Introduction
Lung cancer is one of the most common malignant
tumors worldwide, with smoking and other environmental factors
considered the main risk factors of the disease (1). The disease markedly impairs patient
health and quality of life (2). At
present, treatments include surgical resection, radiotherapy and
chemotherapy, however, molecular targeted therapy that specifically
kills cancer cells with less toxicity to normal cells presents an
additional treatment that may contribute to improving survival and
quality of life in lung cancer patients (3–5).
Gefitinib, a selective inhibitor of the tyrosine kinase, epidermal
growth factor receptor (EGFR), suppresses tumor growth, metastasis
and vascularization and has been demonstrated to induce tumor cell
apoptosis and sensitivity to radiotherapy and chemotherapy
(6–7).
Gefitinib is used for the targeted therapy of lung cancer; however,
the mechanism of action remains unclear. Autophagy is the stress
response exhibited by eukaryotic cells to a changing internal and
external environment, which maintains homeostasis via the breakdown
of intracellular proteins and organelles (8).
Autophagy is considered as a double-edged sword with
regard to genesis, development and the treatment of tumors as it
kills tumor cells but also protect tumor cells against injury
(9). Microenvironment alteration
during tumor formation has been demonstrated to induce autophagy,
limiting tumor metastasis, and thus modulation of the autophagic
signaling pathway may present a potential therapeutic target in
tumor metastasis (10). Autophagy has
been identified as the early response to gefitinib in the treatment
of EGFR-positive breast cancer (11),
and gefitinib induces autophagy in lung cancer via activation of
the AMP-activated protein kinase (AMPK) pathway (12). Furthermore, combined treatment with
protein kinase B (AKT) inhibitors, chloroquine and gefitinib
prevents compensatory autophagy in cells and induces EGFR-mutant
lung cancer cell death (13). By
contrast, autophagy may also promote lung cancer invasion and
metastasis induced by Toll-like receptors, indicating that
suppression of autophagy may present a novel lung cancer treatment
(14). To date, no studies have
confirmed whether autophagy is induced or suppressed during
gefitinib-targeted therapy of lung cancer, and the association
between autophagy and apoptosis remains unclear. Therefore, the
present study investigated the effect of gefitinib on autophagy in
the EGFR wild-type non-small cell lung cancer (NSCLC) A549 cell
line and the A549-gefitinib-resistant (GR) cell line and analyzed
the association between autophagy and apoptosis and the potential
underlying regulatory mechanism of these processes.
Materials and methods
Cell culture
The NSCLC A549 and A549-GR cell lines (Cancer
Research Institute of Southern Medical University, Guangzhou,
China) were cultured in RPMI 1640 medium supplemented with 10%
fetal bovine serum and 1% mycillin (HyClone; GE Healthcare, Logan,
UT, USA) at 37°C in a humidified atmosphere of 5% CO2.
Cells at the exponential growth phase were then incubated with
phosphate-buffered saline (PBS) (blank control group) or 50, 100
200, and 500 nmol/l gefitinib (AstraZeneca, Cambridge, UK) for 3–5
days. The levels of autophagy, proliferation and apoptosis were
then analyzed.
Acridine orange (AO) staining
Cells at the exponential growth phase were suspended
and seeded in a 6-well plate at a density of 1×105
cells/well. After cells became adherent, 1 ml dimethyl sulfoxide
(DMSO) (Beyotime Institute of Biotechnology, Shanghai, China) was
added to the first well (negative control group), 1 ml rapamycin
(Sigma-Aldrich, St. Louis, MO, USA) to the second well (positive
control group), and 50, 100, 200 and 500 nmol/l gefitinib was added
to the third, fourth, fifth and sixth well (study group),
respectively. After 48 h, the plate was stained with 50 µmol/l AO,
incubated for 15 min in the dark and washed 3 times with PBS to
identify the autophagic cells. Autophagic cell death was examined
using a fluorescence microscope (CX41-32RFL; Olympus Corporation,
Tokyo, Japan).
Western blot analysis
Cells were seeded in a 6-well plate at a density of
1×105 cells/well for 24 h. To isolate total protein, 250
µl RIPA buffer (Biosdec, Wuhan, China) was added to each well for
30 min. Expression of associated proteins was analyzed using a
western blot kit (Biosdec, Wuhan, China). Protein samples (50 µg)
were separated by SDS-PAGE gel electrophoresis and transferred to a
0.45 µm polyvinylidene fluoride membrane followed by incubation
overnight at 4°C with rat anti-human antibodies [light chain 3B
(LC3B), phosphatidylinositol 3-kinase (PI3K), Akt, phosphorylated
(p)-Akt, mammalian target of rapamycin (mTOR), p-mTOR, and β-actin]
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at a dilution
of 1:1,000. After decoloration, the samples were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (Biosdec, Wuhan, China) at a 1:5,000 dilution for 1 h at
room temperature and unbound antibody was washed away. Proteins
were visualized following treatment with enhanced chemiluminescence
reagent (Biosdec, Wuhan, China) for 1–2 min and exposure to X-ray
film. Developed films were processed using BandScan software (Glyko
Inc., Novato, CA, USA) to determine optical densities.
