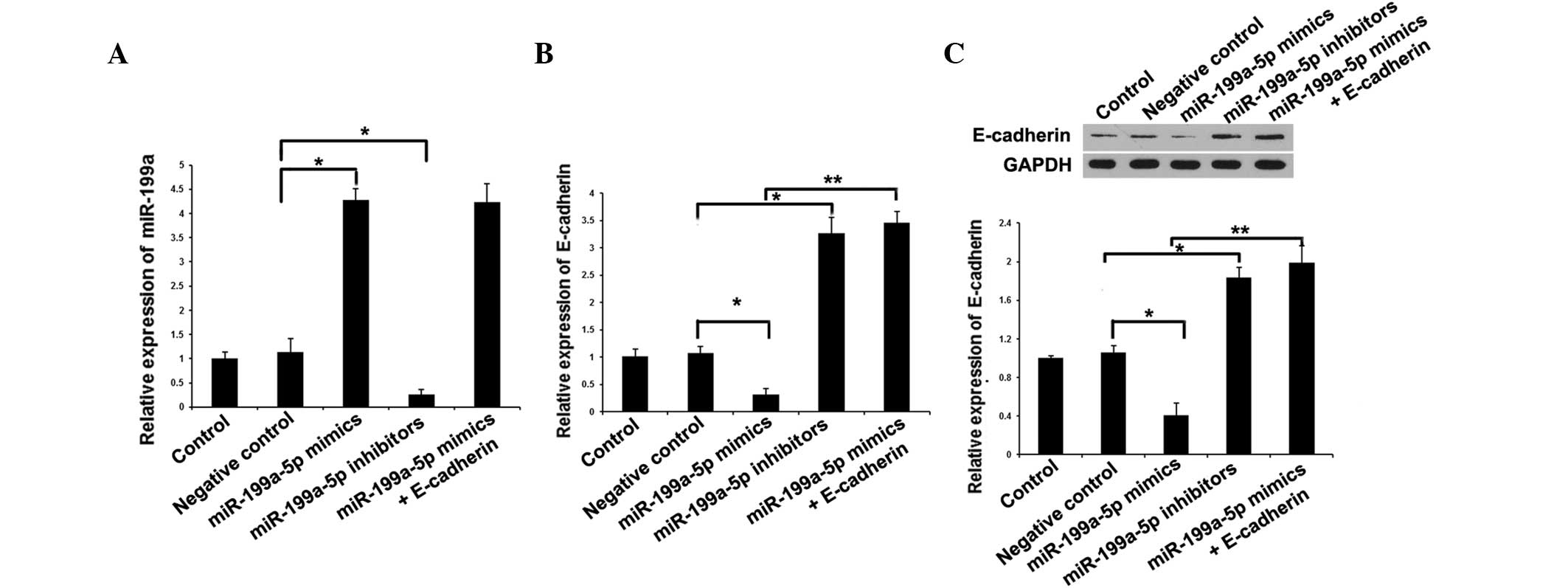
analysis. qPCR was used to measure the expression of miR-199a-5p
following treatment with miR-199a-5p mimics or inhibitor. The qPCR
results revealed that miR-199a-5p mimics efficiently induced the
expression of miR-199a, and miR-199a-5p inhibitor significantly
downregulated the expression of miR-199a (Fig. 1A). qPCR and western blot analysis
revealed that ectopic expression of miR-199a-5p by transfection of
miR-199a-5p mimic led to decreased expression of E-cadherin, and
that knockdown of miR-199a-5p by miR-199a-5p inhibitors upregulated
the expression of E-cadherin (Fig. 1B and
C). Additionally, E-cadherin and miR-199a-5p mimics were
cotransfected into A-431 cells. miR-199a-5p expression was not
affected by E-cadherin transfection. However, the miRNA and protein
expression of E-cadherin were greatly increased in the miR-199a-5p
mimics + E-cadherin group (A-431 cells cotransfected with
E-cadherin and miR-199a-5p mimics) compared with that in the
miR-199a-5p mimics group. Together, the above results confirmed
that E-cadherin is one of the downstream genes of miR-199a-5p in
cSCC cells, and that overexpression of miR-199a-5p induces the
repression and downregulates the expression of E-cadherin.
