Introduction
Adult T-cell leukemia/lymphoma (ATLL) is an
aggressive lymphoid proliferative malignancy associated with the
oncoretrovirus human T-cell leukemia virus type 1 (HTLV-1)
(1–3).
ATLL develops upon a long latency period following viral infection,
and carries poor prognosis due to intrinsic resistance to
chemotherapy and profound immunosuppression (4). Recent advances in the treatment of ATLL
include the introduction of therapies that target C-C chemokine
receptor 4, which is abundantly expressed on the majority of ATLL
cells, and the use of allogeneic hematopoietic stem cell
transplantation for aggressive ATLL (5). However, patients suffer relapse
following the above treatments; thus, alternative or complementary
therapies for the treatment of ATLL are required.
Heat shock protein (HSP) 90 is a molecular chaperone
that enables the correct folding and function of a broad range of
substrate proteins called clients (6). HSP90 client proteins participate in
various ATLL processes, including oncogenic signal transduction
[nuclear factor-κB (NF-κB), Janus kinase/signal transducer and
activator of transcription and Akt], resistance to cell death
(survivin), cell cycle progression [cyclin-dependent kinase (CDK)4
and CDK6] and promotion of cell invasion (matrix
metalloproteinases) (7). These client
proteins may be depleted through the ubiquitin proteasome pathway
using HSP90 inhibitors. Since HSP90 interacts with a multitude of
client proteins, it is assumed that inhibition of HSP90 could
potentiate a greater antitumor effect than that of therapies based
on individual protein targeting. In preclinical studies, HSP90
inhibition alone produced promising results in the treatment of
ATLL (8–10). However, first generation HSP90
inhibitors demonstrated borderline efficacy in clinical trials
(11,12). The borderline therapeutic effect of
the first generation HSP90 inhibitors [which are known as
geldanamycin analogs and include
17-allylamino-17-demethoxygeldanamycin and
17-(dimethylaminoethyl-amino)-17-demethoxygeldanamycin] is
considered to be due to poor solubility and pharmacokinetics,
hepatotoxicity, susceptibility to P-glycoprotein efflux and failure
of metabolism by nicotinamide adenine dinucleotide phosphate
dehydrogenase, quinone 1/deoxythymidine-diaphorase enzymes
(13).
AUY922 is a resorcylic isoxazole amide and a potent
inhibitor of HSP90 (14). Since
AUY922 is a non-geldanamycin analog, it does not exhibit the
aforementioned limitations of the geldanamycin analogs (14), thus being a more promising agent for
clinical testing. AUY922 has demonstrated antitumor activity
against a variety of solid tumors in preclinical mouse models
(14,15). Furthermore, ongoing phase I and II
clinical trials are currently testing the effects of AUY922 in
hematologic malignancies and solid tumors (7). The objective of the present study was to
test the therapeutic efficacy of AUY922 in ATLL.
Materials and methods
Cell lines and inhibitor
The HTLV-1-infected T-cell lines, HUT-102 (Fujisaki
Cell Center, Hayashibara Biomedical Laboratories, Okayama, Japan)
and MT-4 (provided by Professor Naoki Yamamoto, Tokyo Medical and
Dental University, Tokyo, Japan), were cultured in RPMI-1640
(Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) medium containing
10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek,
Israel) and 1% penicillin/streptomycin (Nacalai Tesque, Inc.,
Kyoto, Japan). AUY922 was kindly provided by Novartis Institutes
for BioMedical Research (Basel, Switzerland) for in vivo
study and purchased from Shanghai Biochempartner Co., Ltd.
(Shanghai, China) for in vitro study.
Water-soluble tetrazolium (WST)-8
assay
The effect of AUY922 on cell viability was assayed
by WST-8 assay. Mitochondrial dehydrogenase cleavage of WST-8 to
formazan dye provided a measure of cell viability. WST-8 assays
were conducted according to the manufacturer's protocol (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Briefly,
1×104 HUT-102 and MT-4 cells/well were incubated in
96-well plates at 37°C for 24 h in the presence of different
concentrations of AUY922. A total of 10 µl WST-8/well was added,
and cells were incubated for 4 h. Absorbance was measured at 450 nm
using an iMark™ microplate absorbance reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Xenograft tumor model
Five-week-old female C.B-17/Icr-severe combined
immune deficiency (SCID) mice were obtained from Kyudo, Co., Ltd.
