Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and lethal tumors worldwide (1). HCC is likely to be diagnosed at an
advanced stage, when the liver function of the patients is poor
(2). As a result, the majority of
patients with advanced stage HCC do not fulfill the indications for
surgical resection (3). The
utilization of transcatheter arterial chemoembolization (TACE),
targeted drugs and radiofrequency ablation (RFA) for the treatment
of HCC has achieved good clinical outcomes (4). However, these treatments present a
number of limitations, including tumor location, size, blood supply
and economical reasons (5).
High intensity focused ultrasound (HIFU) is a newly
emerged local thermal ablation therapy that is characterized by
causing minor trauma to patients and enabling a fast recovery
(6). In addition, HIFU enables
three-dimensional conformal treatment of solid tumors and enhances
the body's immune response against the tumor (7). In the present study, a representative
case of unresectable massive HCC treated successfully with HIFU
alone is reported. Although the treatment induced a chest wall
hernia, a satisfying clinical result was observed following HIFU
therapy.
Case report
The patient was a 57-year-old male who was admitted
to the Department of Integrated Oncology of Fudan University
Shanghai Cancer Center (Shanghai, China) in September 2010 with
complaints of occasional abdominal distension that had lasted for 2
years. The patient did not present with jaundice, abdominal pain,
nausea or vomiting upon initial admission.
The medical history indicated that the patient had
suffered hepatitis B for 30 years without receiving any anti-viral
treatment. In September 2010, the patient was subjected to an
abdominal computed tomography (CT) scan, which revealed a mass in
the right lobe of the liver (which was suspected to be HCC with
intrahepatic metastasis), in addition to cirrhosis, splenomegaly
and portal hypertension. Blood test demonstrated α-fetoprotein
(AFP) levels of 3,200 ng/ml (normal range, 0–10 ng/ml).
Subsequently, the patient underwent fine needle aspiration of his
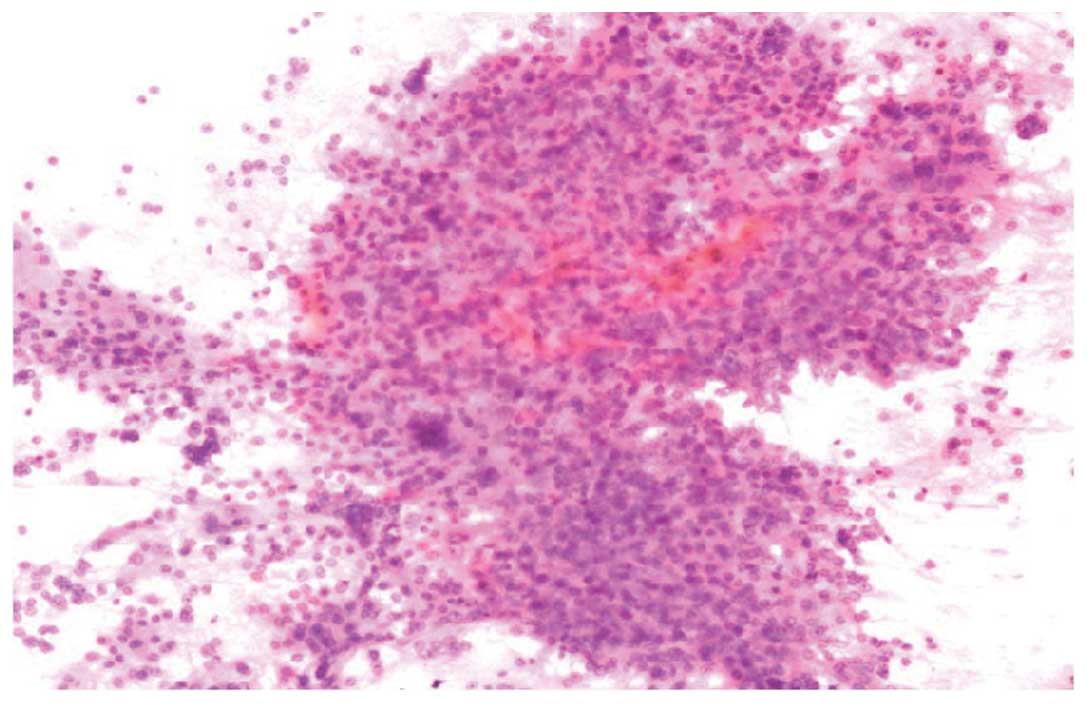
hepatic lesion. The cytomorphological findings revealed the
presence of crowded and giant tumor cells with bizarre shape and
high nuclear-cytoplasmic ratio. The presence of an endothelial cell
lining is one of the histopathological features indicative of HCC
(Fig. 1). In consequence, the patient
was diagnosed with HCC stage B, according to the Barcelona clinic
liver cancer staging classification of HCC (8). Upon obtaining signed written informed
consent, the patient was treated with hepatic arterial infusion
chemotherapy with 150 mg oxaliplatin and 1 g 5-fluorouracil. The
patient did not receive embolization with lipiodol, since no
complete obstruction of the hepatic arterioportal fistula detected
during the angiography was achieved. Following the procedure, the
patient experienced grade 3 hyperemesis, according to the Common
Terminology Criteria for Adverse Events published by the National
Cancer Institute (Bethesda, MD, USA) (9). The patient was discharged upon receiving
supportive care for 4 days.
