Introduction
At present, gastric carcinoma is the fourth most
common human neoplasm and the second most common cause of
carcinoma-associated mortality worldwide, accounting for 986,600
novel cases and 738,000 mortalities annually (1). The incidence and mortality rates of this
disease are higher in China than in other Asian countries (1). The molecular pathogenesis of gastric
carcinoma has been investigated extensively with the aim of
developing more effective therapeutic strategies for this type of
tumor (2). Thus, there is an urgent
requirement for the development of strategies to prevent and treat
this disease.
Fentanyl, which is the most frequently used
analgesic, exhibits minimal cardiovascular effects and does not
increase plasma histamine levels (3).
Due to its relatively short onset of action and duration of effect,
fentanyl is a convenient and widely available drug used in clinical
practice (4). As a result of these
pharmacological properties, fentanyl is commonly used for the
management of severe pain associated with carcinoma (5). A previous study reported that fentanyl
inhibited carcinoma cell proliferation and carcinoma progression
(6), indicating a potential antitumor
role for this drug (7). However,
whether fentanyl affects gastric carcinoma cells remains
unclear.
Nuclear factor-kappa B (NF-κB) is a DNA binding
protein that regulates cellular activities, including cell cycle,
apoptosis, adhesion and angiogenesis, by interacting with its
downstream genes (8). A recent study
reported that NF-κB-dependent microRNA-425 upregulation promotes
gastric carcinoma cell growth by targeting phosphatase and tensin
homolog (PTEN) following interleukin-1β induction (9). NF-κB is important in cell development,
survival and oncogenesis (10,11). The
NF-κB signaling pathway contributes to fentanyl-mediated inhibition
of carcinoma cell proliferation (12). However, at present, the association
between fentanyl and NF-κB remains unclear.
A previous in vitro study by the present
authors revealed that fentanyl inhibits the progression of human
gastric carcinoma MGC-803 cells via the downregulation of NF-κB and
the upregulation of PTEN (7).
However, whether fentanyl administration in vivo exerts
similar effects on the progression of human gastric carcinoma cells
remains unclear. Therefore, the present study was conducted to
investigate the effects of fentanyl on human gastric carcinoma
cells in vivo and to explore the possible mechanism that
underlies these effects.
Materials and methods
Cell culture
The poorly differentiated MGC-803 human gastric
adenocarcinoma cell line was purchased from The Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), penicillin (100 U/ml;
Gibco; Thermo Fisher Scientific, Inc.) and streptomycin (100 µg/ml;
Gibco; Thermo Fisher Scientific, Inc.). The cells were cultured in
an incubator with an atmosphere of 5% CO2 at 37°C, and
the medium was changed every 3 days. A cell suspension was prepared
from the cultured cells using a previously described method
(13). The viability of the cells in
the cell suspension was assessed via trypan blue staining
(Sigma-Aldrich, St. Louis, MO, USA).
Animal model and fentanyl
administration
Male BALB/C nude mice (4 weeks old; weight, 15–20 g;
Vital River Laboratories Co., Ltd., Beijing, China) were used for
all experiments. The mice were bred and maintained under
standardized housing conditions at a constant room temperature with
a 12/12 h light/dark cycle, with access to food and water ad
libitum. The experimental protocol was approved by the Animal
Care and Use Committee of Guangxi Medical University [Nanning,
China; approval no. 2016(KY-E-015)]. A murine model of a
subcutaneous human gastric carcinoma tumor was established by
inoculating the right oxter of each nude mouse with a suspension of
cells in logarithmic phase growth. When tumors had grown to 1 cm in
diameter, a total of 30 nude mice were randomly divided into the
following 6 groups (5 mice per group): Control group (group C),
normal saline group (group N), group F1, group F2, group F3 and
group F4. Group C received no treatment, group N received an
intraperitoneal injection of 1.5 ml/kg saline (daily; GE Healthcare
Life Sciences, Logan, UT, USA), and groups F1, F2, F3 and F4
received intraperitoneal injections of 0.05, 0.1, 0.2 and 0.4 mg/kg
fentanyl (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang,
China), respectively, each day.
Tumor growth curve generation
Following fentanyl administration, the diameter (a)
and length (b) of the tumors were measured using a vernier caliper
(Saben Int'l Trading (Hong Kong) Co., Ltd., Hong Kong, China) every
two days. The tumor volume (TV) was calculated using the following
formula: TV = 1/2 × (a2 × b). The relative tumor volume
(RTV) was calculated using the following formula: RTV =
TVn/TV1 x 100%, where TVn
represents the TV measured at time n, and TV1 represents
the initial TV measurement. The final TV was calculated using the
diameter (a) and length (b) measurements that were obtained
directly after the tumors were completely resected from the mice.
The tumor growth curve was generated using the RTV results.