(Tosu, Japan). The mice were kept in specific pathogen-free
conditions. Animal cages were maintained at a temperature of 24°C
and a humidity of 60%, with a 12 h light/dark cycle. Mice were fed
a standard rodent diet (CE-2; CLEA Japan, Inc., Tokyo, Japan) and
water ad libitum. To induce malignancy, 1×107
HUT-102 cells suspended in 200 µl sterile RPMI-1640 medium were
inoculated subcutaneously into the postauricular region of the SCID
mice, which were then divided randomly into four treatment groups
(n=6/group). AUY922 was solubilized in water containing 5% glucose
(Nacalai Tesque, Inc., Kyoto, Japan) and administered
intraperitoneally every day for 27 days, beginning on the day
subsequent to cell inoculation. The control group received vehicle
(5% glucose solution) only, while the treated groups received
AUY922 at doses of 12.5, 18 or 30 mg/kg. The highest dose of AUY922
(30 mg/kg) was administered for 5–6 days/week with 1–2 days rest,
and the treatment was continued for 4 weeks, while the 12.5 and 18
mg/kg doses, were administered daily for 4 weeks. Tumor diameter
was measured weekly with a shifting caliper, and tumor volume was
calculated. Mice were weighed daily, beginning on day 4. All mice
were sacrificed on day 28, before tumors were able to reach the
ethically allowed maximal size. Subsequently, tumors were excised
and their weight was measured. The present study was performed
according to the Guidelines for Animal Experimentation of the
University of the Ryukyus (Nishihara, Japan), and was approved by
the Animal Care and Use Committee of the University of the
Ryukyus.
Morphological analysis of tumor
tissues and terminal deoxynucleotidyl transferase deoxyuridine
triphosphate nick end labeling (TUNEL) assay
Tumor specimens were collected from the control
group and the 30 mg/kg AUY922-treated group, fixed in formalin
solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan),
dehydrated through graded ethanol series (Japan Alcohol Trading
Co., Ltd., Tokyo, Japan) and embedded in paraffin (Sakura Finetek
Japan Co., Ltd., Tokyo, Japan). The paraffin-embedded specimens of
ATLL tumors were stained with hematoxylin and eosin (H&E; Merck
Millipore, Darmstadt, Germany). Cells were examined under a light
microscope (Axioskop 2 Plus) with an Achroplan ×40/0.65 lens (both