In December 2010, the patient was readmitted into
hospital due to elevated AFP levels (5,732 ng/ml). During the
second admission, the patient refused to receive another hepatic
arterial infusion chemotherapy, due to the previous hyperemesis.
Treatment with sorafenib was then suggested to the patient, but it
was declined due to financial reasons. In consequence, HIFU therapy
was proposed to the patient, and written informed consent was
obtained from the patient for the HIFU procedure.
Admission examination
The results of the biochemical analysis at the time
of readmission were as follows: White blood cell count,
6.0×109 cells/l (normal range, 3.5–9.5×109
cells/l); percentage of neutrophils, 66.5% (normal range, 40–75%);
hemoglobin levels, 140 g/l (normal range, 115–150 g/l); platelet
count, 67×109 cells/l (normal range,
125–350×109 cells/l); albumin levels, 41 g/l (normal
range, 40–55 g/l); total bilirubin levels, 26 µmol/l (normal range,
3.4–17.1 µmol/l); direct bilirubin levels, 8.6 µmol/l (normal
range, 0–3.4 µmol/l); alanine aminotransferase levels, 83 U/l
(normal range, 7–40 U/l); aspartate aminotransferase levels, 76 U/l
(normal range, 13–35 U/l); blood urea nitrogen levels, 6.3 mmol/l
(normal range, 2.6–7.5 mmol/l); and creatinine levels, 68.6 µmol/l
(normal range, 41–73 µmol/l). Electrocardiogram did not report any
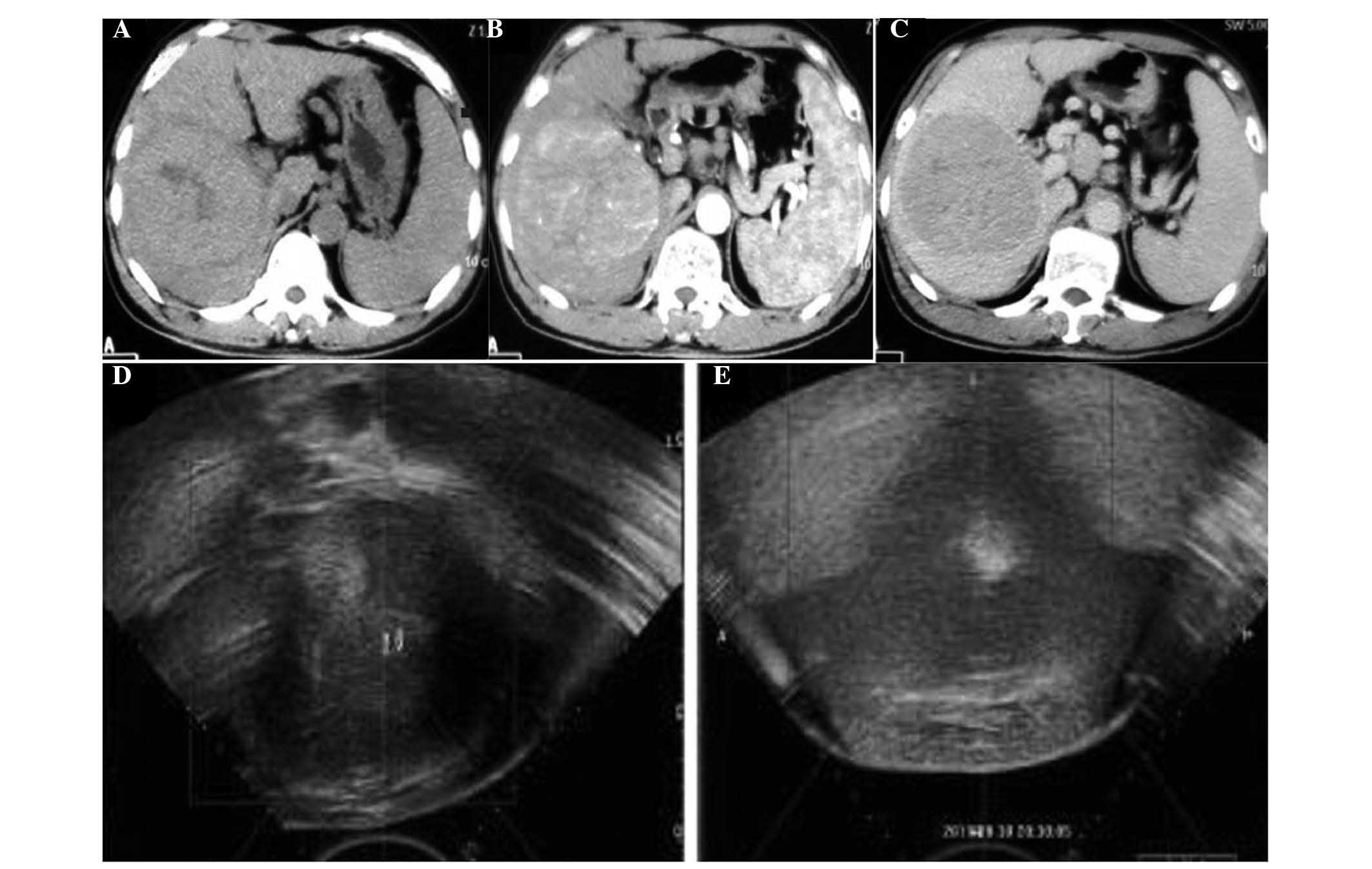
abnormalities. Enhanced abdominal CT revealed a mass located in the
right lobe of the liver with multiple intrahepatic metastases,
liver cirrhosis, splenomegaly and portal hypertension (Fig. 2A–C).
Therapeutic range of HIFU
treatment
