Introduction
Taxane binds to β-tubulin, promotes microtubule
polymerization and prevents depolymerization, thereby arresting
cell division and inducing apoptosis (1). In Japan, docetaxel was approved in June
1997 and paclitaxel was approved in October 1997. Currently, taxane
and anthracycline play a central role in chemotherapy for breast
cancer (2).
There have been numerous studies on the therapeutic
effect of taxanes in metastatic breast cancer (3,4). Jones
et al (3) conducted a
randomized phase III trial to compare the effects of docetaxel and
paclitaxel in metastatic breast cancer, reporting that the overall
response rate (ORR) and median time to progression (TTP) for
docetaxel and paclitaxel were 32% and 5.7 months and 25.0% and 3.6
months, respectively (3). Gradishar
et al reported that the RR and median TTP for taxane as the
first-line therapy were 27% and 19.7 weeks, respectively, and that
the ORR and median TTP were 19% and 16.9 weeks, respectively
(4).
Gene expression profiling using DNA microarrays
classifies breast cancers into five intrinsic subtypes: Luminal A,
luminal B, ER BB2+, normal-like and basal-like (5). Accordingly, immunohistochemical
classification with hormone receptors and human epidermal growth
factor receptor 2 (HER2) may be used to estimate the subtype.
Luminal A breast cancer was defined to express estrogen receptor
(ER) and not express HER2 in the CALGGB 9344 trial (6), express ER and/or progesterone receptor
(PgR) with a low Ki67 labeling index (cut-off, 13%) in the BCIRG
001 trial (7), and express ER with a
low Ki67 labeling index (cut-off, 20%) in the PACS 01 trial
(8). In addition, the St. Gallen
International Conference 2011 proposed that breast cancer
expressing ER and/or PgR, and negative for HER2 with a Ki67
labeling index of <14%, should be defined as luminal A breast
cancer (9).
Numerous studies have reported that chemotherapy is
relatively ineffective in patients with early luminal A breast
cancer. Pritchard et al reported that treatment containing
anthracycline was not superior to cyclophosphamide, methotrexate
and 5-fluorouracil in HER2− breast cancer (10). The BCIRG 001 trial showed that
docetaxel, doxorubicin and cyclophosphamide (TAC) chemotherapy was
more effective compared with fluorouracil, doxorubicin and
cyclophosphamide (FAC) chemotherapy, but failed to show superiority
in patients with luminal A breast cancer (7). Meta analysis of the EBCTCG trials showed
that the proportional risk reductions of chemotherapy were observed
equally in patients with ER+ and ER- breast cancer in a group of
women aged <50 years (11).
However, the NSABP B-30 trial followed up the premenopausal women
of patients who had received chemotherapy, and found that the
hazards ratio for disease-free survival was reduced subsequent to
menopause in premenopausal patients that had received chemotherapy.
This suggested that the chemotherapy-induced depletion of
endogenous estrogen may be more important than the effect of
chemotherapy itself (12). Alba et
al directly compared the efficacy of aromatase inhibitors and
chemotherapy with epirubicin and cyclophosphamide followed by
taxane in neoadjuvant therapy for ER or PgR+ and
HER2− luminal breast cancer, finding that the RR and
complete histological RR of chemotherapy were not significantly
higher than those for endocrine therapy (13).
In addition, several studies have reported the
absence of an additional benefit of taxane treatment for luminal
breast cancer. The CALGB 9344 trial showed that in luminal A breast
cancer, defined as breast cancer positive for ER and negative for
HER2, there was no additional benefit of the addition of paclitaxel
to doxorubicin and cyclophosphamide chemotherapy (6). The BCIRG 001 trial (7), in which luminal A breast cancer was
defined as breast cancer positive for ER or PgR, with a low Ki67
labeling index (cut-off, 13%), reported that the prognosis of
patients treated with TAC was approximately equal to that of
patients treated with FAC. The PACS 01 trial (8) compared 6 cycles of 5-flourouracil,
epirubicin and cyclophosphamide (FEC) and 3 cycles of FEC followed
by 3 cycles of docetaxel. This study found that the recurrence-free
survival of FEC followed by docetaxel was not superior to FEC alone
in patients with luminal A breast cancer (defined as breast cancer
positive for ER with a Ki67 labeling index <20%).