Morphological observation using
microscopy
The nude mice were euthanized via cervical
dislocation 16 days after fentanyl administration, and the tumors
were completely removed. The resected tumor tissues were
paraffin-embedded (Shanghai Huayong Paraffin Co., Ltd., Shanghai,
China), fixed in 10% neutral formaldehyde (Tianjin Kermel Chemical
Reagent Co., Ltd., Tianjin, China) and dehydrated using a graded
ethanol series (Zhejiang Zhongxing Chemical Reagent Co., Ltd,
Lanxi, China). A single ultrathin tumor tissue slice (50 nm) was
obtained from each mouse using Ultrotome V (LKB, Stockholm,
Sweden). The tissue slices were visualized using the Zeiss Axiovert
200 M Inverted Microscope (Zeiss GmbH, Jena, Germany).
Immunohistochemical analysis
Upon fixation with 4% buffered paraformaldehyde
(Tianjin Kermel Chemical Reagent Co., Ltd.), tumor tissues were
embedded in paraffin and cut into 4-µm sections. For the
immunohistochemical analysis of NF-κB, B-cell lymphoma-2 (Bcl-2),
B-cell associated X protein (Bax), vascular endothelial growth
factor-A (VEGF-A) and matrix metalloproteinase-9 (MMP-9)
expression, the sections were deparaffinized using xylene and
rehydrated using an ethanol/H2O gradient, subsequent to
heating in an electro-thermostatic drier (DGG-9070A
Electro-thermostatic Drier; SenXin Experimental Apparatus Co.,
Ltd., Shanghai, China) at 60°C for 20 min, and washed with
phosphate-buffered saline (GE Healthcare Life Sciences). Antigen
repair was then performed using 50 ml citrate buffer (pH 6.5;
Sigma-Aldrich) for 20 min, and the sections were treated with 3%
H2O2 (Shanghai Lianshi Chemical Reagent Co.,
Ltd., Shanghai, China) in methanol (Tianjin Siyou Chemical Reagent
Co., Ltd., Tianjin, China) for 10 min. The sections were then
incubated with rabbit polyclonal anti-NF-κB (dilution, 1:50;
catalog no., sc-7151; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), rabbit monoclonal anti-Bcl-2 (dilution, 1:100; catalog no.,
2870; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal anti-Bax (dilution, 1:100; catalog no., 14796; Cell
Signaling Technology, Inc.), rabbit polyclonal anti-VEGF-A
(dilution, 1:100; catalog no., PA1080; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) and rabbit polyclonal anti-MMP-9
(dilution, 1:100; catalog no., PB9669; Wuhan Boster Biological
Technology, Ltd.) primary antibodies. The antibodies were diluted
in 1% albumin bovine V (Biosharp Company, Hefei, China). Upon
incubation with the donkey anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:10,000;
catalog no., sc-2313; Santa Cruz Biotechnology, Inc.), the sections
were stained with diaminobenzidine (Beijing CellChip Biotechnology
Co., Ltd., Beijing, China).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from tumor tissues using
TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing, China),
according to the manufacturer's protocol. RT was performed by
incubating total RNA (3 µg), random hexamer primer (1 µl;
Invitrogen; Thermo Fisher Scientific, Inc.), 5X reaction buffer (4
µl), RiboLock™ RNase (1 µl), deoxynucleotide triphosphates mix (2
µl) and RevertAid™ M-MuLV reverse transcriptase (1 µl), according
to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). PCR amplification was performed using Taq DNA
polymerase (Invitrogen; Thermo Fisher Scientific, Inc.). The
expression of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
gene served as the internal control. The primer sequences were as
follows: Forward, 5′-GGGAAGGAACGCTGTCAGAG-3′ and reverse,
5′-TAGCCTCAGGGTACTCCATCA-3′ for NF-κB; forward,
5′-GACTTCGCCGAGATGTCCAG-3′ and reverse, 5′-CATCCCAGCCTCCGTTATCC-3′
for Bcl-2; forward, 5′-CCAAGAAGCTGAGCGAGTGT-3′ and reverse,
5′-CCGGAGGAAGTCCAATGTC-3′ for Bax; forward,
5′-TCACCCCACTAATGGCACC-3′ and reverse, 5′-TCCACTTCCCACCAACAGAC-3′
for VEGF-A; forward, 5′-ACGACCACGGACAGAGTAGAA-3′ and reverse,
5′-GAAGGGACTCAATCAGCAACA-3′ for MMP-9; and forward,
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse, 5′-TTTGAGGGTGCAGCGAACTT-3′
for GAPDH. The primers were synthesized by Invitrogen (Thermo
Fisher Scientific, Inc.). The reaction was performed using a
Bio-Rad Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) at 37°C for 15 min and 70°C for 50 min. RT-PCR was performed
as follows: Initial denaturation at 94°C for 4 min; 30 cycles of
denaturation at 94°C for 30 sec, annealing at 55°C for 30 sec and
extension at 72°C for 45 sec; and final extension at 72°C for 5
min. Finally, the PCR amplification products were examined using
1.2% agarose gel electrophoresis.
Western blot analysis
Tumor samples (20 mg) were mechanically homogenized
in 100–200 µl lysis buffer (Wuhan Boster Biological Technology,
Ltd.) and centrifuged (Sorvall ST 16R Centrifuge; Thermo Fisher
Scientific, Inc.) at 11,268 × g for 3–5 min at 4°C. The supernatant
was subsequently collected, and the total protein concentration in
the supernatant was quantified using a Bradford protein assay
(Bio-Rad Laboratories, Inc.). Protein extracts (16 µl/sample) were
heated for 10 min at 95°C and denatured in sodium dodecyl sulfate
sample buffer (Invitrogen; Thermo Fisher Scientific, Inc.). The
samples and the PageRuler™ Prestained Protein Ladder (Thermo Fisher
Scientific, Inc.) were loaded onto a NuPAGE Novex 4–12% Bis-Tris
gel (Invitrogen; Thermo Fisher Scientific, Inc.) for
electrophoresis, and then transferred to a polyvinylidene
difluoride membrane (Bio-Rad Laboratories, Inc.) using the Bio-Rad
Mini-Protein Tetra System (Bio-Rad Laboratories, Inc.). The
membranes were treated with blocking solution containing 5% non-fat
dry milk (Difco™ Skim Milk; Bio-Rad Laboratories, Inc.) in
Tris-buffered saline (TBS; Biosharp Company) with 0.1% Tween-20
(Amresco LLC, Cleveland, OH, USA) for 2 h. The membranes were next
incubated with the rabbit polyclonal anti-NF-κB (dilution,
1:1,000), rabbit polyclonal Bcl-2 (dilution, 1:1,000), rabbit
polyclonal anti-Bax (dilution, 1:1,000), rabbit polyclonal
anti-VEGF-A (dilution, 1:500) and rabbit polyclonal anti-MMP-9
(dilution, 1:500) primary antibodies overnight for ~12 h at 4°C.
Following three washes with TBS containing Tween-20, the membranes
were incubated with donkey anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (dilution, 1:10,000) for
1 h. The immunoreactive bands were visualized using ECL kit
(Bio-Rad Laboratories, Inc.). Briefly, the membranes were washed
three times with TBS containing Tween-20 for 10 sec each, and
incubated with a chemiluminescence reagent for 30–60 sec. The
membranes were then exposed to X-ray film in a dark room for 10
sec-15 min. X-ray films were visualized using a gel electrophoresis
scanning X-ray imaging analysis system (Gel-Doc XR System; Bio-Rad
Laboratories, Inc.), and analyzed using Quantity One analysis
software (version 4.6.2; Bio-Rad Laboratories, Inc.). The relative
concentrations of NF-κB, Bcl-2, Bax, VEGF-A and MMP-9 were
normalized to GAPDH and expressed as a ratio compared with the
control.
Statistical analysis
All data were analyzed using SPSS version 16.0
statistical software (SPSS, Inc., Chicago, IL, USA). Tumor growth
data were analyzed using one-way analysis of variance and the
Bonferroni method. Western blotting data were statistically
analyzed using a two-tailed Student's t-test. All data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Tumor growth curve
Following fentanyl administration, the diameter (a)
and length (b) of the tumors were measured every two days. No
significant differences were identified in the RTV of groups C or N
at any time point (P>0.05). The RTV of groups F1, F2, F3 and F4
were significantly reduced compared with that of groups C and N
(P<0.05), however, no statistically significant differences were
identified among the fentanyl treatment groups (F1, F2, F3 and F4)
(P>0.05) at the first four time points. On day 10 (corresponding
to the fifth time point), the RTV of the F3 and F4 groups were
significantly reduced compared with the RTV of F1 and F2 groups
(P<0.05). On day 16 (eighth time point), the RTV of group F4 was
significantly reduced compared with that of groups F1, F2 and F3
(P<0.05) (Fig. 1).