MTT cell proliferation/viability
assay
Cells (2×103 cells/well) at the
exponential phase were seeded in a 96-well plate. After cells
became adherent, the medium was replaced with 5 mg/ml MTT solution
(Beyotime Institute of Biotechnology) and incubated in the dark for
4 h at 37°C. The supernatant was aspirated from the plate and 150
µl DMSO (Beyotime Institute of Biotechnology) was added to each
well. Next, the plate was agitated for 10 min at room temperature
and absorbance was measured at a wavelength of 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Flow cytometry analysis
Cells (1×106/well) were cultured in a
6-well plate. After the cells became adherent, the medium was
aspirated and cells were suspended, centrifuged (4,000 × g for 5
min) and fixed in 75% precooled ethanol overnight at −20°C.
Following centrifugation, the supernatant was discarded and the
sediment was washed twice with PBS, resuspended in PBS (480
µl/well) and stained with 10 µl Annexin-V and propidium iodide (PI;
Beyotime Institute of Biotechnology). Cellular apoptosis was
analyzed by flow cytometry (BD FACSCalibur; BD Biosciences, San
Diego, CA, USA).
Inhibition of the PI3K/AKT/mTOR
signaling pathway
The PI3K inhibitor, LY294002 (10 nmol/l), was added
to the culture medium. Cellular autophagy, apoptosis and
proliferation were analyzed 48 h after culturing.
Statistical analysis
Data were expressed as the mean ± standard deviation
and analyzed by paired t-test using SPSS 16.0 software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Gefitinib induces autophagy in A549
cells
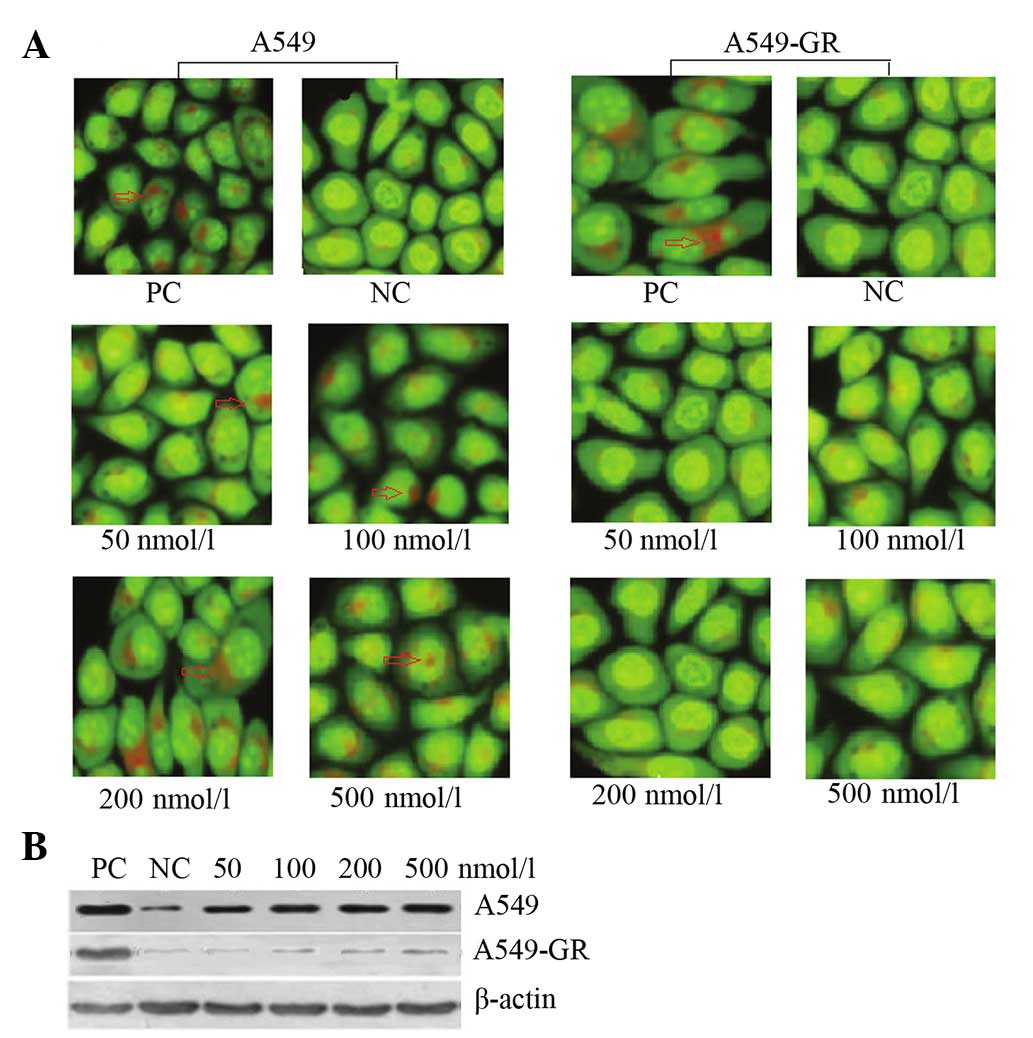
AO staining revealed that gefitinib induced the
formation of autophagic vacuoles in A549 cells in a dose-dependent
manner. By contrast, no significant autophagy was identified in the
A549-GR cells (Fig. 1A). Furthermore,
the expression of LC3B, an autophagy-related protein, varied
between the two lung cancer cell lines. Following gefitinib
treatment, LC3B expression exhibited a dose-dependent increase in
A549 cells, however, no significant difference in LC3B expression
was identified in A549-GR cells (Fig.