Overall, numerous studies have suggested that taxane
therapy is relatively ineffective in patients with operable early
luminal breast cancer. However, to the best of our knowledge, no
studies have reported whether taxane also lacks efficacy as a
treatment for luminal metastatic breast cancer. Therefore, the
therapeutic effect of taxanes on metastatic breast cancer in
various immunohistochemical subtypes was retrospectively
investigated.
Materials and methods
Patient selection
Of the 527 patients with metastatic breast cancer
treated in The Cancer Institute Hospital of The Japanese Foundation
for Cancer Research (Tokyo, Japan) between January 2000 and August
2008, 293 patients received treatment with taxanes. Out of these
patients, 159 patient tissues were classified as luminal type
(ER+ and/or PgR+ and HER2−), 28
patient tissues were classified as luminal HER2 type
(ER+ and/or PgR+ and HER2+), 57
patient tissues were classified as HER2 type (ER−,
PgR− and HER2+), and 49 patient tissues were
classified as triple-negative type (ER−, PgR−
and HER2−) breast cancer.
Methods
Comprehensive consent for the use of specimen
materials was obtained preoperatively from all patients
participating as subjects in the present study. The study was
approved by the Institutional Review Board of The Cancer Institute
Hospital of The Japanese Foundation for Cancer Research (Tokyo,
Japan).
The immunohistochemical subtypes were determined by
biopsy of the primary lesion at stage IV and the surgical materials
in recurrent breast cancer. Biopsy specimens were embedded in
paraffin and cut into 4-µm slices. The specimens were stained with
hematoxylin and eosin (HE) and were immunohistochemically examined
for ER, PgR and HER2 expression. Using HE-stained slices, the
histological subtypes of cancer and the nuclear grade of cancer
cells were evaluated according to the general rules for clinical
and pathological assessment of breast cancer, as edited by the
Japanese Breast Cancer Society (14).
The histological subtype was divided into three groups, consisting
of papillotubular carcinoma, solid-tubular carcinoma and scirrhous
carcinoma, based on the size and structure of invasive components.
The definitions of the histological subtypes were as follows:
Papillotubular carcinoma, an invasive carcinoma characterized by
large invasive components with papillary structures and/or tubular
formations; solid-tubular carcinoma, an invasive carcinoma
characterized by large solid invasive components; and scirrhous
carcinoma, an invasive carcinoma characterized by small scattered
invasive components or trabecular invasive nests with a
desmoplastic stroma. The nuclear grade was evaluated using a
combination of nuclear atypia and mitotic counts. Nuclear grade was
divided into three groups, consisting of nuclear grades 1, 2 and 3,
in order of increasing atypia.
Immunohistochemical assessment of ER and PgR
expression was performed using mouse anti ERα monoclonal antibody
(product no. IR08461, clone, 1D5; Dako Japan Co., Ltd., Tokyo,
Japan. This product is supplied in 0.05 mol/l Tris/HCl, pH 7.2,
containing 15 mol/l sodium azide and fetal calf serum protein (1 ml
total volume). Anti-ER, 1D5 used at a dilution of 1:35 when
performing IHC using the Dako EnVision) and mouse anti PgR
monoclonal antibody (product no. IS06830, clone, PgR636; Dako Japan
Co., Ltd. Ready-to-use monoclonal mouse antibody provided in liquid
form in a buffer containing stabilizing protein and 0.015 mol/l
NaN3. The target concentration of Anti-PR, clone PgR636
is 0.5 µg/ml; the acceptable concentration range of Anti-PR, clone
PgR636 is 0.4–0.6 µg/ml). Positive reactions for ER and PgR were
defined as nuclear staining in ≥10% of cancer cells, and no
reaction was defined as staining in <10% of cancer cells.