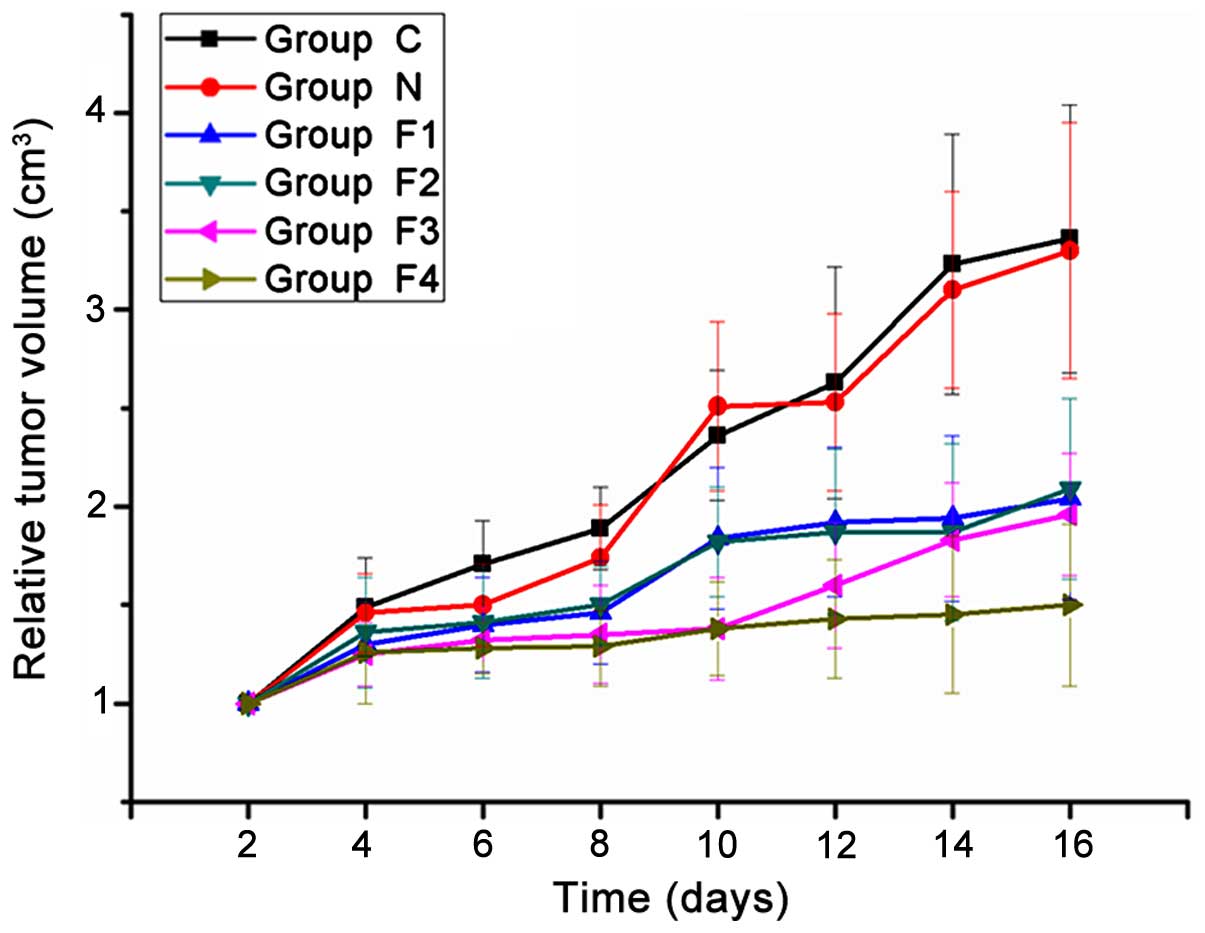 | Figure 1.Tumor growth curve. A total of 30
mice were bred and maintained under the same conditions, prior to
being divided into 6 groups (n=5 mice/group). Daily intraperitoneal
injections of 0.05, 0.1, 0.2 and 0.4 mg/kg fentanyl were
administered to groups F1, F2, F3 and F4, respectively, while 1.5
ml/kg saline was administered to group N. Group C received no
treatment. The tumor volume was measured every 2 days over the
period of 16 days, with 8 time points in total. The differences
between the final tumor volumes were assessed for significance.
Among the different treatment groups, group F4 showed the maximum
effect. The data are expressed as the mean ± standard deviation of
5 mice in each group. Significant differences (P<0.05) were
identified as follows: Group C vs. groups F1, F2, F3 and F4; group
N vs. groups F1, F2, F3 and F4; and group F4 vs. groups F1, F2 and
F3. |
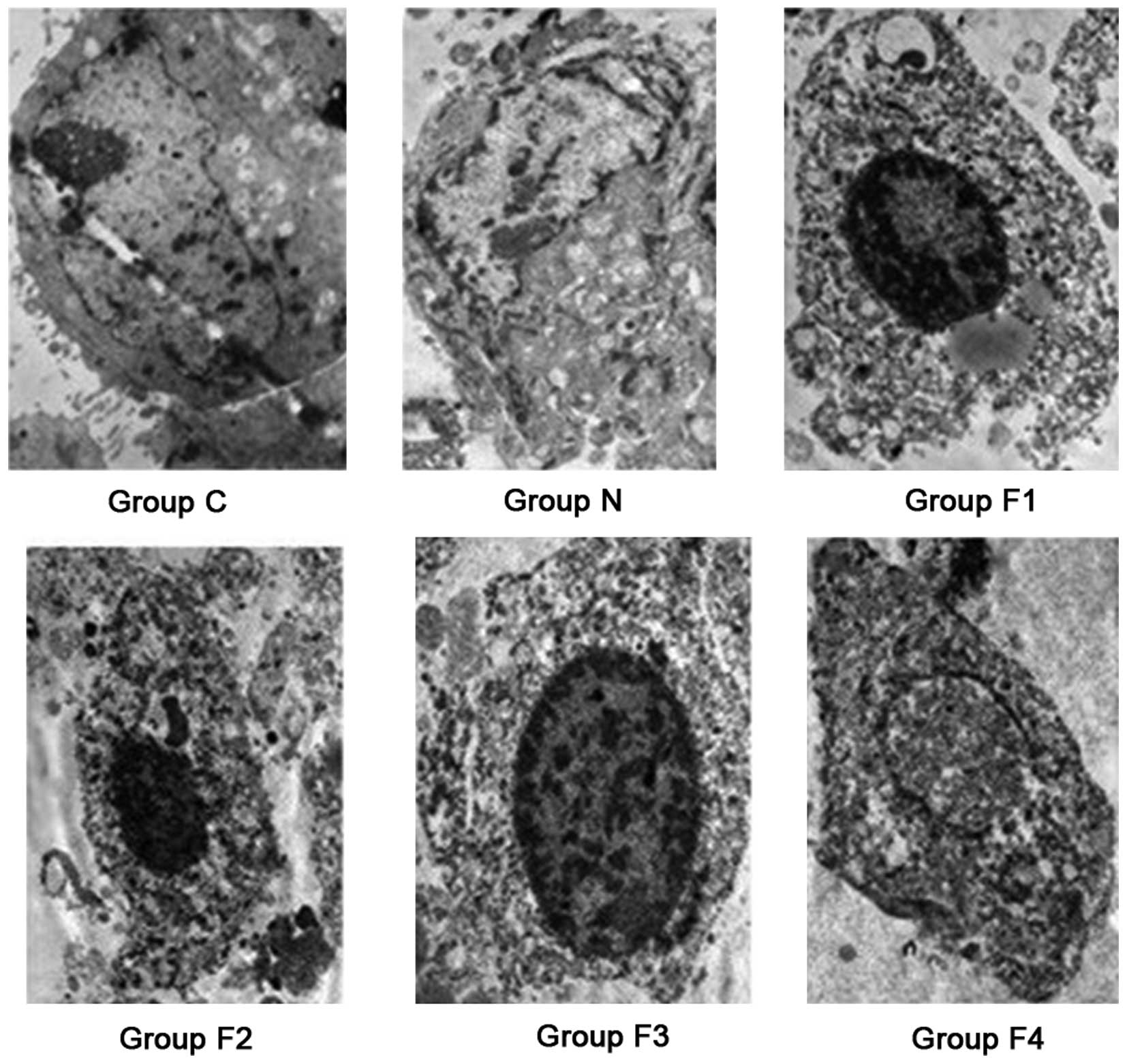
Subcutaneous tumor morphology
Microscopy was used to analyze the morphology of the
tumors. In group C, the subcutaneous gastric carcinoma tumor cells
exhibited an irregular shape with clear chromatin, increased
nucleoli and intact nuclear and cell membranes. Apoptotic
morphological changes of varying degrees were observed in groups
F1, F2, F3 and F4, where pyknosis, karyolysis, nuclear membrane
rupture, cytoplasmic vacuoles and apoptotic bodies were identified.