The therapeutic range of HIFU treatment for the
lesion covered the neoplastic lesion and extended to the area of
normal tissue located within ~2.0-cm distance from the lesion.
HIFU treatment parameters and
procedure
The procedure was completed under the guidance of
real-time ultrasound using the JC-HIFU system (Chongqing
Haifu-HIFU-Tech, Chongqing, China). The treatment parameters were
as follows: Frequency, 0.85 MHz; focal diameter, 3 mm; focal field
length, 8 mm; focal length, 151 mm; and sound therapy peak power,
400 W. The patient was placed in the right lateral position with
full contact of the skin in the degassed water. Following
anesthesia, sedation and immobilization of the patient, ultrasound
(frequency, 2.5 MGz) was applied to identify the location, size and
boundary of the lesion, in order to determine the treatment target
of the tumor. Under ultrasound guidance and monitored in real time,
the lesion was gradually ablated three-dimensionally to achieve a
complete ablation of the entire tumor in order to avoid any
residual malignancy. After the whole lesion was ablated thoroughly,
the tumor tissue underwent coagulation necrosis and exhibited a
marked change of increased echo reflectance (Fig. 2E). Each slice of the targeted lesion
was carefully and completely ablated from the deepest to the
shallowest regions of the tumor, with the following parameters:
Treatment power, 400 W; line scan, 3 mm/sec; scanning distance, 5
mm; number of irradiations, 3; treatment length, 4 h; irradiation
time, 4,850 sec; treatment intensity, 1,212 kJ; total energy
therapy, 1,940,000 J; and treatment volume, 11.43
cm3.