Hormone receptor positivity was defined as showing positivity for
ER or PgR. Immunohistochemical detection of the HER2 protein was
performed using the HercepTest (product no. K520411, Dako Japan
Co., Ltd.).
Immunohistochemical detection of the HER2 protein
was performed using the HercepTest (Dako Japan Co., Ltd.).
Expression of the HER2 protein was classified into four groups: 0;
1+; 2+; and 3+. In the tissues that were classified as 2+, HER2
genetic testing by fluorescent in situ hybridization (FISH)
was performed using a PathVysion HER2-DNA Probe kit (Abbott
Molecular, Inc., Des Plaines, IL, USA). The protein and genetic
status were each estimated based on the guidelines for HER2 testing
in breast cancer, as edited by the American Society of Clinical
Oncology/College of American Pathologists (15). HER2 positivity was defined as HER2
protein expression of 3+ or HER2 gene amplification.
Based on the combination of ER, PgR and HER2
expression, patients were classified into four subtypes, defined as
follows: Luminal subtype, ER+ and/or PgR+,
and HER2−; luminal HER2 subtype, ER+ and/or
PgR+, and HER2+; HER2 type, ER−,
PgR− and HER2+; and triple-negative subtype,
ER−, PgR− and HER2−.
Treatment
The taxane regimens employed in the present study
included a weekly paclitaxel (wPAC) regimen at a dose of 80
mg/m2 and a triweekly docetaxel (3wDOC) regimen at a
dose of 60 mg/m2, which is the approved dose of
docetaxel in Japan. wPAC and 3wDOC were administered to 59.1 and
40.9% of the patients with luminal type breast cancer, 78.6 and
21.4% of the patients with luminal-HER2 type breast cancer, 82.5
and 17.5% of the patients with HER2 type breast cancer, and 55.1
and 44.9% of the patients with triple-negative type breast cancer,
respectively. Concurrent trastuzumab therapy was administered to
89.3% of the patients with luminal-HER2 type breast cancer, 94.7%
of the patients with HER2 type breast cancer and 1.9% of the
patients with luminal type breast cancer who were later found to
not express HER2 by FISH. In patients who received wPAC and 3wDOC
for the treatment of metastatic breast cancer, the therapeutic
effect of the initial regimen including a taxane was evaluated.
Response evaluation
Radiological tumor assessments were performed by
computed tomography every 3–4 months during treatment. Local lesion
and lymph node metastases were measured by echogram. The response
to chemotherapy was assessed according to the Response Evaluation
Criteria in Solid Tumors (RECIST) version 1.1 (16). TTP was defined as the time interval
between the start of taxane treatment and disease progression or
failure of treatment due to adverse events.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
used for statistical analysis in the present study. The ORR and
clinical benefit rate (CBR) were analyzed using the χ2
test. TTP was tested by the log-rank test using the Kaplan-Meier
method. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The characteristics of all 293 patients are listed
in Table I. All evaluated patients
were diagnosed with invasive breast cancer. The average age was 54
years. The patient numbers according to the age group were as
follows: ≤49 years old, 112 patients (38.2%); and ≥50 years old,
181 patients (61.8%). The most common sites of distant metastasis
were the lymph nodes, which was identified in 190 patients (64.8%).
The sites of metastases was 3 (P=0.016). The number of patients
that received one previous chemotherapy regimen was 103 patients
(35.2%; P=0.003); 143 patients (48.8%) had a history of receiving
anthracyclines (P<0.001).
 | Table I.Patient characteristics (n=293). |
Table I.
Patient characteristics (n=293).