In group N, swollen tumor tissue cells with an irregular shape,
large and clear nucleoli and an integrated nuclear membrane were
observed (Fig. 2).
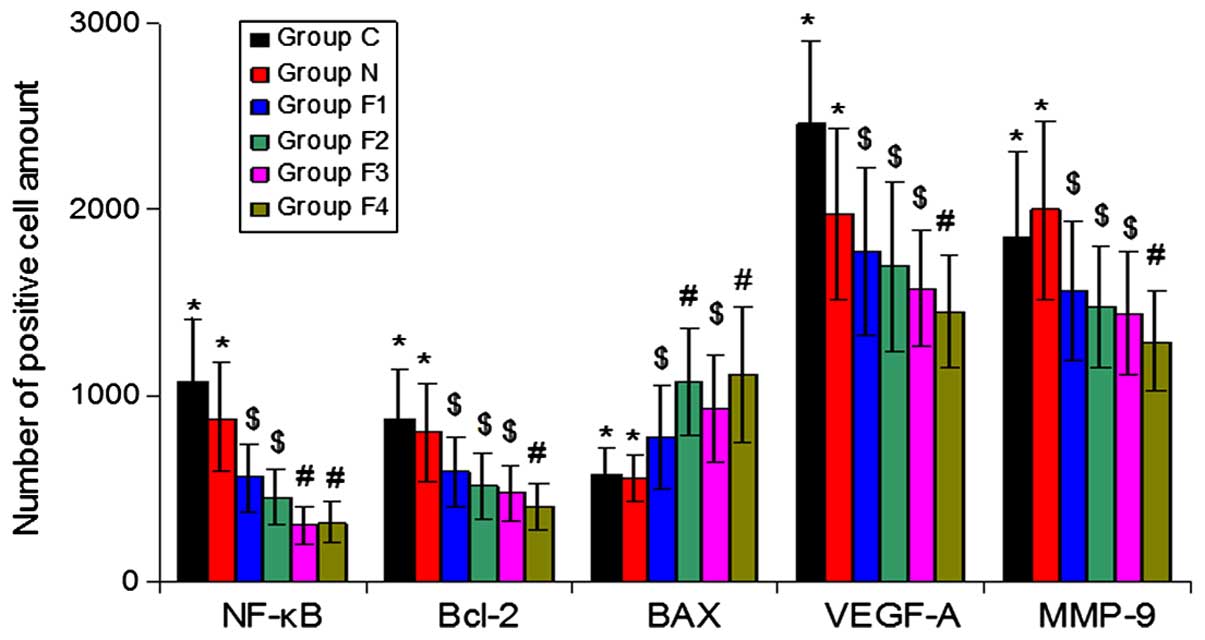
Immunohistochemical analysis of NF-κB,
Bcl-2, Bax, VEGF-A and MMP-9 protein expression
The percentage of cells exhibiting positive NF-κB,
Bcl-2, VEGF-A and MMP-9 expression was reduced in groups F1, F2, F3
and F4 compared with groups C and N. By contrast, Bax expression
was increased in groups F1, F2, F3 and F4 compared with groups C
and N (P<0.05) (Fig. 3).
NF-κB protein was located in the nucleus, whereas
Bcl-2, Bax, VEGF-A and MMP-9 were located in the cytoplasm. In
groups C and N, NF-κB, Bcl-2, VEGF-A and MMP-9 were widely
distributed and exhibited strong positive staining throughout the
cells, whereas Bax was less widely distributed and exhibited weak
staining. By contrast, in groups F1, F2, F3 and F4, NF-κB, Bcl-2,
VEGF-A and MMP-9 were less widely distributed and exhibited weak
staining, whereas Bax was widely distributed and exhibited strong
staining (Fig. 4).
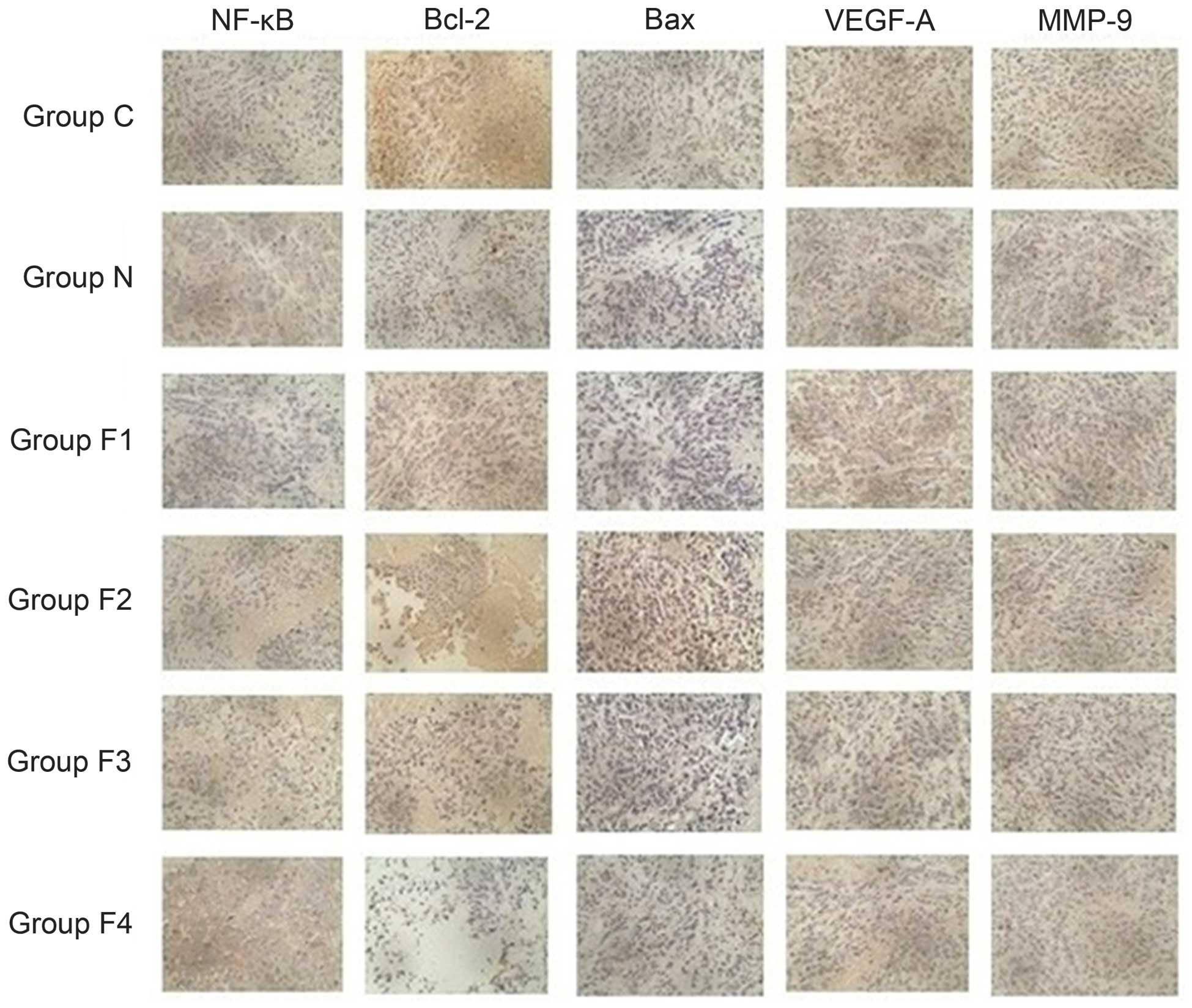 | Figure 4.Immunohistochemical staining with
3,3′-diaminobenzidine and hematoxylin indicates positive cells with
brown dots (magnification, ×40). In groups C and N, intense
brown-stained cells indicated overexpression of NF-κB, Bcl-2,
VEGF-A and MMP-9. These proteins demonstrated moderate and weak
expression in groups F1, F2, F3 and F4. Groups C and N showed a
decreased number of brown-stained cells, indicating weak expression
of Bax, which was moderately and strongly expressed in groups F1,
F2, F3 and F4. NF-κB, nuclear factor-kappa B; Bcl-2, B-cell
lymphoma-2; Bax, Bcl-2 associated X protein; VEGF-A, vascular
endothelial growth factor-A; MMP-9, matrix metalloproteinase-9. |
NF-κB, Bcl-2, Bax, VEGF-A and MMP-9
messenger RNA (mRNA) expression
Gel electrophoresis of the RT-PCR products of groups
F1, F2, F3 and F4 revealed specific bands corresponding to NF-κB,
Bcl-2, VEGF-A and MMP-9 at 321, 259, 321 and 221 bp, respectively.