1B).
Gefitinib induces apoptosis in A549
cells
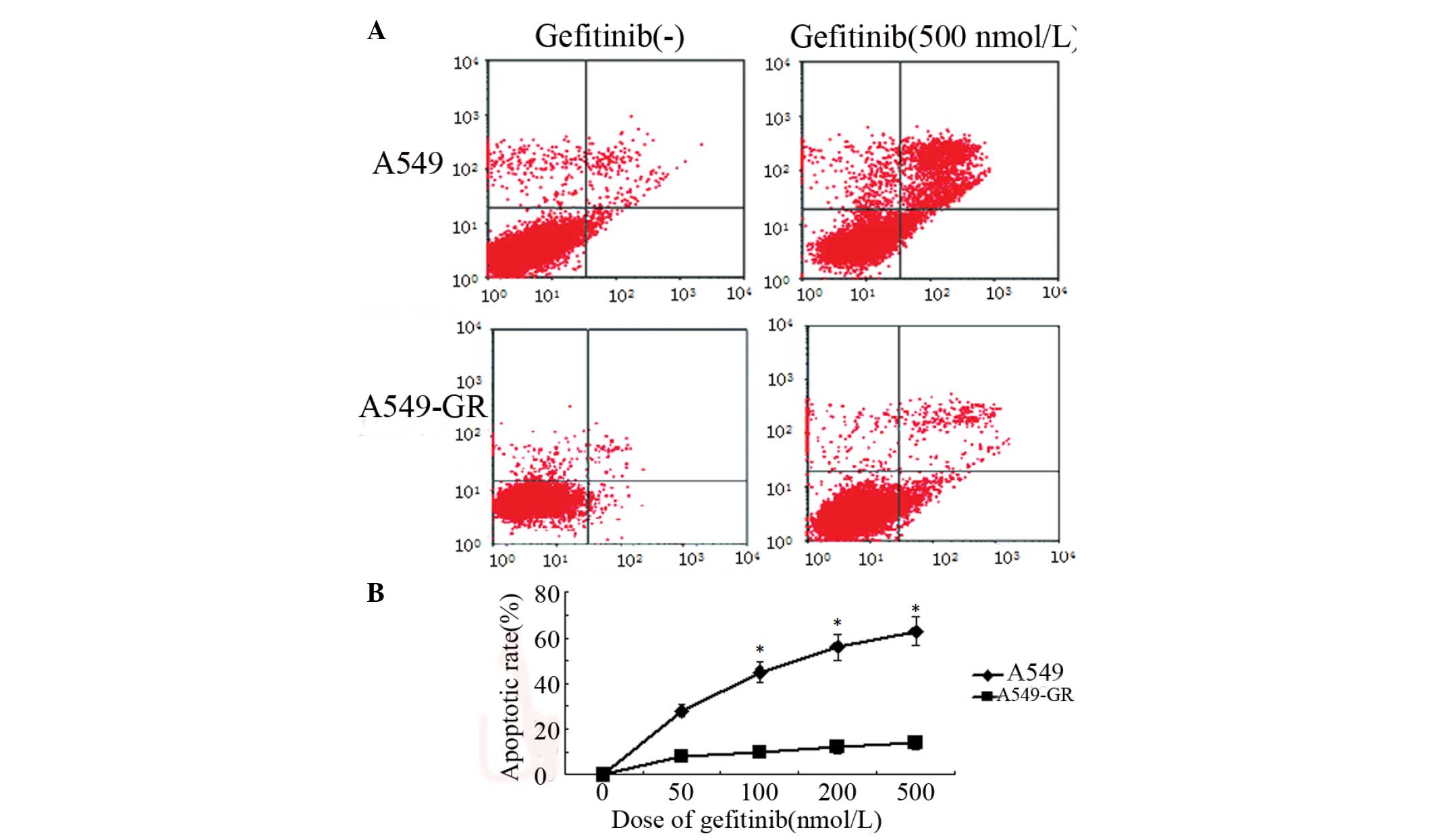
Annexin V- and PI-positive cells were determined by
flow cytometry. Apoptotic cells were located in the right upper
quadrant. The results revealed that gefitinib increased the
apoptotic rate of A549 cells in a dose-dependent manner. Following
treatment with 500 nmol/l gefitinib, the apoptosis rate was 60.2
and <10% in A549 and A549-GR cells, respectively (Fig. 2). No significant changes in apoptosis
rate were identified in A549-GR cells following gefitinib treatment
therapy (Fig. 2).