from Carl Zeiss AG, Oberkochen, Germany). Images were captured with
an AxioCam MRc camera and AxioVision 4.7 software (Carl Zeiss AG).
Analysis of DNA fragmentation by TUNEL assay was performed using a
commercial kit (Roche Applied Science, Penzberg, Germany).
Biomarker analysis
Serum concentrations of human soluble interleukin-2
receptor (sIL-2R; R&D Systems, Inc., Minneapolis, MN, USA) and
human soluble cluster of differentiation 30 (sCD30; BioVendor Inc.,
Brno, Czech Republic) were measured by enzyme-linked immunosorbent
assay, according to the manufacturer's protocol.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Differences between groups and between treatments were
tested for statistical significance by the Mann-Whitney U test and
Student's t test, as appropriate. P<0.05 was considered to
indicate a statistical significant difference.
Results
AUY922 reduces cell viability of
HTLV-1-infected T-cell lines in vitro
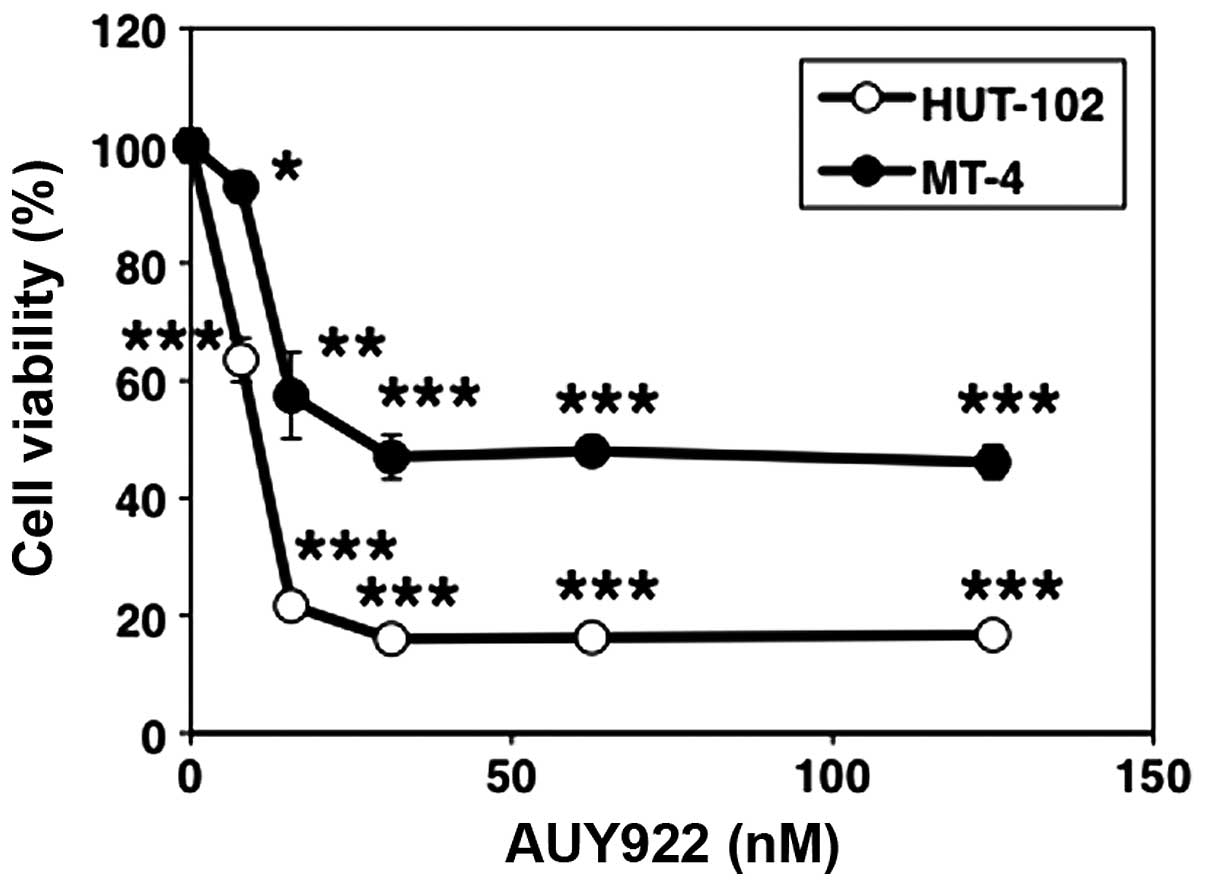
Two HTLV-1-infected T-cell lines (HUT-102 and MT-4)
were used to determine the efficacy of AUY922 against ATLL. The two
cell lines were treated with various concentrations of AUY922 for
24 h, and cell viability was measured using the WST-8 assay. AUY922