The band intensity indicated that NF-κB, Bcl-2, VEGF-A and MMP-9
mRNA expression levels were increased and BAX mRNA expression
levels were decreased in group C and N compared with the
fentanyl-treated groups (F1, F2, F3 and F4). In addition,
tumor-bearing mice treated with different doses (0.05, 0.1, 0.2 and
0.4) of fentanyl (groups F1, F2, F3 and F4) showed decreased mRNA
expression of NF-κB, Bcl-2, VEGF-A and MMP-9, and increased
expression of BAX compared with mice in groups C and N, which
indicates suppression of tumor growth. Semiquantitative grey ratio
analysis of the specific bands and their internal controls was
performed using Quantity One software version 4.6.2. The results
demonstrated that NF-κB, Bcl-2, VEGF-A and MMP-9 mRNA expression
was significantly reduced in groups F1, F2, F3 and F4 compared with
groups C and N. In addition, Bax expression was significantly
increased in groups F1, F2, F3 and F4 compared with groups C and N
(P<0.05). No significant differences in NF-κB, Bcl-2, Bax,
VEGF-A and MMP-9 mRNA expression were identified between groups C
and N (Fig. 5).
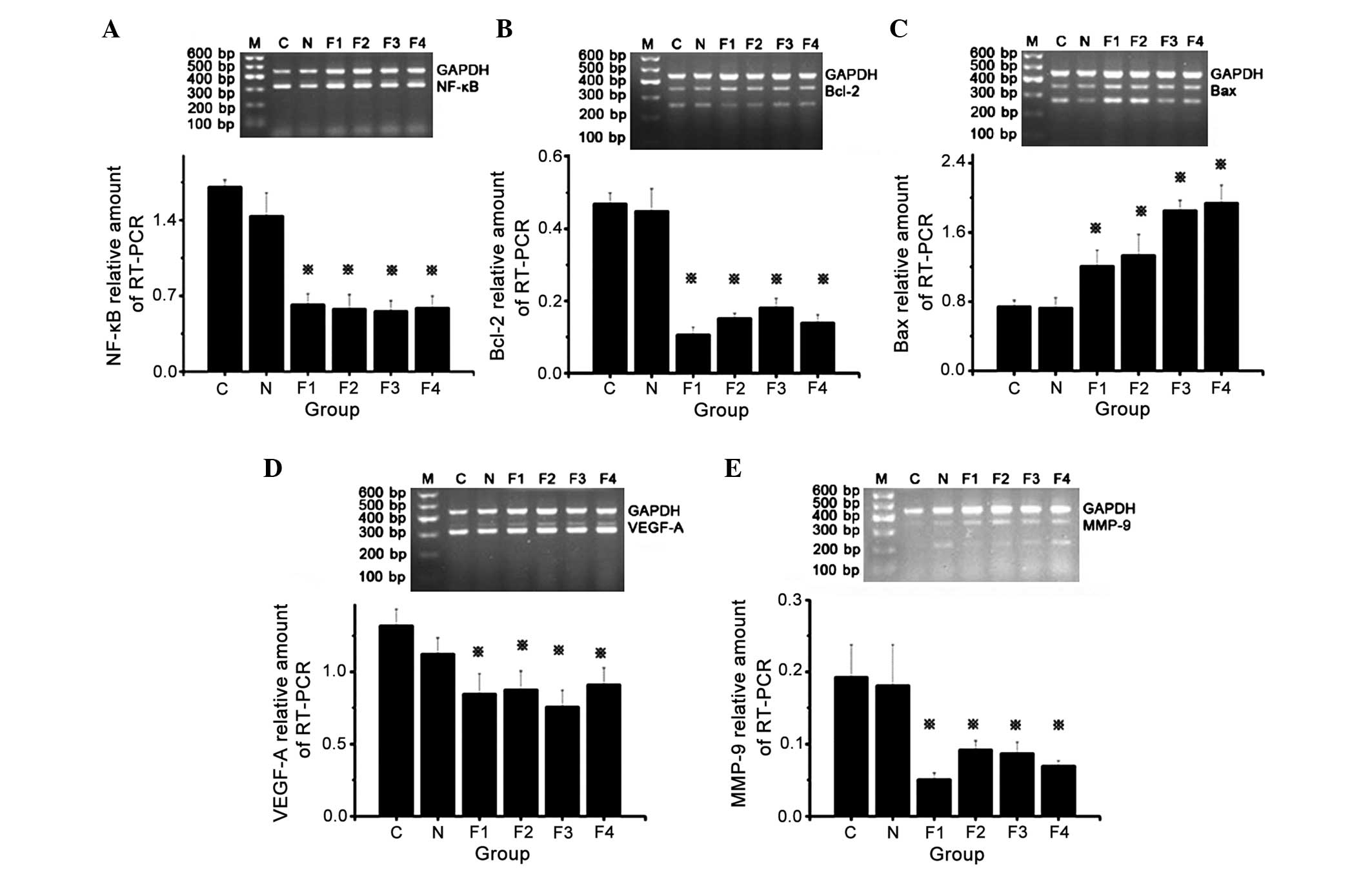 | Figure 5.Messenger RNA expression levels of
NF-κB, Bcl-2, Bax, VEGF-A and MMP-9 in groups C, N, F1, F2, F3 and
F4. (A) Top panel: RT-PCR results of NF-κB and GAPDH expression.
Bottom panel: Relative quantification of NF-κB expression.