Gefitinib inhibits proliferation of
A549 cells
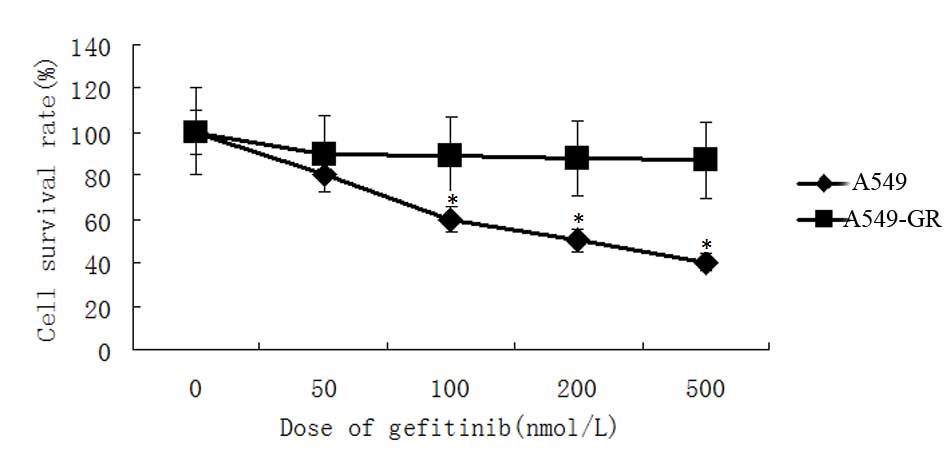
MTT assay revealed gefitinib significantly
attenuated A549 cell proliferation. The survival rate of A549 cells
was reduced by gefitinib in a dose-dependent manner. Survival rate
of A549 cells decreased to 40% following treatment with 500 nmol/l
gefitinib. By contrast, cell proliferation of A549-GR cells was not
affected by gefitinib treatment and no significant differences in
survival rate were identified in A549-GR cells following treatment
with gefitinib (Fig. 3).
Gefitinib mediates autophagy and
apoptosis of A549 cells via the PI3K/AKT/mTOR pathway
To elucidate the underlying molecular mechanism of
gefitinib-mediated autophagy and apoptosis, the expression of
proteins associated with the PI3K/AKT/mTOR pathway was measured
using western blot analysis prior to and following gefitinib
treatment in A549 and A549-GR cells. The results demonstrated that
PI3K, AKT, pAKT, mTOR and p-mTOR protein expression was
significantly downregulated in A549 cells following treatment with
500 nmol/l gefitinib. By contrast, no significant differences in
PI3K, AKT, pAKT, mTOR and p-mTOR protein expression were identified