at 0–31 nM concentration resulted in a dose-dependent decrease in
cell viability of both tested cell lines. However, cell viability
reached a plateau level at concentrations >31 nM (Fig. 1).
AUY922 delays ATLL tumor growth in
vivo
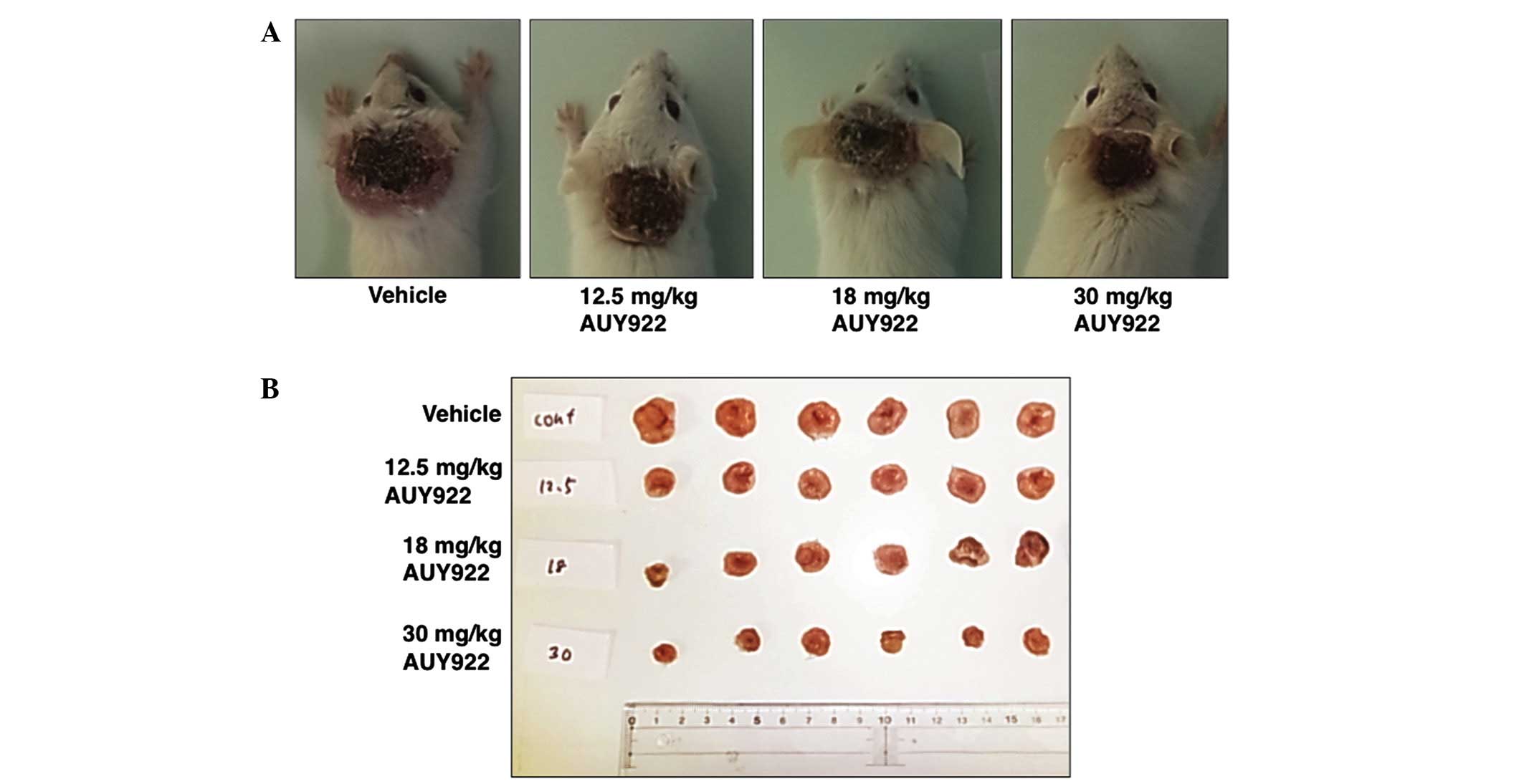
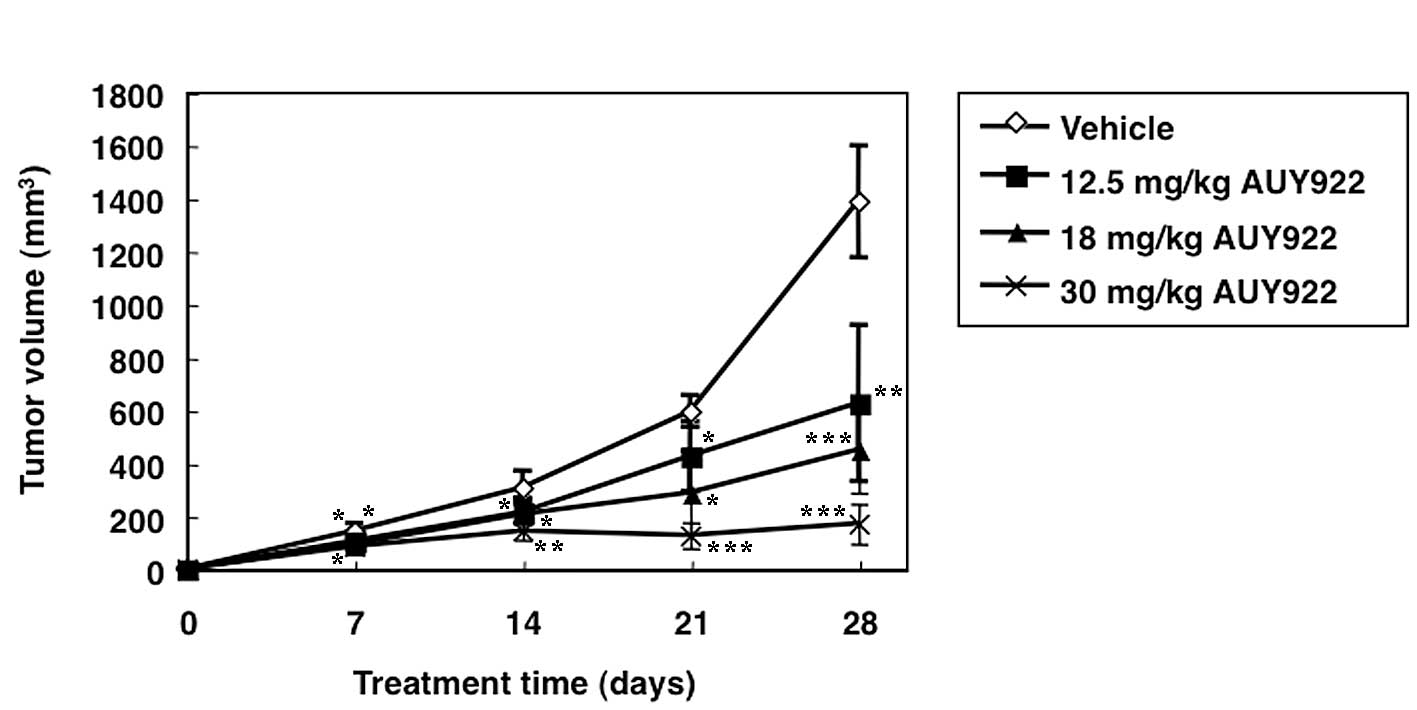
Treatment of mice harboring HUT-102 ATLL tumors with
AUY922 resulted in significant reduction in tumor volume, compared
with vehicle-treated mice. All three doses of AUY922 used in the
present study (12.5, 18 and 30 mg/kg) resulted in significant
suppression of the growth rate of tumors, compared with
vehicle-treated mice (Figs. 2A and
3). The most efficient antitumor
effect was observed in mice treated with 30 mg/kg AUY922, with
almost complete stasis. This reduction in tumor growth was also
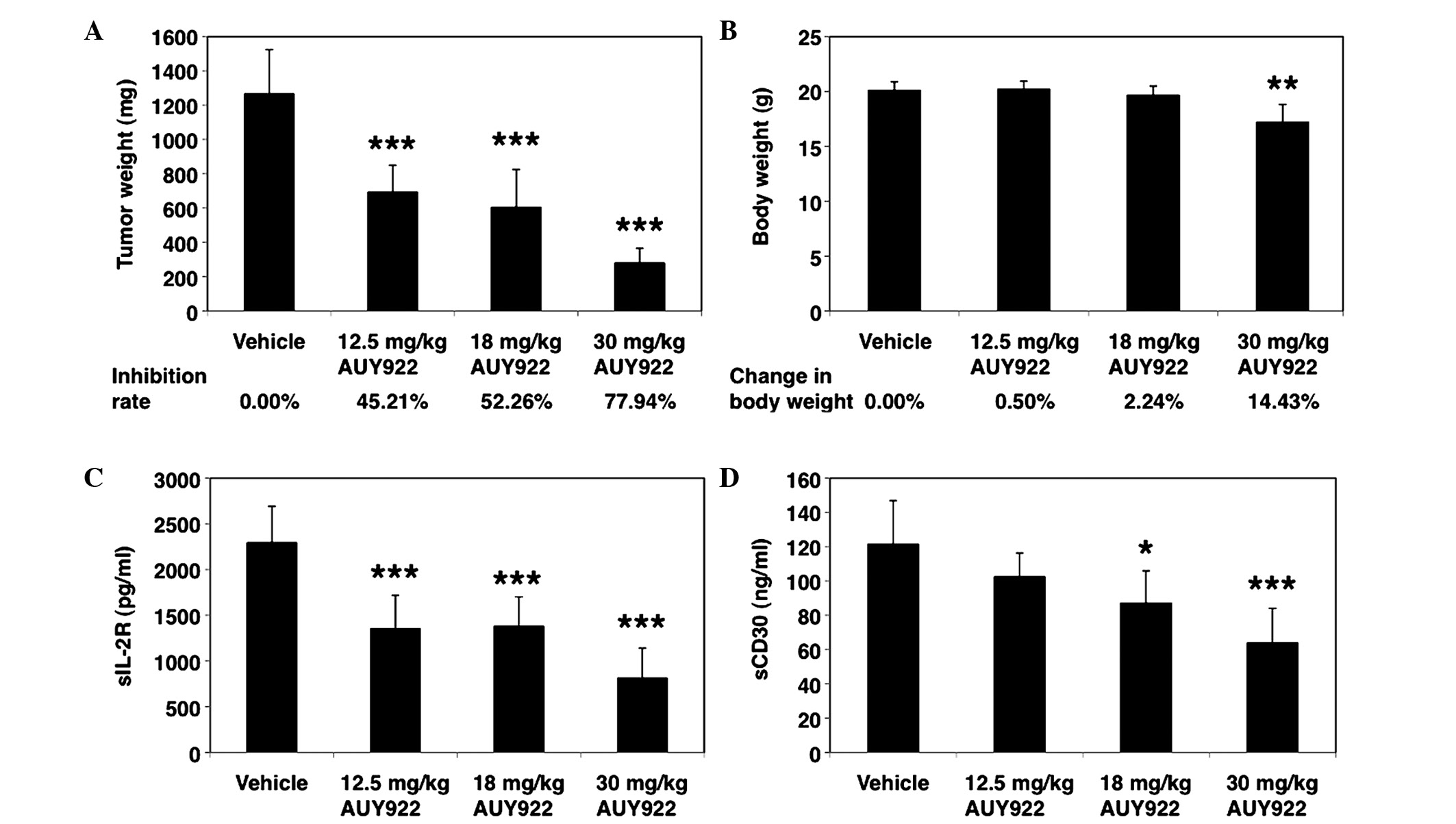
reflected in the weight of the excised tumor harvested on day 28,
which was significantly lower in the AUY922-treated groups than in
the control groups (Figs. 2B and
4A). The tumor inhibition rates for
12.5, 18 and 30 mg/kg AUY922 were 45.21, 52.26 and 77.94%,
respectively (Fig. 4A).