Overexpression of miR-199a-5p
represses cSCC cell invasion
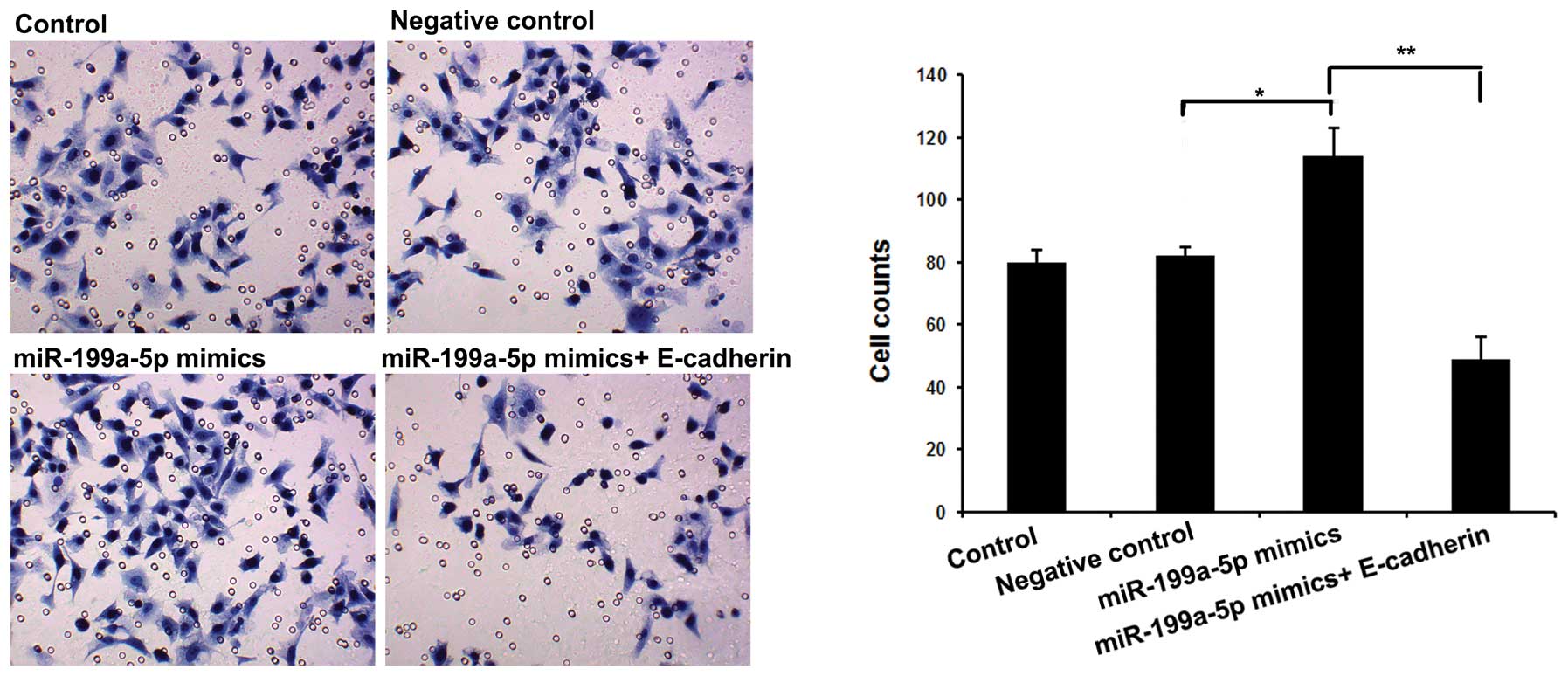
To further explore the function of miR-199a-5p in
cSCC cells, we evaluated cell invasion by Transwell following
transfection with miR-199a-5p mimics alone or combined with
E-cadherin. As shown in Fig. 2, we
observed that overexpression of miR-199a-5p significantly increased
cell invasion, which was reversed by cotransfection with
miR-199a-5p mimics and E-cadherin. Together, the above results may
indicate that miR-199-5p performs an oncogenic role by suppressing
E-cadherin expression in cSCC.