in A549-GR cells following treatment with gefitinib (Fig. 4).
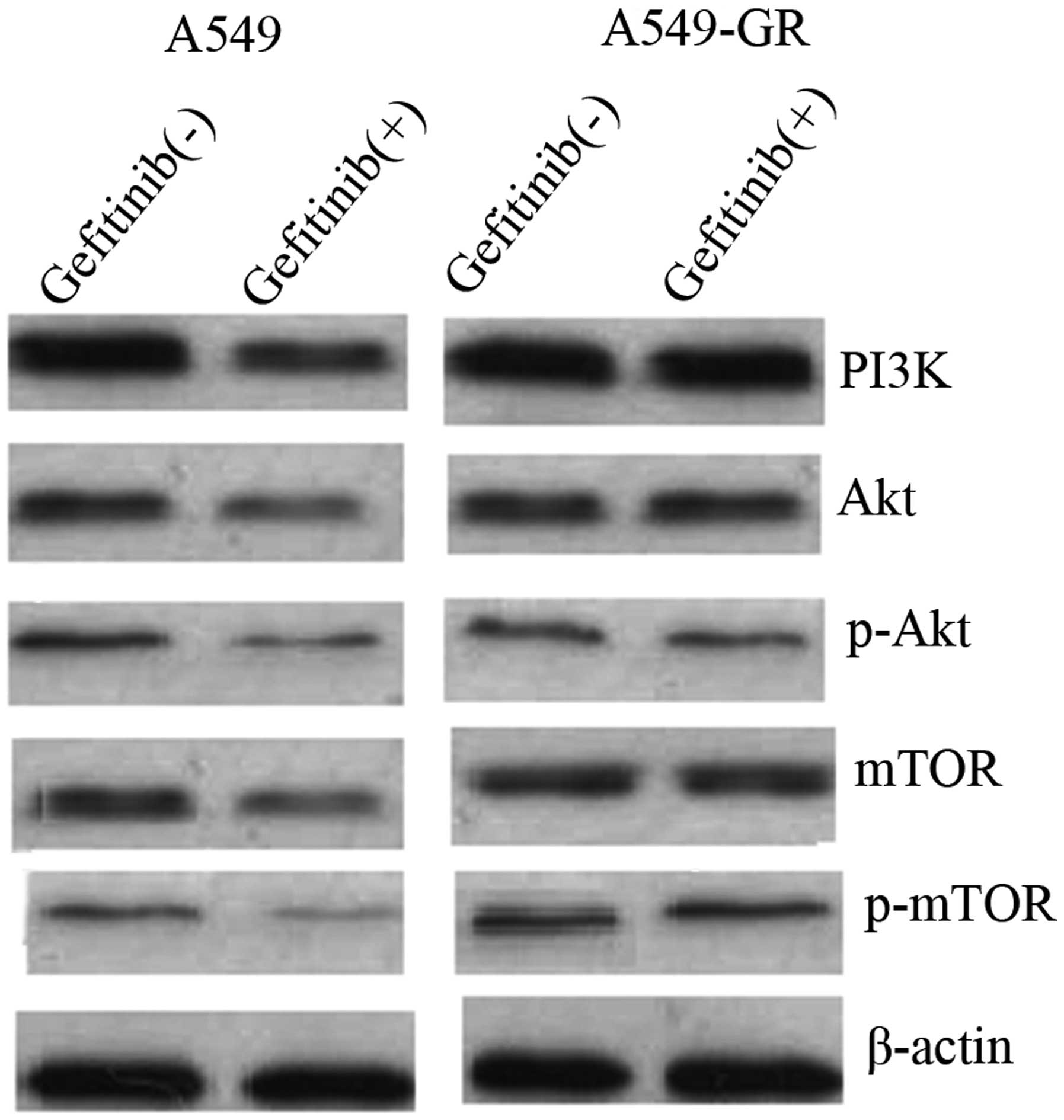 | Figure 4.Gefitinib mediates autophagy and
apoptosis of A549 cells via suppression of the PI3K/AKT/mTOR
pathway. Western blot analysis showing reduced protein expression
of PI3K, AKT, pAKT, mTOR and p-mTOR in A549 cells following
gefitinib treatment, however, no differences in protein expression
were identified in A549-GR cells. GR, gefitinib-resistant; PI3K,
phosphatidylinositol 3-kinase; AKT, protein kinase B; mTOR,
mammalian target of rapamycin; p, phosphorylated. |
Effect of gefitinib on autophagy and
apoptosis in A549 cells following blockade of the PI3K/AKT/mTOR
pathway
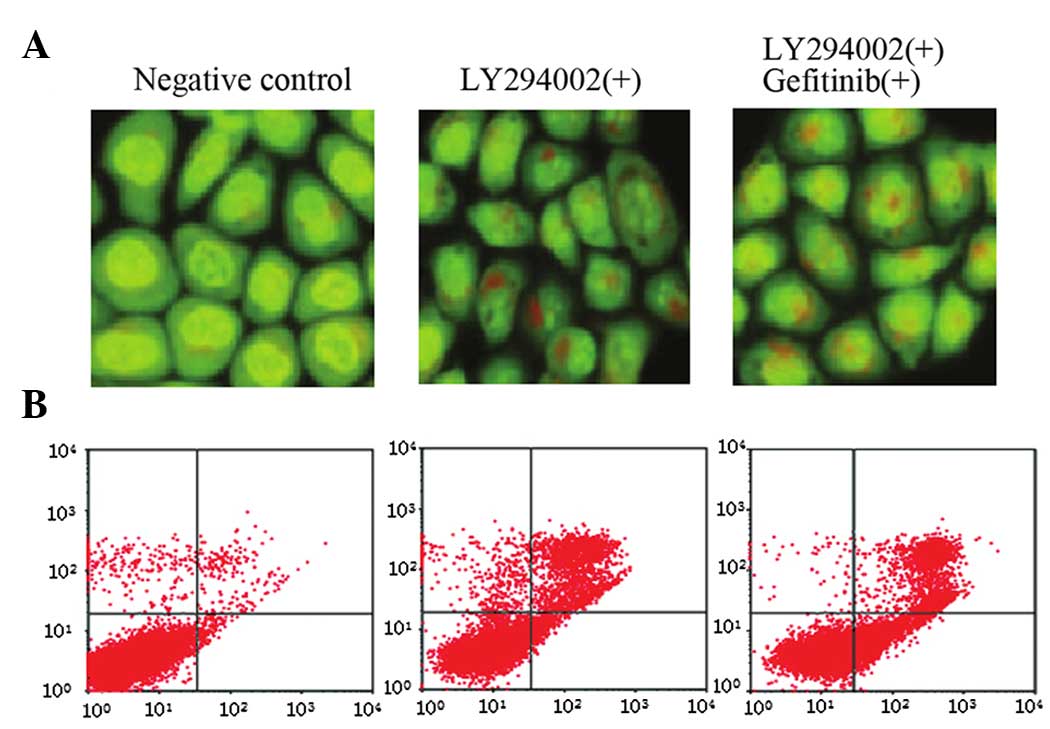
AO staining indicated that LY294002 treatment alone
activated autophagy in A549 cells. Although LY294002 inhibited the
PI3K/AKT/mTOR pathway, no significant differences in autophagic
cell death were identified in A549 cells following combined
treatment with gefitinib and LY294002 when compared with LY294002
alone (Fig. 5A). Flow cytometry
revealed that LY294002 activated A549 cell apoptosis, however, no
synergistic effect was observed in LY294002-induced apoptosis
following combined treatment with gefitinib (Fig. 5B).