| Patient
characteristics | Value, n (%) |
|---|
| Age, years |
|
|
Median | 54 |
|
Range | 28–84 |
| Age distribution |
|
|
<50 | 112 (38.2) |
| ≥50 | 181 (61.8) |
| Status |
|
| Stage
IV | 92
(31.4) |
|
Recurrence | 201 (68.6) |
| DFI,
days | 1,323 |
| Number of
metastasis |
|
|
Median | 3 |
|
Range | 0–8 |
| Sites of
metastasis |
|
| Lymph
node | 190 (64.8) |
|
Liver | 138 (47.1) |
| Lung | 156 (53.2) |
| Bone | 178 (60.8) |
|
Brain | 69
(23.5) |
|
Local | 64
(21.8) |
|
Others | 51
(17.4) |
| Prior chemotherapy
regimens |
|
| None | 65
(22.2) |
| 1 | 103 (35.2) |
| 2 | 70
(23.9) |
| 3 | 32
(10.9) |
| ≥4 | 23 (7.8) |
| Prior
anthracycline | 143 (48.8) |
| For
metastasis | 135 (46.1) |
| NAC or
adjuvant | 8
(2.7) |
| Estrogen
receptor |
|
|
Positive | 177 (60.4) |
|
Negative | 116 (39.6) |
| Progesterone
receptor |
|
|
Positive | 133 (45.4) |
|
Negative | 160 (54.6) |
| HER2 status |
|
| IHC
0 | 114 (38.9) |
| IHC
1+ | 77
(26.3) |
| IHC
2+ | 32
(10.9) |
|
FISH negative | 18
(6.1) |
|
FISH positive | 14
(4.8) |
| IHC
3+ | 70
(23.9) |
Distribution of immunohistochemical
subtypes and clinicopathological features
Associations between the distribution of
immunohistochemical subtypes and clinicopathological features are
shown in Table II. While there was
no significant difference in age among the 4 subtypes, significant
differences between subtype distribution, number of metastases,
number of prior chemotherapy regimens and history of anthracycline
administration were observed. Luminal type tumors were associated
with a significantly increased incidence of bone metastasis
(P<0.001), while the HER2 and triple-negative types showed a
significantly increased frequency of brain metastasis (both
P<0.001). The patients with luminal type tumors had received
more prior chemotherapy compared with other subtypes, and the
luminal type included a significantly increased proportion of
patients with a history of receiving anthracyclines
(P<0.001).
 | Table II.Immunohistochemical subtypes
distribution. |
Table II.
Immunohistochemical subtypes
distribution.
| Characteristic | Luminal, n (%) | Luminal-HER2, n