Overexpression of miR-199a-5p affects
activity of MMP2 and MMP9 in cSCC
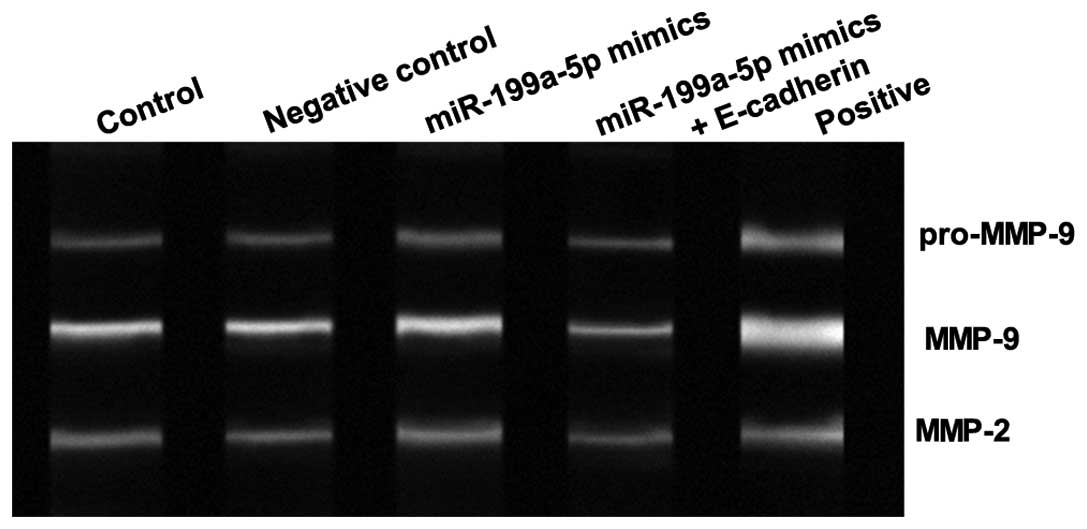
In addition, we assessed the effects of miR-199a-5p
on the activity of MMP2 and MMP9. As shown in Fig. 3, the activity of MMP2 and MMP9 was
significantly upregulated by miR-199a-5p mimics compared with the
scramble control, and this was reversed by cotransfection with
miR-199a-5p mimics and E-cadherin. This suggested that upregulation
of miR-199a-5p increased the activity of MMP2 and MMP9 by
regulating E-cadherin expression in cSCC cells.
Discussion
Overall, the morbidity of cSCC has been increasing
worldwide over the past 10 years. The 5-year overall survival rate
of patients with metastatic cSCC is ~50% lower than that of
patients with regional or localized disease (20). A better understanding of the genes or
molecular alterations involved in cSCC progression is likely to be
useful in early detection and future targeted treatment strategies.
Previously, multiple studies have revealed that miRNA expression is
a crucial regulator of cSCC progression (9,21,22). In this study, we demonstrated that
miR-199a-5p was an upstream regulator of E-cadherin, and that it
suppressed the expression of E-cadherin in cSCC cells. In addition,
miR-199a-5p significantly decreased cell invasion and the activity
of MMP2 and MMP9 in cSCC cells.
E-cadherin is a 120 kDa calcium-dependent
transmembrane glycoprotein encoded by the CDH1 gene. It is
expressed in the majority of epithelial cells. E-cadherin, as a
crucial protein-mediated cell-cell adhesion molecule, plays an
essential role in establishing cell polarity and in maintaining
normal tissue architecture. Loss of E-cadherin decreases cellular
adhesion, resulting in an increase in cellular motility (23). Accumulating studies have demonstrated
that E-cadherin is closely associated with tumor invasion and
metastasis (24). E-cadherin is
involved in the process of epithelial-to-mesenchymal transition,
which contributes to intercellular adhesion, acquisition of a
mesenchymal phenotype and enhanced migratory potential in tumors.
It has been reported that downregulation of E-cadherin in
esophageal carcinoma was associated with an increased invasive and
metastatic potential (25). A
previous study has revealed that E-cadherin, as an epithelial
marker, is downregulated in cSCC (26). As lower expression of E-cadherin in
cSCC tumors and cells has been confirmed in previous studies
(19,27), the direct upstream miRNAs of
E-cadherin have become increasing significant in understanding the
mechanism of E-cadherin-involved tumor progression. In a previous
study, the downregulated expression of miRNA-199a was observed in
liver, breast and bladder cancer (28). It was reported that inducing the
expression of miRNA-199a was responsible for decreasing the
proliferation of gastric cancer cells by targeting the mTOR
signaling pathway (29). The abundant
expression of miR-199a-5p has been observed in liver tissue, and in
epithelial as well as non-epithelial tissues (30). Inhibition of miR-199a-5p impaired the
metastatic potential of gastric cancer cells, and E-cadherin was
identified as a direct and functional target of miR-199a-5p in
gastric cancer cells (31). In the
present study, we confirmed that E-cadherin was one of the
downstream genes of miR-199a-5p in cSCC cells, and that
overexpression of miR-199a-5p induced the repression and
downregulated the expression of E-cadherin in cSCC.