Discussion
Advances in molecular biological techniques have
resulted in the development of targeted therapies, which are
important for the treatment of cancer. Gefitinib is a selective
tyrosine kinase inhibitor that interrupts EGFR activation via
binding to ATP-competitive binding sites of the extracellular
domain, which further inhibits cell proliferation and
neovascularization and stimulates cell apoptosis (15–18). In
May 2003, the Food and Drug Administration (USA) approved gefitinib
for late stage platinum and paclitaxel-resistant NSCLC. Currently,
gefitinib is used for the treatment of NSCLC (19–21),
however, the mechanism of action remains unclear. In the present
study, gefitinib-sensitive (A549) and GR (A549-GR) lung cancer
cells were used to examine the effect of gefitinib on NSCLC and the
underlying mechanism of action.
The results of the present study demonstrated that
gefitinib stimulates autophagy in lung cancer cells (Fig. 1). Autophagy, also known as type II
programmed cell death, is closely associated with tumorigenicity
and tumor development. Autophagy is able to promote or inhibit cell
apoptosis, suggesting that autophagy exhibits different functions
in a variety of histocytes. For example, in previous studies
autophagy inhibited apoptosis in breast carcinoma and the
suppression of autophagy promoted apoptosis in breast carcinoma
(22,23). However, the stimulation of autophagy
promoted apoptosis in colon carcinoma (24). Mimulone was reported to induce
autophagic cell death in lung cancer A549 cells (25), indicating that interference with
autophagy in lung cancer cells may present a potential treatment
for lung cancer (26,27). Bortezomib-induced apoptosis in lung
cancer cells is promoted by the suppression of autophagy (28) and autophagy in lung cancer cells is
regulated via interference with the AMPK pathway (29). In the present study, gefitinib induced
autophagy in lung cancer A549 cells while enhancing apoptosis and
suppressing proliferation. By contrast, A549-GR cells rarely
exhibited autophagy or apoptosis and cell proliferation was not
suppressed in response to gefitinib therapy. These findings suggest
that gefitinib exhibits antitumor activity in lung cancer via the
induction of autophagy and apoptosis.
The PI3K/AKT/mTOR pathway is one of the most
important downstream signaling pathways activated following EGFR
activation with multiple biological functions that are involved in
cell cycle regulation (30,31). The PI3K/AKT/mTOR pathway exhibits an
important function in the regulation of autophagy (32,33).
Therefore, autophagy is promoted by blockade of the PI3K/AKT/mTOR
pathway (34) and impaired by
activation of the pathway (35). In
the present study, gefitinib decreased the expression of PI3K, AKT,
pAKT, mTOR and p-mTOR in A549 lung cancer cells and blockade of the
PI3K/AKT pathway following treatment with LY294002 induced
autophagy and apoptosis, indicating that gefitinib induces
autophagy and apoptosis in lung cancer cells via inhibition of the
PI3K/AKT/mTOR pathway. Furthermore, the results of the present
study demonstrated that combined gefitinib treatment did not
exhibit a synergistic effect on the autophagy and apoptosis induced
in lung cancer cells following the inactivation of PI3K/AKT/mTOR
pathway by LY294002. It is hypothesized that blockade of the
PI3K/AKT/mTOR pathway interrupts targets of gefitinib, which in
turn affect the activity of gefitinib. Previous studies have
reported that gefitinib suppressed EGF-induced cellular migration
(36) and induced apoptosis in breast
cancer cells (37) via PI3K pathway
interference. Furthermore, the PI3K pathway affects lung cancer
cell sensitivity to gefitinib (38,39).
Gefitinib contributes to tumor suppression by
inducing autophagy and apoptosis and inhibiting proliferation in
lung cancer cells via the blockade of PI3K/AKT/mTOR pathway.
However, the association between gefitinib-induced autophagy and
apoptosis in lung cancer cells and the underlying mechanism of such
requires further study.