(%) | HER2, n (%) | Triple-negative, n
(%) | P-value |
|---|
| Total | 159
(100.0) | 28
(100.0) | 57
(100.0) | 49
(100.0) |
|
| Age, years |
|
|
|
|
|
|
Median | 53 | 51 | 55 | 55 |
|
|
Range | 28–84 | 33–83 | 34–74 | 29–84 |
|
| Age
distribution |
|
|
|
| 0.155 |
| <50
years | 70
(44.0) | 10 (35.7) | 17 (29.8) | 15 (30.6) |
|
| ≥50
years | 89
(56.0) | 18 (64.3) | 40 (70.2) | 34 (69.4) |
|
| Status |
|
|
|
| 0.133 |
| Stage
IV | 42
(26.4) | 13 (46.4) | 21 (36.8) | 16 (32.7) |
|
|
Recurrence | 117 (73.6) | 15 (53.6) | 36 (63.2) | 33 (67.3) |
|
| DFI,
days | 1,766 | 1,293 | 769 | 694 |
|
| No. of metastases,
n |
|
|
|
|
|
|
Median | 2.3 | 2.8 | 3.1 | 4.0 |
|
|
Range | 1–6 | 1–8 | 1–7 | 1–7 |
|
| Site of
metastasis |
|
|
|
| 0.016 |
| Lymph
node | 96
(60.4) | 18 (64.3) | 40 (70.2) | 36 (73.5) |
|
|
Liver | 80
(50.3) | 12 (42.9) | 25 (43.9) | 21 (42.9) |
|
|
Lung | 80
(50.3) | 11 (39.3) | 33 (57.9) | 32 (65.3) |
|
|
Bone | 114 (71.7) | 15 (53.6) | 24 (42.1) | 25 (51.0) |
|
|
Brain | 22
(13.8) | 5
(17.9) | 22 (38.6) | 20 (40.8) |
|
|
Local | 28
(17.6) | 4
(14.3) | 14 (24.6) | 18 (36.7) |
|
|
Other | 26
(16.4) | 5
(17.9) | 8
(14.0) | 12 (24.5) |
|
| No. of chemotherapy
regimens |
|
|
|
| 0.003 |
|
None | 21
(13.2) | 8
(28.6) | 20 (35.1) | 16 (32.7) |
|
| 1 | 55
(34.6) | 9
(32.1) | 19 (33.3) | 20 (40.8) |
|
| 2 | 39
(24.5) | 9
(32.1) | 12 (21.1) | 10 (21.4) |
|
| 3 | 26
(16.4) | 1 (3.6) | 4 (7.0) | 1 (2.0) |
|
| ≥4 | 18
(11.3) | 1 (3.6) | 2 (3.5) | 2 (4.1) |
|
| Prior anthracycline
administration |
|
|
|
| <0.001 |
| For
metastasis | 90
(56.6) | 8
(28.6) | 24 (42.1) | 17 (34.7) |
|
| NAC or
adjuvant | 34
(21.4) | 1 (3.6) | 0 (0.0) | 3 (6.1) |
|
| Chemotherapy
regimen |
|
|
|
|
|
| Weekly
Paclitaxel | 94
(59.1) | 22 (78.6) | 47 (82.5) | 27 (55.1) |
|
|
Triweekly Docetaxel | 65
(40.9) | 6
(21.4) | 10 (17.5) | 22 (44.9) |
|
| With
Trastuzumab | 3
(1.9) | 25 (89.3) | 54 (94.7) | 0 (0.0) |
|
RR
The RR and CBR in the 4 subtypes are shown in
Table III. The RR was 33.1% and the
CBR was 56.3% in all patients with metastatic breast cancer. The RR
and CBR in the 4 subtypes were significantly different between the