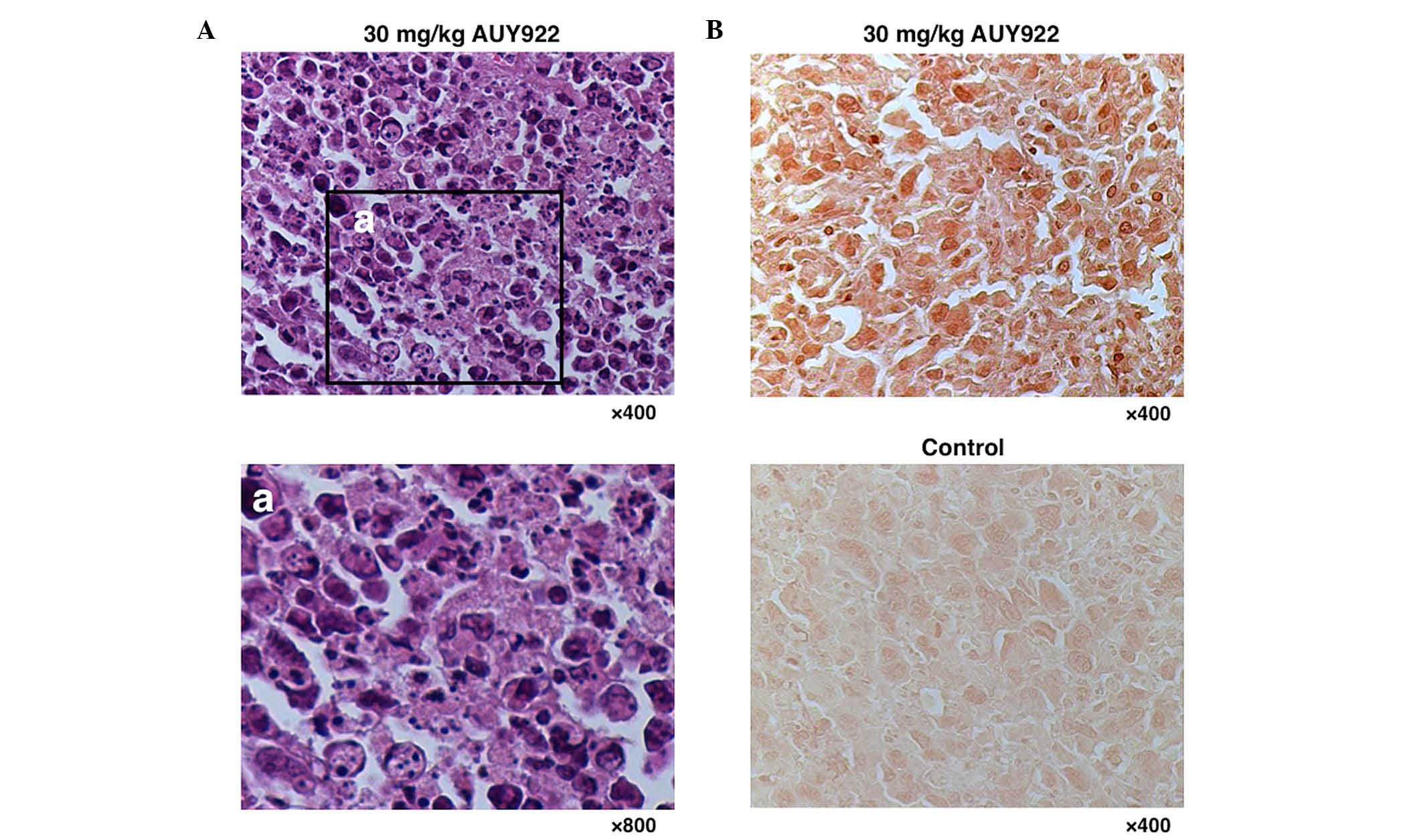
To test whether apoptosis is the major process
underlying HSP90 inhibition by AUY922, H&E-stained and
TUNEL-labeled slides were examined. As represented in Fig. 5A, apoptotic HUT-102 cells were
observed in the AUY922 treatment group (30 mg/kg), which were
characterized by cytoplasmic condensation, chromatin
hyperchromatism and condensation, and nuclear fragmentation. TUNEL
staining revealed abundant apoptotic cells in the tumors of the
AUY922-treated group, compared with only a few apoptotic cells in
the tumors of the control group (Fig.