References
|
1
|
Hernández-Hernández JR, de Moreno
Vega-Herrero MB, Iglesias-Heras M, García-García R,
Hernández-Terciado F and Celdrán-Gil J: Lung cancer in avila
province, Spain. Incidence rates, epidemiolgy of the year 2012 and
trends in the last 20 years. Semergen. 41:362–369. 2015.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostroff JS: Quality lung cancer screening
protects quality of life: No harm, no foul. Cancer. 120:3275–3276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M: Lung cancer: Progress in
diagnosis and treatments. Topics: iii. Treatment; 3. Chemotherapy
for patients with non-small cell lung cancer, 2) Driver mutation
and molecular-targeted therapy in lung cancer. Nihon Naika Gakkai
Zasshi. 103:1314–1321. 2014.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez P, Martinez-Marti A, Navarro A,
Cedrés S and Felip E: Molecular targeted therapy for early-stage
non-small-cell lung cancer: Will it increase the cure rate? Lung
Cancer. 84:97–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Printz C: Targeted therapy in lung cancer:
Survival, quality of life improved for some patients. Cancer.
120:2625–2626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Fan Y, Ma S, Song X, Han B, Cheng
Y, Huang C, Yang S, Liu X, Liu Y, et al: Final overall survival
results from a phase iii, randomised, placebo-controlled,
parallel-group study of gefitinib versus placebo as maintenance
therapy in patients with locally advanced or metastatic
non-small-cell lung cancer (INFORM; C-tong 0804). J Thorac Oncol.
10:655–664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu H, Zhang J, Wu X, Luo Z, Wang H, Sun S,
Peng W, Qiao J, Feng Y, Wang J and Chang J: A phase II randomized
trial evaluating gefitinib intercalated with pemetrexed/platinum
chemotherapy or pemetrexed/platinum chemotherapy alone in
unselected patients with advanced non-squamous non-small cell lung
cancer. Cancer Biol Ther. 15:832–839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang Z and Klionsky DJ: Eaten alive: A
history of macroautophagy. Nat Cell Biol. 12:814–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Apel A, Zentgraf H, Büchler MW and Herr I:
Autophagy-A double-edged sword in oncology. Int J Cancer.
125:991–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu Y, Xia X and Pan H: Active autophagy in
the tumor microenvironment: A novel mechanism for cancer
metastasis. Oncol Lett. 5:411–416. 2013.PubMed/NCBI
|
|
11
|
Dragowska WH, Weppler SA, Wang JC, Wong
LY, Kapanen AI, Rawji JS, Warburton C, Qadir MA, Donohue E, Roberge
M, et al: Induction of autophagy is an early response to gefitinib
and a potential therapeutic target in breast cancer. PLoS One.
8:e765032013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Z, Hang J, Hu J and Gao B: Gefitinib,
an egfr tyrosine kinase inhibitor, activates autophagy through AMPK
in human lung cancer cells. J BUON. 19:466–473. 2014.PubMed/NCBI
|
|
13
|
Bokobza SM, Jiang Y, Weber AM, Devery AM
and Ryan AJ: Combining AKT inhibition with chloroquine and
gefitinib prevents compensatory autophagy and induces cell death in
EGFR mutated NSCLC cells. Oncotarget. 5:4765–4778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H and Liu Z: Autophagy facilitates TLR4- and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CH: A mechanistic study on
gefitinib-induced apoptosis reveals a new link between EGFR and
hTERT in breast cancers. Arch Pharm Res. 32:1333–1334. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang CY, Shen CC, Su HL and Chen CJ:
Gefitinib induces apoptosis in human glioma cells by targeting bad
phosphorylation. J Neurooncol. 105:507–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Min R, Wu M and Chen W: Gefitinib
induces mitochondrial-dependent apoptosis in Saccharomyces
cerevisiae. Mol Med Rep. 4:357–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu PH, Kuo TC, Chang KC, Chang CH and Chu
CY: Gefitinib-induced epidermal growth factor receptor-independent
keratinocyte apoptosis is mediated by the JNK activation pathway.
Br J Dermatol. 164:38–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karim NA, Musaad S, Zarzour A, Patil S and
Jazieh AR: Phase II clinical trial of gefitinib for the treatment
of chemonaive patients with advanced non-small cell lung cancer
with poor performance status. Clin Med Insights Oncol. 8:121–128.
2014.PubMed/NCBI
|
|
20
|
Tamiya A, Tamiya M, Shiroyama T, Saijo N,
Nakatani T, Minomo S, Tsuji T, Takeuchi N, Omachi N, Kurata K, et
al: Phase II trial of carboplatin, S-1 and gefitinib as first-line
triplet chemotherapy for advanced non-small cell lung cancer
patients with activating epidermal growth factor receptor
mutations. Med Oncol. 32:402015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ko R, Kenmotsu H, Hisamatsu Y, Akamatsu H,
Omori S, Nakashima K, Oyakawa T, Wakuda K, Shukuya T, Ono A, et al:
The effect of gefitinib in patients with postoperative recurrent
non-small cell lung cancer harboring mutations of the epidermal
growth factor receptor. Int J Clin Oncol. 20:668–673. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun WL, Lan D, Gan TQ and Cai ZW:
Autophagy facilitates multidrug resistance development through
inhibition of apoptosis in breast cancer cells. Neoplasma.