*P<0.05 vs. groups C and N. (B) Top panel: RT-PCR results of
Bcl-2 and GAPDH expression. Bottom panel: Relative quantification
of Bcl-2 expression. *P<0.05 vs. groups C and N. (C) Top panel:
RT-PCR results of Bax and GAPDH expression. Bottom panel: Relative
quantification of Bax expression. *P<0.05 vs. groups C and N.
(D) Top panel: RT-PCR results of VEGF-A and GAPDH expression.
Bottom panel: Relative quantification of VEGF-A expression.
*P<0.05 vs. groups C and N. (E) Top panel: RT-PCR results of
MMP-9 and GAPDH expression. Bottom panel: Relative quantification
of MMP-9 expression. *P<0.05 vs. groups C and N. NF-κB, nuclear
factor-kappa B; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2 associated X
protein; VEGF-A, vascular endothelial growth factor-A; MMP-9,
matrix metalloproteinase-9; RT-PCR, reverse
transcription-polymerase chain reaction; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
NF-κB, Bcl-2, Bax, VEGF-A and MMP-9
protein expression
Analysis of protein expression in groups F1, F2, F3
and F4 revealed specific bands for NF-κB, Bcl-2, VEGF-A and MMP-9
at 65, 26, 27 and 78 kDa, respectively. The western blot analysis
showed increased protein expression of NF-κB, Bcl-2, VEGF-A and
MMP-9 and decreased expression of BAX protein in groups C and N as
compared to fentanyl treated groups (F1, F2, F3 and F4). In
addition, treatment of tumor-bearing mice with different doses
(0.05, 0.1, 0.2 and 0.4) of fentanyl (F1, F2, F3 and F4) showed
decreased protein expression of NF-κB, Bcl-2, VEGF-A and MMP-9, and
increased expression of BAX protein compared to mice in groups C
and N, which indicates suppression of tumor growth.
Semiquantitative analysis demonstrated that NF-κB, Bcl-2, VEGF-A
and MMP-9 protein expression in groups F1, F2, F3 and F4 was
significantly decreased, while Bax protein expression was
significantly increased, compared with groups C and N (P<0.05).
No significant differences in NF-κB, Bcl-2, Bax, VEGF-A and MMP-9
protein expression were identified between groups C and N (Fig. 6).
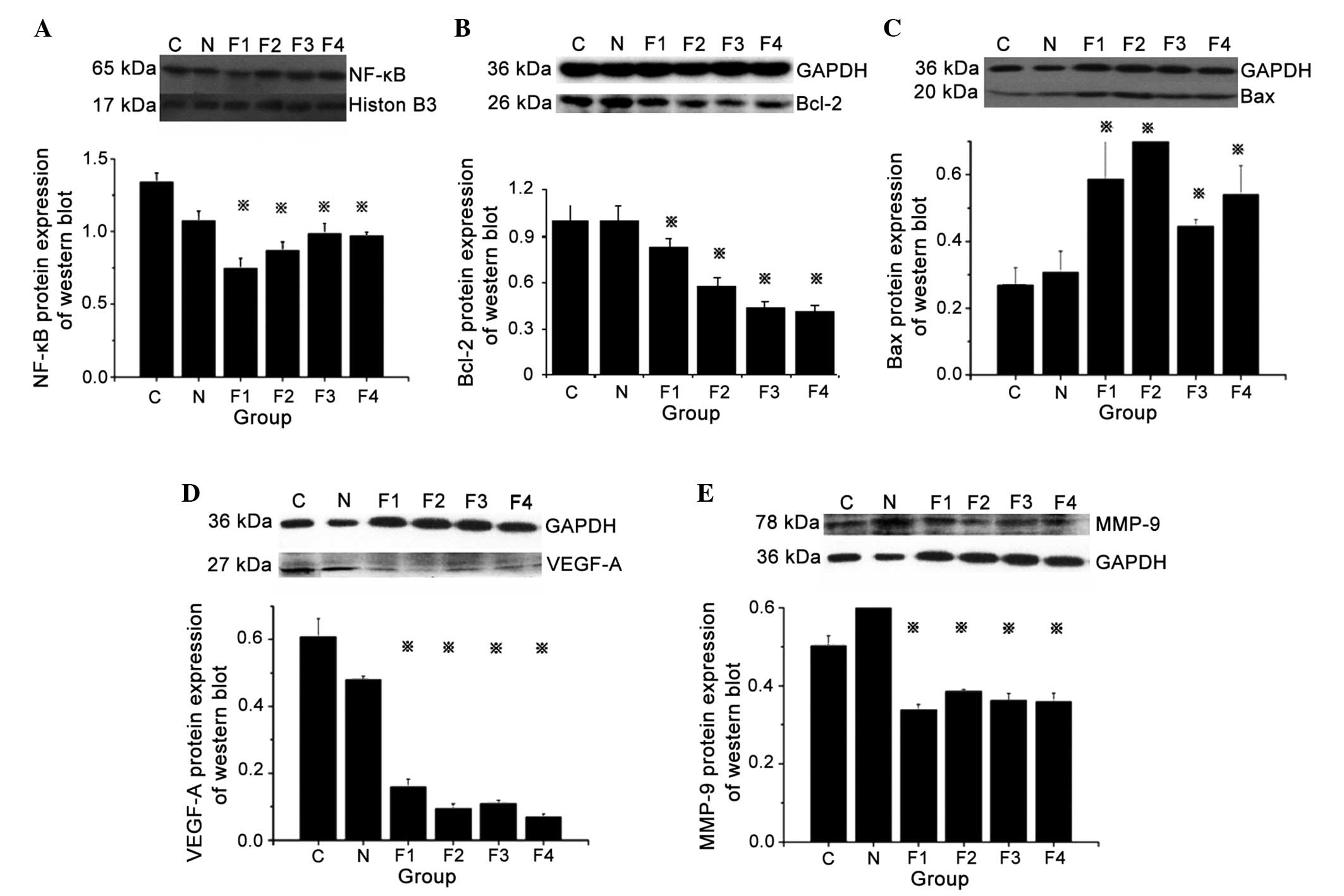 | Figure 6.Protein expression levels of NF-κB,
Bcl-2, Bax, VEGF-A and MMP-9, as analyzed by western blotting. (A)
Top panel: Protein expression of NF-κB and internal control
histone. Bottom panel: Relative quantification of NF-κB protein
expression. *P<0.05 vs. groups C and N. (B) Top panel: Protein
expression of Bcl-2 and internal control GAPDH. Bottom panel:
Relative quantification of Bcl-2 protein expression. *P<0.05 vs.
groups C and N. (C) Top panel: Protein expression of Bax and
internal control GAPDH. Bottom panel: Relative quantification of
Bax protein expression. *P<0.05 vs. groups C and N. (D) Top
panel: Protein expression of VEGF-A and internal control GAPDH.
Bottom panel: Relative quantification of VEGF-A protein expression.
*P<0.05 vs. groups C and N. (E) Top panel: Protein expression of
MMP-9 and internal control GAPDH. Bottom panel: Relative
quantification of MMP-9 protein expression. *P<0.05 vs. groups C
and N. NF-κB, nuclear factor-kappa B; Bcl-2, B-cell lymphoma-2;
Bax, Bcl-2 associated X protein; VEGF-A, vascular endothelial
growth factor-A; MMP-9, matrix metalloproteinase-9; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
In a previous in vitro study, the present
authors demonstrated that fentanyl inhibits the progression of
human gastric carcinoma MGC-803 cells via NF-κB downregulation and
PTEN upregulation (7). In the present
study, a xenograft MGC-803 tumor mouse model was established
following the intraperitoneal administration of various doses of
fentanyl to nude mice. Subsequently, NF-κB, Bcl-2, Bax, VEGF-A and
MMP-9 expression was measured in the subcutaneous tumor tissues.