5B).
Importantly, the treatment regimens were well
tolerated, and the mean body weight of the animals increased during
the study in the 12.5 and 18 mg/kg treatment groups (Fig. 4B). However, the body weight was
significantly different between the AUY922 (30 mg/kg) and the
control groups (Fig. 4B). AUY922 at
30 mg/kg caused considerable loss of body weight (14.43%) due to
diarrhea, whereas at 12.5 and 18 mg/kg, it was well tolerated, with
a final mean change in body weight of 0.50 and 2.24%, respectively
(Fig. 4B).
The serum levels of the surrogate tumor markers
sIL-2R (16) and sCD30 (17) were also measured in order to determine
the therapeutic efficacy of AUY922. Compared with the vehicle
control group, there was a significant reduction in serum sIL-2R
and sCD30 levels in the animals of the AUY922 groups (Fig. 4C and D). These results are consistent
with the dose-dependent antitumor efficacy observed in the HUT-102
ATLL in vivo model. Taken together, the above results
indicate that treatment with AUY922 results in substantial
inhibition of in vivo growth of ATLL cells through its
direct effects on tumor cells.
Discussion
Novel therapies aimed at simultaneous targeting of
multiple signaling pathways have been considered for the treatment
of ATLL, since such approaches could prevent the development of
molecular escape mechanisms towards selective targeted therapy and
aid to overcome chemoresistance (18). In this context, the use of HSP90
inhibitors, which is based on interference with a broad range of
oncogenic signaling components in ATLL cells, has gained momentum
(8–10). In the present study, the anti-ATLL
efficacy of AUY922, a second generation synthetic HSP90 inhibitor,
was demonstrated. Previous studies have reported that AUY922
induces cell-cycle arrest and apoptosis in ATLL cell lines and
primary ATLL cells in vitro (19). HSP90 blockade resulted in the
inhibition of NF-κB, Akt and proviral integration site for Moloney
murine leukemia virus family (19).
The present study investigated the effects of AUY922 on mice
harboring ATLL tumor cells. The results demonstrated that AUY922
has significant anti-ATLL properties. Compared with the control
group, AUY922 significantly decreased tumor volume and weight, and
increased tumor inhibition rate, as demonstrated by morphological
changes indicative of apoptosis and increased tumor cell apoptosis.
Furthermore, the present results revealed the dose-dependent
effects of AUY922, whose maximum effect was noted at a dose of 30
mg/day. These results suggest that HSP90 blockade with the novel
inhibitor AUY922 represents an efficacious approach for the
treatment of ATLL.
In contrast to previous studies that investigated
the chemotherapeutic effects of AUY922 in other malignancies, this
agent was used at doses of 12.5 and 18 mg/kg/day, or 30 mg/kg for
5–6 days/week in the present study, which is below the maximum
tolerated dose for AUY922, instead of daily injections of 50 mg/kg
reported in other studies (14). This
aspect is important in minimizing potential side-effects of
HSP90-targeted therapy. In fact, diarrhea occurred in mice injected
with AUY922 at 30 mg/kg for 5–6 days/week, and it has been reported
that the most common AUY922-related toxicity was diarrhea in phase
I dose-escalation studies involving patients with advanced solid
tumors (20,21). Taken together, the present data
support the selection of AUY922 as a novel anti-ATLL candidate for
clinical evaluation.
Acknowledgements
The authors would like to thank Novartis Institutes
for BioMedical Research for kindly providing AUY922.
References
|
1
|
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn
PA, Minna JD and Gallo RC: Detection and isolation of type C
retrovirus particles from fresh and cultured lymphocytes of a
patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA.
77:7415–7419. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hinuma Y, Nagata K, Hanaoka M, Nakai M,
Matsumoto T, Kinoshita KI, Shirakawa S and Miyoshi I: Adult T-cell
leukemia: Antigen in an ATL cell line and detection of antibodies
to the antigen in human sera. Proc Natl Acad Sci USA. 78:6476–6480.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida M, Miyoshi I and Hinuma Y:
Isolation and characterization of retrovirus from cell lines of
human adult T-cell leukemia and its implication in the disease.
Proc Natl Acad Sci USA. 79:2031–2035. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bazarbachi A, Suarez F, Fields P and
Hermine O: How I treat adult T-cell leukemia/lymphoma. Blood.