62:199–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alayev A, Berger SM, Kramer MY, Schwartz
NS and Holz MK: The combination of rapamycin and resveratrol blocks
autophagy and induces apoptosis in breast cancer cells. J Cell
Biochem. 116:450–457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song X, Kim SY, Zhang L, Tang D, Bartlett
DL, Kwon YT and Lee YJ: Role of AMP-activated protein kinase in
cross-talk between apoptosis and autophagy in human colon cancer.
Cell Death Dis. 5:e15042014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
An HK, Kim KS, Lee JW, Park MH, Moon HI,
Park SJ, Baik JS, Kim CH and Lee YC: Mimulone-induced autophagy
through p53-mediated AMPK/mTOR pathway increases caspase-mediated
apoptotic cell death in A549 human lung cancer cells. PLoS One.
9:e1146072014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu HY, Chang YJ, Fan NC, Wang LS, Lai NC,
Yang CM, Wu LC and Ho JA: Synergism through combination of
chemotherapy and oxidative stress-induced autophagy in A549 lung
cancer cells using redox-responsive nanohybrids: A new strategy for
cancer therapy. Biomaterials. 42:30–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee TG, Jeong EH, Kim SY, Kim HR and Kim
CH: The combination of irreversible EGFR TKIs and SAHA induces
apoptosis and autophagy-mediated cell death to overcome acquired
resistance in EGFR T790m-mutated lung cancer. Int J Cancer.
136:2717–2729. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu G, Li H, Ji Z, Jiang X, Lei Y and Sun
M: Inhibition of autophagy by autophagic inhibitors enhances
apoptosis induced by bortezomib in non-small cell lung cancer
cells. Biotechnol Lett. 36:1171–1178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Z, Hang J, Hu J and Gao B: Gefitinib,
an EGFR tyrosine kinase inhibitor, activates autophagy through AMPK
in human lung cancer cells. J BUON. 19:466–473. 2014.PubMed/NCBI
|
|
30
|
Davis NM, Sokolosky M, Stadelman K, Abrams
SL, Libra M, Candido S, Nicoletti F, Polesel J, Maestro R, D'Assoro
A, et al: Deregulation of the EGFR/PI3K/PTEN/AKT/mTORC1 pathway in
breast cancer: Possibilities for therapeutic intervention.
Oncotarget. 5:4603–4650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duan H, Qu L and Shou C: Activation of
EGFR-PI3K-AKT signaling is required for mycoplasma
hyorhinis-promoted gastric cancer cell migration. Cancer Cell Int.
14:1352014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heras-Sandoval D, Pérez-Rojas JM,
Hernández-Damián J and Pedraza-Chaverri J: The role of
PI3K/AKT/mTOR pathway in the modulation of autophagy and the
clearance of protein aggregates in neurodegeneration. Cell Signal.
26:2694–2701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014.PubMed/NCBI
|
|
34
|
Wang C, Zhang X, Teng Z, Zhang T and Li Y:
Downregulation of PI3K/Akt/mTOR signaling pathway in
curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J
Pharmacol. 740:312–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Datta K, Suman S and Fornace AJ: Radiation
persistently promoted oxidative stress, activated mTOR via
PI3K/AKT, and downregulated autophagy pathway in mouse intestine.
Int J Biochem Cell Biol. 57:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shien T, Doihara H, Hara H, Takahashi H,
Yoshitomi S, Taira N, Ishibe Y, Teramoto J, Aoe M and Shimizu N:
PLC and PI3K pathways are important in the inhibition of
EGF-induced cell migration by gefitinib (‘Iressa’, ZD1839). Breast
Cancer. 11:367–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng M, Wang J, Chen Y, Zhang L and Liu D:
Combination of SF1126 and gefitinib induces apoptosis of
triple-negative breast cancer cells through the PI3K/AKT-mTOR
pathway. Anticancer Drugs. 26:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jeannot V, Busser B, Brambilla E, Wislez
M, Robin B, Cadranel J, Coll JL and Hurbin A: The PI3K/AKT pathway
promotes gefitinib resistance in mutant KRAS lung adenocarcinoma by
a deacetylase-dependent mechanism. Int J Cancer. 134:2560–2571.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li H, Schmid-Bindert G, Wang D, Zhao Y,
Yang X, Su B and Zhou C: Blocking the PI3K/AKT and MEK/ERK
signaling pathways can overcome gefitinib-resistance in non-small
cell lung cancer cell lines. Adv Med Sci. 56:275–284. 2011.
View Article : Google Scholar : PubMed/NCBI
|