The results revealed that fentanyl inhibits the growth of
subcutaneous human gastric carcinoma tumors in nude mice in
vivo, and promotes gastric carcinoma cell apoptosis by
inhibiting the NF-κB signaling pathway and altering the Bcl-2/Bax
ratio. Furthermore, the results confirmed that fentanyl inhibits
gastric subcutaneous carcinoma invasion and angiogenesis by
downregulating VEGF-A and MMP-9 expression.
Fentanyl, which is a potent µ-opioid receptor (MOR)
agonist, is considered to be an effective analgesic for cancer pain
in terminal cancer patients (14).
Lennon et al (15) reported
that MOR promotes opioid- and growth factor-induced proliferation,
migration and epithelial-mesenchymal transition in human lung
cancer. A recent study confirmed that fentanyl inhibits tumor
growth, increases the expression of sirtuin 1 and decreases he
expression of acetyl-p65 in colorectal carcinoma cells via the
inhibition of NF-κB activation (16).
Thus, the potential antitumor activity of fentanyl must be
considered in the management of carcinoma pain. The current study
demonstrated that fentanyl-mediated inhibition of tumor cell
proliferation and tumor growth is not dose- or time-dependent. In a
study by Kampa et al (17),
opioid alkaloids and casomorphin peptides decreased the
proliferation of prostatic carcinoma cell lines in a dose-dependent
manner. This discrepancy may be attributed to the different types
of carcinoma that were investigated in the two studies. Notably,
the present study demonstrated that fentanyl alters cellular
morphology, induces cell apoptosis and reduces human gastric
carcinoma cell migration.
The transcription factor NF-κB is a DNA binding
protein that augments the transcription of various genes that are
involved in cell proliferation (18).
NF-κB exhibits an important function in cell development (10), survival and oncogenesis (11), which is mediated by the formation of
homodimers or heterodimers containing NF-κB/Rel family members,
including RelA/p65, RelB, c-Rel, NF-κB1/p50 and NF-κB2/p52
(10,11,19). A
variety of different stimuli, including cytokines, oxidative
stress, apoptosis-inducing stimuli and drugs used in anticancer
treatment, are able to activate NF-κB (20,21).
Previous studies have demonstrated that morphine directly inhibits
NF-κB function via the release of nitric oxide (NO) (22,23).
Similarly, in the present study, fentanyl inhibited NF-κB
expression in human gastric carcinoma cells. However, whether
fentanyl inhibits NF-κB expression and induces antiproliferative
and apoptotic effects via the release of NO or via other mechanisms
requires further investigation.
Bcl-2, which is a classical anti-apoptotic gene,
encodes a 26-kDa transmembrane protein that suppresses apoptosis
and subsequently enhances cell survival (24). Bax, which acts as a tumor suppressor,
belongs to the Bcl-2 family subgroup of pro-apoptotic genes
(25). The Bax protein is a homolog
of Bcl-2 and promotes cell death via apoptosis (25). Bax may bind to Bcl-2, forming
Bax/Bcl-2 heterodimers, or to itself, forming Bax/Bax homodimers
(24). Apoptosis is regulated
according to the ratio of these two proteins; specifically,
apoptosis is induced by Bax and inhibited by the formation of
Bax/Bcl-2 heterodimers (26).
Alterations in Bcl-2 and Bax mRNA and protein expression patterns,
which typically reflect different prognostic profiles for carcinoma
patients, have been identified in human malignancies (27). Bcl-2 is strongly regulated by NF-κB
activity (7). NF-κB potentially
reduces the Bcl-2/Bax ratio, inducing gastric carcinoma cell
apoptosis (28).
The VEGF superfamily critically influences
tumor-related angiogenesis (29,30). VEGF
promotes neovascularization and migration, and increases vascular
permeability (31). VEGF-A is
considered the most potent angiogenic factor, and functions by
activating the receptor tyrosine kinases VEGF receptor-1 (VEGFR-1)
and VEGFR-2 (32). Lee et al
(33) demonstrated that VEGF
suppresses T-lymphocyte infiltration in the tumor microenvironment
via the inhibition of NF-κB-induced endothelial activation. The
present study revealed that the mRNA and protein expression of
NF-κB and VEGF-A decreased in tumor tissues upon fentanyl
administration; however, the association between these two proteins
remains unclear.
Tumor cells degrade extracellular matrix (ECM)
components to invade surrounding tissues (34). This process is tightly controlled by
ECM-degrading enzymes, including MMPs (35). MMPs, which are a family of
closely-related enzymes that degrade ECM, are involved in tumor
invasion and migration, and may be associated with the invasion,
lymph node metastasis and survival of gastric carcinoma (36,37). MMP-9
is a zinc-containing enzyme that exhibits potent proteolytic
activity against a wide range of ECM components, including laminin
subunit alpha-5 and type IV collagen, which are the major
constituents of basement membranes (38). A study performed by Yang et al
(39) reported positive MMP-9
expression in 60.7% of gastric carcinoma samples. In the present
study, positive MMP-9 expression was identified in gastric
carcinoma tissues, and it was observed that MMP-9 protein and mRNA
expression levels in tumor tissues decreased following fentanyl
administration.
In conclusion, fentanyl is recommended as an opioid
analgesic in the management of pain in carcinoma patients. The
results of present study indicate that fentanyl inhibits the
progression of human gastric carcinoma MGC-803 cells by modulating
NF-κB-dependent gene expression in vivo. Thus, fentanyl is
promising for the pain management of cancer patients. However, the
mechanism of fentanyl modulation of NF-κB-dependent gene expression
requires additional research.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of China (Beijing, China; grant nos.
81160289 and 81560500) and the Guangxi Science Research and
Technology Development Program (Nanning, China; grant no.
1355005-1-6).
Glossary
Abbreviations
Abbreviations:
|
NF-κB
|
nuclear factor-kappa B
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bax
|
Bcl-2-associated X protein
|
|
VEGF-A
|
vascular endothelial growth
factor-A
|
|
MMP-9
|
matrix metalloproteinase-9
|
|
RT-PCR
|
reverse transcription-polymerase chain
reaction
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsamandas AC, Kardamakis D, Tsiamalos P,
Liava A, Tzelepi V, Vassiliou V, Petsas T, Vagenas K, Zolota V and
Scopa CD: The potential role of Bcl-2 expression, apoptosis and
cell proliferation (Ki-67 expression) in cases of gastric carcinoma
and correlation with classic prognostic factors and patient
outcome. Anticancer Res. 29:703–709. 2009.PubMed/NCBI
|
|
3
|
Stanley TH: The fentanyl story. J Pain.
15:1215–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mystakidou K, Katsouda E, Parpa E, Vlahos
L and Tsiatas ML: Oral transmucosal fentanyl citrate: Overview of
pharmacological and clinical characteristics. Drug Deliv.
13:269–276. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seifeldin R and Grossman P: Fentanyl
transdermal system and oxycodone hydrochloride. J Manag Care Pharm.