Post-operation treatment and follow-up
information
Following HIFU treatment, the patient presented with
swelling of the skin on his right chest. In consequence, an ice
pack was applied intermittently for 2 h, while liver protective and
infection preventive intravenous drips were also administered to
the patient, until the swelling of the regional skin gradually
disappeared. Following discharge from the hospital, the patient
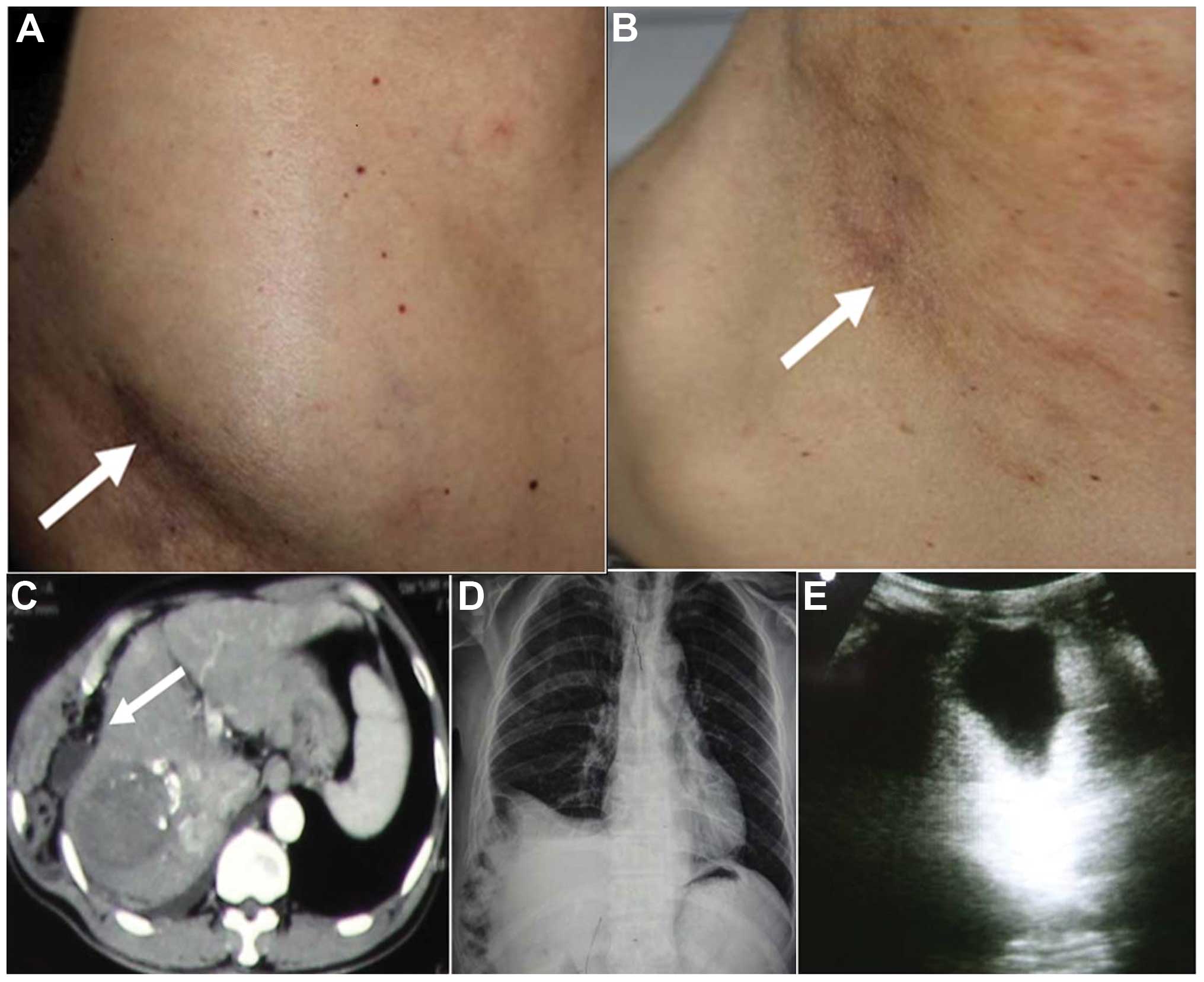
noticed a reducible mass on his right chest wall 6 months later
(Fig. 3A). The mass protruded outward
while exhaling, and flattened upon massaging and inhaling (Fig. 3B). However, no treatment was received
for this symptom, as it did not affect the patient's quality of
life or cause any discomfort. In May 2014, abdominal CT scan
(Fig. 3C) reported a tumor mass
measuring 40×45×40 mm located in the right lobe of the liver, which
was significantly reduced in size compared with the previous
follow-up. The patient's chest X-ray (Fig. 3D) suggested right pleural effusion,
right rib bone interruption and bone sclerotin discontinuation.