subtypes (P<0.001). In the luminal-HER2 and HER2 types, the RRs
were 57.1 and 52.6%, respectively, and the CBRs were 78.6 and
71.9%, respectively. In the luminal type, the RR and CBR were 28.3
and 51.6%, respectively. Between the luminal and triple-negative
types, the RR and CBR of patients with luminal type tumors were
increased compared with the patients with triple-negative type
tumors.
 | Table III.Response rate. |
Table III.
Response rate.
| Response | Luminal, n | Luminal-HER2,
n | HER2, n | Triple-negative,
n | Total, n |
|---|
| CR | 2 | 0 | 3 | 0 | 5 |
| PR | 43 | 16 | 27 | 6 | 92 |
| SD | 78 | 8 | 18 | 25 | 129 |
| Long SD | 37 | 6 | 11 | 14 | 68 |
| PD | 36 | 4 | 9 | 18 | 67 |
| RR, % | 28.3 | 57.1 | 52.6 | 12.2 | 33.1 |
| CBR, % | 51.6 | 78.6 | 71.9 | 40.8 | 56.3 |
TTP according to subtypes
The median follow-up time was 9.2 months. TTP events
had occurred in 117 patients with luminal type tumors (73.6%), 19
patients with luminal-HER2 type tumors (67.9%), 38 patients with
HER2 type tumors (66.7%) and 45 patients with triple-negative type
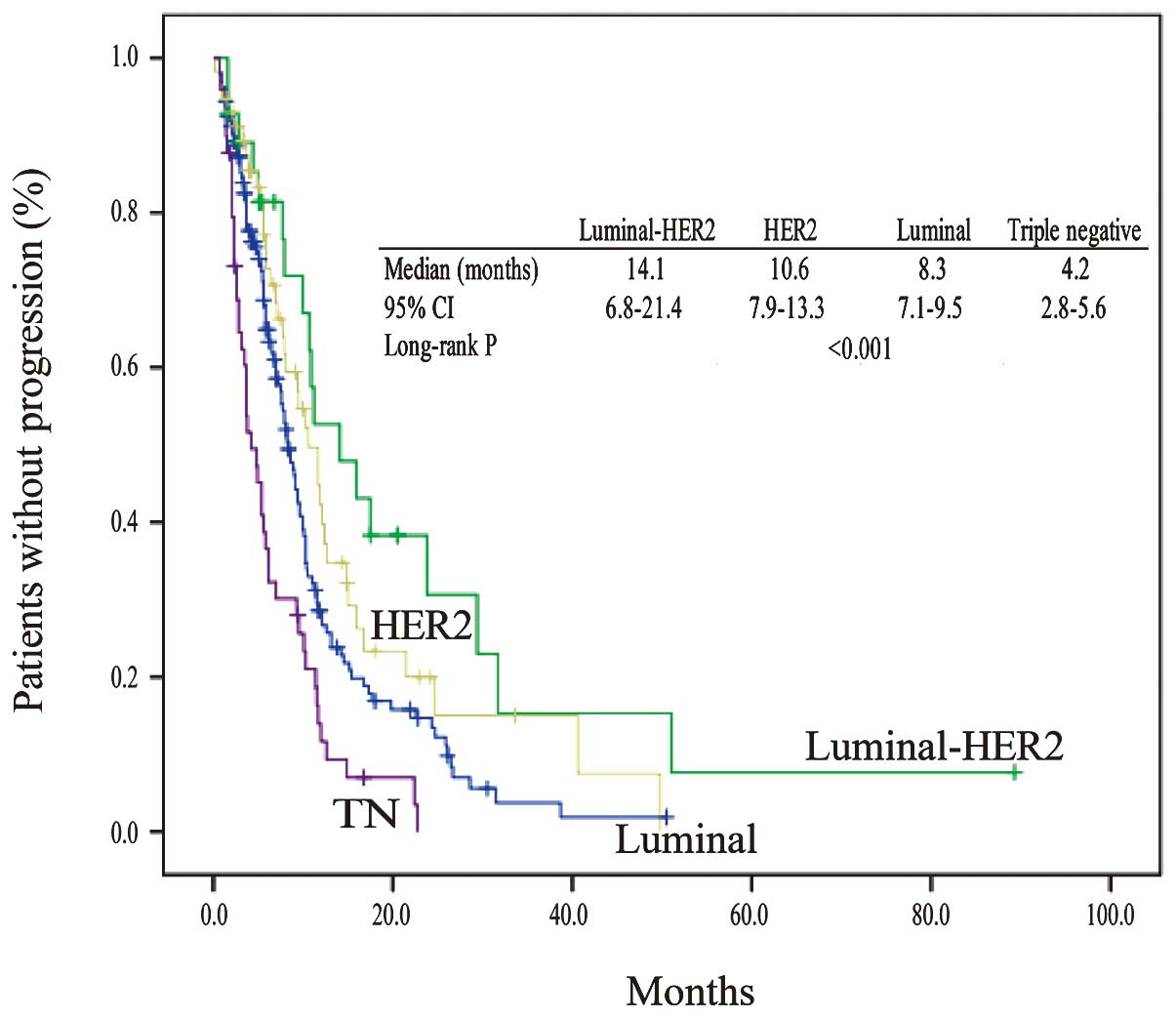
tumors (91.8%). The TTP in the 4 subtypes is shown in Fig. 1. There were significant differences in
TTP between subtypes (median TTP, 8.3 months in the luminal, 14.1
months in the luminal-HER2, 10.6 months in the HER2, and 4.2 months
in the triple-negative types; P<0.001). Among the four subtypes,
patients with the luminal-HER2 type had a significantly improved
TTP. The TTP of patients with luminal-HER2 tumors was better than
the TTP of patients with triple-negative tumors.
Adverse events leading to the
discontinuation of taxanes
In 30 (10.2%) of the 293 patients, taxane
administration was discontinued due to adverse events (Grade 3 and
4; Table IV). PAC was discontinued
due to events including peripheral neuropathy, which was most
frequent at 6.3%, worsening of the general condition, neutropenia
and interstitial pneumonia. DOC was discontinued due to edema,
which was most frequent at 6.8%, nasolacrimal duct stenosis, muscle
pain and heart failure.
 | Table IV.Adverse events leading to the
discontinuation of taxanes. |
Table IV.