118:1736–1745. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishitsuka K and Tamura K: Human T-cell
leukaemia virus type I and adult T-cell leukaemia-lymphoma. Lancet
Oncol. 15:e517–e526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banerji U: Heat shock protein 90 as a drug
target: Some like it hot. Clin Cancer Res. 15:9–14. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong DS, Banerji U, Tavana B, George GC,
Aaron J and Kurzrock R: Targeting the molecular chaperone heat
shock protein 90 (HSP90): Lessons learned and future directions.
Cancer Treat Rev. 39:375–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan P, Qing G, Qu Z, Wu CC, Rabson A and
Xiao G: Targeting autophagic regulation of NFkappaB in HTLV-I
transformed cells by geldanamycin: Implications for therapeutic
interventions. Autophagy. 3:600–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurashina R, Ohyashiki JH, Kobayashi C,
Hamamura R, Zhang Y, Hirano T and Ohyashiki K: Anti-proliferative
activity of heat shock protein (Hsp) 90 inhibitors via
beta-catenin/TCF7L2 pathway in adult T cell leukemia cells. Cancer
Lett. 284:62–70. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikebe E, Kawaguchi A, Tezuka K, et al:
Oral administration of an HSP90 inhibitor, 17-DMAG, intervenes
tumor-cell infiltration into multiple organs and improves survival
period for ATL model mice. Blood Cancer J. 3:e1322013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banerji U, O'Donnell A, Scurr M, Pacey S,
Stapleton S, Asad Y, Simmons L, Maloney A, Raynaud F, Campbell M,
et al: Phase I pharmacokinetic and pharmacodynamic study of
17-allylamino, 17-demethoxygeldanamycin in patients with advanced
malignancies. J Clin Oncol. 23:4152–4161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramanathan RK, Egorin MJ, Erlichman C, et
al: Phase I pharmacokinetic and pharmacodynamic study of
17-dimethylaminoethylamino-17-demethoxygeldanamycin, an inhibitor
of heat-shock protein 90, in patients with advanced solid tumors. J
Clin Oncol. 28:1520–1526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelland LR, Sharp SY, Rogers PM, Myers TG
and Workman P: DT-diaphorase expression and tumor cell sensitivity
to 17-allylamino, 17-demethoxygeldanamycin, an inhibitor of heat
shock protein 90. J Natl Cancer Inst. 91:1940–1949. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eccles SA, Massey A, Raynaud FI, Sharp SY,
Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall
F, et al: NVP-AUY922: A novel heat shock protein 90 inhibitor
active against xenograft tumor growth, angiogenesis, and
metastasis. Cancer Res. 68:2850–2860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jensen MR, Schoepfer J, Radimerski T,
Massey A, Guy CT, Brueggen J, Quadt C, Buckler A, Cozens R,
Drysdale MJ, et al: NVP-AUY922: A small molecule HSP90 inhibitor
with potent antitumor activity in preclinical breast cancer models.
Breast Cancer Res. 10:R332008. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamihira S, Atogami S, Sohda H, Momita S,
Yamada Y and Tomonaga M: Significance of soluble interleukin-2
receptor levels for evaluation of the progression of adult T-cell
leukemia. Cancer. 73:2753–2758. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishioka C, Takemoto S, Kataoka S,
Yamanaka S, Moriki T, Shoda M, Watanabe T and Taguchi H: Serum
level of soluble CD30 correlates with the aggressiveness of adult
T-cell leukemia/lymphoma. Cancer Sci. 96:810–815. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W and Neckers L: Targeting the
molecular chaperone heat shock protein 90 provides a multifaceted
effect on diverse cell signaling pathways of cancer cells. Clin
Cancer Res. 13:1625–1629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi H, Hasegawa H, Sasaki D, Ando K,
Sawayama Y, Imanishi D, Taguchi J, Imaizumi Y, Hata T, Tsukasaki K,
et al: Heat shock protein 90 inhibitor NVP-AUY922 exerts potent
activity against adult T-cell leukemia-lymphoma cells. Cancer Sci.
105:1601–1608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sessa C, Shapiro GI, Bhalla KN, Britten C,
Jacks KS, Mita M, Papadimitrakopoulou V, Pluard T, Samuel TA,
Akimov M, et al: First-in-human phase I dose-escalation study of
the HSP90 inhibitor AUY922 in patients with advanced solid tumors.
Clin Cancer Res. 19:3671–3680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doi T, Onozawa Y, Fuse N, Yoshino T,
Yamazaki K, Watanabe J, Akimov M, Robson M, Boku N and Ohtsu A:
Phase I dose-escalation study of the HSP90 inhibitor AUY922 in
Japanese patients with advanced solid tumors. Cancer Chemother
Pharmacol. 74:629–636. 2014. View Article : Google Scholar : PubMed/NCBI
|