Ultrasonography of the chest wall mass (Fig. 3E) revealed a hernia in the
subcutaneous tissue. At the time of the last follow-up, conducted
on May 2014, the patient had survived for 44 months since the
diagnosis of HCC. The chest wall hernia had no effect on the
quality of life of the patient, whose levels of AFP in serum had
reduced to 182 ng/ml.
Discussion
Surgery is the most effective treatment for HCC
(10). However, the majority of cases
of HCC are diagnosed at a late stage, resulting in the majority of
patients unable to receive surgical resection (11). TACE has been used for the treatment of
HCC, and achieves its therapeutic effect by utilizing lipiodol for
the embolization of the major arterial supply to the liver tumor,
thus inducing ischemic necrosis of the tumor (12). However, the present patient did not
undergo lipiodol embolization, due to the inability of achieving
complete obstruction of the hepatic arterioportal fistula. RFA is
also useful for the treatment of HCC (13). However, the size of the tumor in the
present case was too large to undergo RFA. The cost of targeted
therapy for HCC in China is relatively high, thus the majority of
Chinese patients are unable to afford it. Therefore, an effective
modality with acceptable financial cost for the treatment of HCC is
required.
HIFU is a relatively novel emerged local thermal
ablation modality (14). By using
local high temperature produced by ultrasound focusing on the
targeted lesion, the temperature at the targeted lesion site
rapidly rises to 65–100°C, which induces coagulation necrosis in
the lesion (15). HIFU achieves its
three-dimensional conformal ablation and completely resects the
lesion by moving the ultrasound applicator in order to vary the
focused region inside the body (16).
Via effective ablation, the tumor tissue undergoes coagulation
necrosis and loss of replication, infiltration and metastasis
capacity, eventually becoming fibrous degenerated or calcified
tissue. In addition, multidrug resistant cancer cells and those in
G0 phase are also sensitive to HIFU treatment. Furthermore, HIFU is
able to stimulate the body's anti-tumor immune response subsequent
to the procedure (17). HIFU is
considered a superior treatment in terms of controlling tumor
progression and prolonging survival, due to its advantages, which
include minimal trauma, no risk of radiation and repeatable
modality (18).
However, common complications following HIFU
treatment have been reported, including pain in the treated area,
subcutaneous edema, rib fractures and skin empyrosis (19). Due to the ultrasonic energy
accumulation and deposition on the ribs, necrosis was previously
reported to occur in all the layers of subcutaneous, costal and
intercostal tissue following HIFU treatment. In that study, aseptic
necrosis was prevented from spreading to the superficial skin by
various treatments and nursing, thus avoiding skin ulceration and
cellulitis of the necrotic subcutaneous tissue (17). In the present patient, the necrotic
subcutaneous tissue was absorbed, which led to thinning of the
chest wall structure and resulted in the occurrence of chest wall
hernia. To date, chest wall hernia has not been reported in the
literature as a complication of HIFU treatment.
In conclusion, despite certain complications
associated with HIFU, this treatment may be an effective
alternative modality for HCC patients who are not suitable for
TACE, RFA or sorafenib treatment.
Acknowledgements
The authors would like to thank Dr Sonya Vargulick
(Albany College of Pharmacy and Health Sciences, Albany, NY, USA)
for editing the original manuscript.
References
|
1
|
Flores A and Marrero JA: Emerging trends
in hepatocellular carcinoma: Focus on diagnosis and therapeutics.