Adverse events leading to the
discontinuation of taxanes.
| Adverse event | Paclitaxel, n
(%) | Docetaxel, n
(%) |
|---|
| Sensory
neuropathy | 12 (6.3) | 1 (1.0) |
| Nail pain | 1
(0.5) | 0 (0.0) |
|
Thrombocythemia | 1
(0.5) | 0 (0.0) |
| Neutropenia | 1
(0.5) | 0 (0.0) |
| Fatigue | 1
(0.5) | 0 (0.0) |
| Fever | 1
(0.5) | 0 (0.0) |
| Intestinal
pneumonia | 1
(0.5) | 0 (0.0) |
| Edema | 1
(0.5) | 7 (6.8) |
| Obstruction of
nasolacrimal canal | 0
(0.0) | 1 (1.0) |
| Muscular pain | 0
(0.0) | 1 (1.0) |
| Heart failure | 0
(0.0) | 1 (1.0) |
| Total | 19
(10.0) | 13 (12.6) |
Discussion
In the present study, the therapeutic effect of
taxanes on metastatic breast cancer was retrospectively
investigated in various immunohistochemical subtypes. It was found
that the immunohistochemical subtypes were associated with the
therapeutic effect of taxanes for metastatic breast cancer. In
addition, , taxane yielded the therapeutic effect of RR; 28.3% and
TTP; 8.3 months in luminal metastatic breast cancer. The results of
the present analysis were not comparable to the numerous previous
studies (6–8), which showed the ineffectiveness of
taxane anticancer drugs for operable early luminal breast
cancer.
Possible causes of the difference in the results of
pre- and postoperative taxane treatment for early breast cancer and
those of taxane treatment for luminal metastatic breast cancer
include the following factors. The first is the increased
heterogeneity of luminal-type cancer cells. ER+ and
HER2− luminal breast cancer grows slowly, showing a low
apoptosis rate and low genetic instability (17). Foulkes et al (17) proposed the hypothesis that
slow-growing tumors cause heterogeneity within the tumor, such that
numerous tumor cells acquire the ability for distant metastasis.
Therefore, in recurrent disease, luminal breast cancer cells would
be expected to be more aggressive and proliferative, and then
sensitive to chemotherapy.
The second possibly cause is the heterogeneity of
breast cancer biopsy specimens, which may cause phenotypic changes
associated with metastasis or recurrence. Commonly, in stage IV
disease, a biopsy of the primary lesion is performed in only a
small portion of the primary cancer. By contrast, in patients with
recurrent disease, the whole primary lesion is evaluated for the
cancer subtype, but different subtypes may occur at the sites of
metastasis and recurrence. Amir et al (18) reported that the phenotypes of hormone
receptors and HER2 were different between the primary and
metastatic or recurrent lesions, and that ER, PgR and HER2
phenotypes were different between the primary and metastatic
lesions in 16, 30 and 13% of patients, respectively (18). Since biopsies were not generally
performed during treatment in the present study, the status of
immunohistochemical expression of hormone receptors and HER2 at the
site of metastasis was confirmed in only 9 patients with luminal
breast cancer. The status of ER expression was identical in all
primary and metastatic lesions, but the status of PgR expression
was different between the primary and metastatic lesions in 6
patients. The HER2 expression status in the metastatic lesions in 2
patients was 2+ according to immunohistochemical analysis, but FISH
analysis showed that the expression of HER2 was amplified and
absent in 1 lesion each. Although no association between
discordance of phenotypes and therapeutic effect has been
elucidated, it may be important in the future to perform numerous
tissue biopsies and re-evaluate the phenotypes of hormone receptor
and HER2 expression. The phenotype of metastatic lesions may alter
during cancer progression. Future investigation is required to
address whether information about metastatic sites obtained from
biopsies may aid in the prediction of response or outcome.