MMPs are considered to play an essential role in the
metastasis of tumor cells by decomposing the extracellular matrix
and destroying the basement membrane of blood vessels. The
dissolution of the basement membrane caused by MMP in tumors is a
notable process in the movement of tumor cells. A significant
correlation has been reported between the activation of MMP and the
migration and invasion of tumor cells (32,33). The
expression of MMP2 and MMP9 was observed to be higher in patients
with lymph node metastases compared with patients without lymph
node involvement in oropharyngeal SCC (34). By gelatin zymography, we observed that
transfection of miR-199a-5p mimic induced the activity of MMP2 and
MMP9. Inversely, the activity of MMP2 and MMP9 was reduced by
cotransfection with miR-199a-5p mimic and E-cadherin. Thus, the
oncogenic role of miR-199a-5p in cell invasion in cSCC cells may be
associated with the activity of MMP2 and MMP9.
In conclusion, we demonstrated that miR-199a-5p
specifically suppressed the expression of E-cadherin at the mRNA
and protein level in cSCC cells. Moreover, the oncogenic role of
miR-199a in cell invasion in cSCC cells was also associated with
the activity of MMP2 and MMP9. This miR-199a-5p/E-cadherin/MMP
pathway may therefore offer a novel perspective for cSCC metastasis
and may represent an effective therapeutic target for cSCC
treatment.
References
|
1
|
Alam M and Ratner D: Cutaneous
squamous-cell carcinoma. N Engl J Med. 344:975–983. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ratushny V, Gober MD, Hick R, Ridky TW and
Seykora JT: From keratinocyte to cancer: the pathogenesis and
modeling of cutaneous squamous cell carcinoma. J Clin Invest.
122:464–472. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang S and He X: The role of microRNAs in
liver cancer progression. Br J Cancer. 104:235–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kent OA and Mendell JT: A small piece in
the cancer puzzle: microRNAs as tumor suppressors and oncogenes.
Oncogene. 25:6188–6196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer - A brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ,
Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG and Bae DS: Altered
MicroRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruegger C, Kempf W, Spoerri I, Arnold AW,
Itin PH and Burger B: MicroRNA expression differs in cutaneous
squamous cell carcinomas and healthy skin of immunocompetent
individuals. Exp Dermatol. 22:426–428. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sand M, Skrygan M, Georgas D, Sand D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Microarray analysis of
microRNA expression in cutaneous squamous cell carcinoma. J
Dermatol Sci. 68:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou M, Liu W, Ma S, Cao H, Peng X, Guo L,
Zhou X, Zheng L, Guo L, Wan M, et al: A novel onco-miR-365 induces
cutaneous squamous cell carcinoma. Carcinogenesis. 34:1653–1659.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang A, Landén NX, Meisgen F,
Lohcharoenkal W, Ståhle M, Sonkoly E and Pivarcsi A: MicroRNA-31 is
overexpressed in cutaneous squamous cell carcinoma and regulates
cell motility and colony formation ability of tumor cells. PLoS
One. 9:e1032062014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gastaldi C, Bertero T, Xu N,
Bourget-Ponzio I, Lebrigand K, Fourre S, Popa A, Cardot-Leccia N,
Meneguzzi G, Sonkoly E, et al: MiR-193b/365a cluster controls
progression of epidermal squamous cell carcinoma. Carcinogenesis.
35:1110–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang L, Lin JX, Yu YH, Zhang MY, Wang HY
and Zheng M: Downregulation of six microRNAs is associated with
advanced stage, lymph node metastasis and poor prognosis in small
cell carcinoma of the cervix. PLoS One. 7:e337622012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen Q, Cicinnati VR, Zhang X, Iacob S,
Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G and
Beckebaum S: Role of microRNA-199a-5p and discoidin domain receptor
1 in human hepatocellular carcinoma invasion. Mol Cancer.
9:2272010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F
and Xia Q: Cisplatin-induced downregulation of miR-199a-5p
increases drug resistance by activating autophagy in HCC cell.
Biochem Biophys Res Commun. 423:826–831. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He XJ, Ma YY, Yu S, Jiang XT, Lu YD, Tao
L, Wang HP, Hu ZM and Tao HQ: Up-regulated miR-199a-5p in gastric
cancer functions as an oncogene and targets klotho. BMC cancer.