Clin Med Insights Oncol. 8:71–76. 2014.PubMed/NCBI
|
|
2
|
Chang PE, Ong WC, Lui HF and Tan CK: Is
theprognosis of young patients with hepatocellular carcinoma poorer
than the prognosis of older patients? A comparative analysis of
clinical characteristics, prognostic features, and survival
outcome. J Gastroenterol. 43:881–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rasool M, Rashid S, Arooj M, Ansari SA,
Khan KM, Malik A, Naseer MI, Zahid S, Manan A, Asif M, et al: New
possibilities in hepatocellular carcinoma treatment. Anticancer
Res. 34:1563–1571. 2014.PubMed/NCBI
|
|
4
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rust C and Gores GJ: Locoregional
management of hepatocellular carcinoma. Surgical and ablation
therapies. Clin Liver Dis. 5:161–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown MR, Farquhar-Smith P, Williams JE,
ter Haar G and deSouza NM: The use of high-intensity focused
ultrasound as a novel treatment for painful conditions - a
description and narrative review of the literature. Br J Anaesth.
115:520–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Deng J, Feng J and Wu F:
Enhancement of antitumor vaccine in ablated hepatocellular
carcinoma by high-intensity focused ultrasound. World J
Gastroenterol. 16:3584–3591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang L, Yan L, Wen T, Li B, Zeng Y, Yang
J, Wang W, Xu M and Wu H: Comparison of outcomes of hepatic
resection and radiofrequency ablation for hepatocellular carcinoma
patients with multifocal tumors meeting the Barcelona-Clinic Liver
Cancer stage A classification. J Am Coll Surg. 221:951–961. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong X, Lim EA, Hershman DL, Moinpour CM,
Unger J, Lee SM, Zhong X, Lim EA, Hershman DL, Moinpour CM, et al:
ReCAP: Identifying severe adverse event clusters using the National
Cancer Institute's Common Terminology Criteria for Adverse Events.
J Oncol Pract. 12:245–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song T: Recent advances in surgical
treatment of hepatocellular carcinoma. Drug Discov Ther. 9:319–330.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hołówko W, Wróblewski T, Wojtaszek M, Grąt
M, Kobryń K, Ziarkiewicz-Wróblewska B and Krawczyk M: Transarterial
chemoembolization prior to liver transplantation in patients with
hepatocellular carcinoma. Ann Transplant. 20:764–768. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyahara K, Nouso K and Yamamoto K:
Chemotherapy for advanced hepatocellular carcinoma in the sorafenib
age. World J Gastroenterol. 20:4151–4159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SH, Kim SU, Jang JW, Bae SH, Lee S,
Kim BK, Park JY, Kim Do Y, Ahn SH and Han KH: Use of transient
elastography to predict de novo recurrence after radiofrequency
ablation for hepatocellular carcinoma. Onco Targets Ther.
8:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ten Eikelder HM, Bošnački D, Elevelt A,
Donato K, Di Tullio A, Breuer BJ, van Wijk JH, van Dijk EV, Modena
D, Yeo SY and Grüll H: Modelling the temperature evolution of bone
under high intensity focused ultrasound. Phys Med Biol.
61:1810–1828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Greef M, Schubert G, Wijlemans JW,
Koskela J, Bartels LW, Moonen CT and Ries M: Intercostal high
intensity focused ultrasound for liver ablation: The influence of
beam shaping on sonication efficacy and near-field risks. Med Phys.
42:4685–4697. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li CC, Wang YQ, Li YP and Li XL:
High-intensity focused ultrasound for treatment of pancreatic
cancer: A systematic review. J Evid Based Med. 7:270–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu
H and Wang ZB: Pathological changes in human malignant carcinoma
treated with high-intensity focused ultrasound. Ultrasound Med
Biol. 27:1099–1106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blana A, Walter B, Rogenhofer S and
Wieland WF: High-intensity focused ultrasound for the treatment of
localized prostate cancer: 5-year experience. Urology. 63:297–300.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Wang Y, Wang T, Wang J, Wang L and
Tang J: Safety and efficacy of US-guided high-intensity focused
ultrasound for treatment of submucosal fibroids. Eur Radiol.
22:2553–2558. 2012. View Article : Google Scholar : PubMed/NCBI
|