9:457–459. 2003.PubMed/NCBI
|
|
6
|
Huffman DM, Grizzle WE, Bamman MM, Kim JS,
Eltoum IA, Elgavish A and Nagy TR: SIRT1 is significantly elevated
in mouse and human prostate cancer. Cancer Res. 67:6612–6618. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qin Y, Li L, Chen J, Tang X, Liao C, Xie Y
and Xiao Q: Fentanyl inhibits progression of human gastric cancer
MGC-803 cells by NF-kappaB downregulation and PTEN upregulation in
vitro. Oncol Res. 20:61–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Wang H, Jiang Z, Hu A, Chu L, Sun
Y and Han J: MicroRNA-19a mediates gastric carcinoma cell
proliferation through the activation of nuclear factor-κB. Mol Med
Rep. 12:5780–5786. 2015.PubMed/NCBI
|
|
9
|
Ma J, Liu J, Wang Z, Gu X, Fan Y, Zhang W,
Xu L, Zhang J and Cai D: NF-kappaB-dependent microRNA-425
upregulation promotes gastric cancer cell growth by targeting PTEN
upon IL-1β induction. Mol Cancer. 13:402014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beg AA, Sha WC, Bronson RT, Ghosh S and
Baltimore D: Embryonic lethality and liver degeneration in mice
lacking the RelA component of NF-kappa B. Nature. 376:167–170.
1995. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baeuerle PA and Baltimore D: NF-kappa B:
Ten years after. Cell. 87:13–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi J and Luo J: SIRT1 and p53, effect on
cancer, senescence and beyond. Biochim Biophys Acta.
1804:1684–1689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fabry ME, Kaul DK, Davis L, Gore JC, Brown
M and Nagel RL: An animal model for sickle cell vaso-occlusion: A
study using NMR and technetium imaging. Prog Clin Biol Res.
240:297–304. 1987.PubMed/NCBI
|
|
14
|
Fustéi Gamisans M and Busquet Duran X:
Impact of the commercialization of transdermic fentanyl on the home
care of terminal cancer patients. Aten Primaria. 29:316–317.
2002.(In Spanish). PubMed/NCBI
|
|
15
|
Lennon FE, Mirzapoiazova T, Mambetsariev
B, Poroyko VA, Salgia R, Moss J and Singleton PA: The Mu opioid
receptor promotes opioid and growth factor-induced proliferation,
migration and epithelial mesenchymal transition (EMT) in human lung
cancer. PLoS One. 9:e915772014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang XL, Chen ML and Zhou SL: Fentanyl
increases colorectal carcinoma cell apoptosis by inhibition of
NF-κB in a Sirt1-dependent manner. Asian Pac J Cancer Prev.
15:10015–10020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kampa M, Bakogeorgou E, Hatzoglou A,
Damianaki A, Martin PM and Castanas E: Opioid alkaloids and
casomorphin peptides decrease the proliferation of prostatic cancer
cell lines (LNCaP, PC3 and DU145) through a partial interaction
with opioid receptors. Eur J Pharmacol. 335:255–265. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verma IM, Stevenson JK, Schwarz EM, Van
Antwerp D and Miyamoto S: Rel/NF-kappa B/I kappa B family: Intimate
tales of association and dissociation. Genes Dev. 9:2723–2735.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boland MP, Foster SJ and O'Neill LA:
Daunorubicin activates NFkappaB and induces kappaB-dependent gene
expression in HL-60 promyelocytic and Jurkat T lymphoma cells. J
Biol Chem. 272:12952–12960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang CK, Llanes S and Schumer W: Effect
of dexamethasone on NF-κB activation, tumor necrosis factor
formation, and glucose dyshomeostasis in septic rats. J Surg Res.
72:141–145. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Welters ID, Fimiani C, Bilfinger TV and
Stefano GB: NF-kappaB, nitric oxide and opiate signaling. Med
Hypotheses. 54:263–268. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welters ID, Menzebach A, Goumon Y, Cadet
P, Menges T, Hughes TK, Hempelmann G and Stefano GB: Morphine
inhibits NF-kappaB nuclear binding in human neutrophils and
monocytes by a nitric oxide-dependent mechanism. Anesthesiology.
92:1677–1684. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scopa CD, Vagianos C, Kardamakis D,
Kourelis TG, Kalofonos HP and Tsamandas AC: Bcl-2/bax ratio as a
predictive marker for therapeutic response to radiotherapy in
patients with rectal cancer. Appl Immunohistochem Mol Morphol.
9:329–334. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Crowson AN, Magro CM, Kadin ME and Stranc
M: Differential expression of the bcl-2 oncogene in human basal
cell carcinoma. Hum Pathol. 27:355–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong SH, Lee HW, Han JH, Kang SY, Choi
JH, Jung YM, Choi H, Oh YT, Park KJ, Hwang SC, et al: Low
expression of Bax predicts poor prognosis in resected non-small
cell lung cancer patients with non-squamous histology. Jpn J Clin
Oncol. 38:661–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin Z, Yan W, Jin H, Ge C and Xu Y:
Differential effect of psoralidin in enhancing apoptosis of colon
cancer cells via nuclear factor-kappaB and B-cell lymphoma-2/B-cell
lymphoma-2-associated X protein signaling pathways. Oncol Lett.
11:267–272. 2016.PubMed/NCBI
|
|
29
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Spyridopoulos I, Luedemann C, Chen D,
Kearney M, Chen D, Murohara T, Principe N, Isner JM and Losordo DW:
Divergence of angiogenic and vascular permeability signaling by
VEGF: Inhibition of protein kinase C suppresses VEGF-induced
angiogenesis, but promotes VEGF-induced, NO-dependent vascular
permeability. Arterioscler Thromb Vasc Biol. 22:901–906. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee SJ, Kim JG, Sohn SK, Chae YS, Moon JH,
Kim SN, Bae HI, Chung HY and Yu W: No association of vascular
endothelial growth factor-A (VEGF-A) and VEGF-C expression with
survival in patients with gastric cancer. Cancer Res Treat.
41:218–223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hansson J, Lind L, Hulthe J and Sundström
J: Relations of serum MMP-9 and TIMP-1 levels to left ventricular
measures and cardiovascular risk factors: A population-based study.
Eur J Cardiovasc Prev Rehabil. 16:297–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao
X, Wetterskog D, Funa K, Bråkenhielm E and Cao Y: Angiogenic
factors FGF2 and PDGF-BB synergistically promote murine tumor
neovascularization and metastasis. J Clin Invest. 117:2766–2777.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cao R, Björndahl MA, Religa P, Clasper S,
Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, et
al: PDGF-BB induces intratumoral lymphangiogenesis and promotes
lymphatic metastasis. Cancer Cell. 6:333–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun WH, Sun YL, Fang RN, Shao Y, Xu HC,
Xue QP, Ding GX and Cheng YL: Expression of cyclooxygenase-2 and
matrix metalloproteinase-9 in gastric carcinoma and its correlation
with angiogenesis. Jpn J Clin Oncol. 35:707–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang Q, Ye ZY, Zhang JX, Tao HQ, Li SG and
Zhao ZS: Expression of matrix metalloproteinase-9 mRNA and vascular
endothelial growth factor protein in gastric carcinoma and its
relationship to its pathological features and prognosis. Anat Rec
(Hoboken). 293:2012–2019. 2010. View Article : Google Scholar : PubMed/NCBI
|