14:2182014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Liu J, Jiang B, Chen J, Fu Z, Bai F,
Jiang J and Tang Z: MiR-199a-5p loss up-regulated DDR1 aggravated
colorectal cancer by activating epithelial-to-mesenchymal
transition related signaling. Dig Dis Sci. 59:2163–2172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrette K, Van Kelst S, Wouters J,
Marasigan V, Fieuws S, Agostinis P, van den Oord J and Garmyn M:
Epithelial-mesenchymal transition during invasion of cutaneous
squamous cell carcinoma is paralleled by AKT activation. Br J
Dermatol. 171:1014–1021. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanemura A, Terando AM, Sim MS, van Hoesel
AQ, de Maat MF, Morton DL and Hoon DS: CpG island methylator
phenotype predicts progression of malignant melanoma. Clin Cancer
Res. 15:1801–1807. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu N, Zhang L, Meisgen F, Harada M,
Heilborn J, Homey B, Grandér D, Ståhle M, Sonkoly E and Pivarcsi A:
MicroRNA-125b down-regulates matrix metallopeptidase 13 and
inhibits cutaneous squamous cell carcinoma cell proliferation,
migration and invasion. J Biol Chem. 287:29899–29908. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nelson WJ and Nusse R: Convergence of Wnt,
beta-catenin and cadherin pathways. Science. 303:1483–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng QW, He BS, Pan YQ, Sun HL, Xu YQ, Gao
TY, Li R, Song GQ and Wang SK: Roles of E-cadherin (CDH1) genetic
variations in cancer risk: a meta-analysis. Asian Pac J Cancer
Prev. 15:3705–3713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimada Y, Hashimoto Y, Kan T, Kawamura J,
Okumura T, Soma T, Kondo K, Teratani N, Watanabe G, Ino Y, et al:
Prognostic significance of dysadherin expression in esophageal
squamous cell carcinoma. Oncology. 67:73–80. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toll A, Masferrer E, Hernández-Ruiz ME,
Ferrandiz-Pulido C, Yébenes M, Jaka A, Tuneu A, Jucglà A, Gimeno J,
Baró T, et al: Epithelial to mesenchymal transition markers are
associated with an increased metastatic risk in primary cutaneous
squamous cell carcinomas but are attenuated in lymph node
metastases. J Dermatol Sci. 72:93–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brouxhon SM, Kyrkanides S, Raja V,
Silberfeld A, Teng X, Trochesset D, Cohen J and Ma L:
Ectodomain-specific E-cadherin antibody suppresses skin SCC growth
and reduces tumor grade: a multitargeted therapy modulating RTKs
and the PTEN-p53-MDM2 axis. Mol Cancer Ther. 13:1791–1802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang J, Gusev Y, Aderca I, Mettler TA,
Nagorney DM, Brackett DJ, Roberts LR and Schmittgen TD: Association
of MicroRNA expression in hepatocellular carcinomas with hepatitis
infection, cirrhosis and patient survival. Clin Cancer Res.
14:419–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng W, Chen ZY, Wang L, Wang Z and Li J:
MicroRNA-199a-3p is downregulated in gastric carcinomas and
modulates cell proliferation. Genet Mol Res. 12:3038–3047. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang Y, Ridzon D, Wong L and Chen C:
Characterization of microRNA expression profiles in normal human
tissues. BMC Genomics. 8:1662007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao X, He L, Li T, Lu Y, Miao Y, Liang S,
Guo H, Bai M, Xie H, Luo G, et al: SRF expedites metastasis and
modulates the epithelial to mesenchymal transition by regulating
miR-199a-5p expression in human gastric cancer. Cell Death Differ.
21:1900–1913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ryzhakova OS and Solov'eva NI: Matrix
metalloproteinases (MMP)-MMP-1,-2,-9 and its endogenous activity
regulators in transformed by E7 oncogene HPV16 and HPV18 cervical
carcinoma cell lines. Biomed Khim. 59:530–540. 2013.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohtasham N, Babakoohi S, Shiva A, Shadman
A, Kamyab-Hesari K, Shakeri MT and Sharifi-Sistani N:
Immunohistochemical study of p53, Ki-67, MMP-2 and MMP-9 expression
at invasive front of squamous cell and verrucous carcinoma in oral
cavity. Pathol Res Pract. 209:110–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Burduk PK, Bodnar M, Sawicki P, Szylberg
Ł, Wiśniewska E, Kaźmierczak W, Martyńska M and Marszałek A:
Expression of metalloproteinases 2 and 9 and tissue inhibitors 1
and 2 as predictors of lymph node metastases in oropharyngeal
squamous cell carcinoma. Head Neck. 37:418–422. 2015. View Article : Google Scholar : PubMed/NCBI
|