In the present study, 89.3 and 94.7% of luminal-HER2
and HER2 type breast cancer patients, respectively, received a
treatment regimen including trastuzumab with taxanes. Adding
trastuzumab to taxane therapy would be expected to improve the TTP
in these subtypes. Marty et al (19) reported the efficacy of trastuzumab
combined with docetaxel as the first-line treatment in
HER2+ metastatic breast cancer (19). Trastuzumab plus docetaxel was
significantly superior to docetaxel alone in terms of overall RR
(61 vs. 34%) and time to disease progression (median, 11.7 vs. 6.1
months). Although the proportion of patients administered with
taxanes and trastuzumab as the first-line treatment was 28.6 and
35.1% in the luminal HER2 and HER2 types, respectively, the results
of the present analysis were comparable to previous studies
(19). The TTP of the patients with
triple-negative type breast cancer was significantly shorter
compared with the TTP of patients with the other three subtypes of
breast cancer. The poor prognosis of triple-negative breast cancer
may be affected by the short disease-free interval.
The limitation of the present study is that there is
no information on the proliferative marker index, such as Ki-67, of
the primary sites. The patients with luminal breast cancer that
were examined in the present study harbored the two subtypes of
low-proliferative luminal A and high-proliferative luminal B. The
proliferative index of primary sites may aid the identification of
the response to taxanes. However, in recurrent breast cancer,
breast cancer diagnosed as luminal A at the time of surgery was
treated with endocrine therapy (20).
Even for luminal B breast cancer, if not life-threatening,
endocrine therapy is chosen along the algorithm of Hortobagyi
(20). The present study showed that
the inactivity of taxane in hormone-receptor positive or
HER2− breast cancer could not be predicted at the
primary site. Therefore, metastatic hormone receptor-positive
breast cancer was defined as luminal breast cancer without the
strict distinction between luminal A and luminal B at the time of
surgery.
In taxane therapy, adverse events may cause dose
reduction or discontinuation of treatment. The main adverse events
that disturb treatment include paclitaxel-associated peripheral
neuropathy or docetaxel-associated generalized edema. In the
present study, taxane administration was discontinued due to
adverse events in 30 (10.2%) of the 293 patients. Therefore, the
effect of toxicities on the efficacy of treatment with taxane in
the present study may be minimal. This observation is clinically
important because the present study showed that taxane remains a
reasonable choice for the treatment of luminal metastatic breast
cancer. Additional investigations are required to elucidate the
predictive markers of taxane therapy for the patients with
metastatic breast cancer in each immunohistochemical subtype.
References
|
1
|
De Weger VA, Beijnen JH and Schellens JH:
Cellular and clinical pharmacology of the taxanes docetaxel and
paclitaxel - A review. Anticancer Drugs. 5:488–494. 2014.
View Article : Google Scholar
|
|
2
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Invasive Breast Cancer Version 1.2016, NCCN
Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw.
14:324–354. 2016.PubMed/NCBI
|
|
3
|
Jones SE, Erban J, Overmoyer B, Budd GT,
Hutchins L, Lower E, Laufman L, Sundaram S, Urba WJ, Pritchard KI,
et al: Randomized phase III study of docetaxel compared with
paclitaxel in metastatic breast cancer. J Clin Oncol. 23:5542–5551.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gradishar WJ, Tjulandin S, Davidson N,
Shaw H, Desai N, Bhar P, Hawkins M and O'Shaughnessy J: Phase III
trial of nanoparticle albumin-bound paclitaxel compared with
polyethylated castor oil-based paclitaxel in women with breast
cancer. J Clin Oncol. 23:7794–7803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumors. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayes DF, Thor AD, Dressler LG, Weaver D,
Edgerton S, Cowan D, Broadwater G, Goldstein LJ, Martino S, Ingle
JN, et al: Cancer and Leukemia Group B (CALGB) Investigators: HER2
and response to paclitaxel in node-positive breast cancer. N Engl J
Med. 357:1496–1506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hugh J, Hanson J, Cheang MC, Nielsen TO,
Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et
al: Breast cancer subtypes and response to docetaxel in
node-positive breast cancer: Use of an immunohistochemical
definition in BCIRG 001 trial. J Clin Oncol. 27:1168–1176. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Penault-Llorca F, André F, Sagan C,
Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC,
Bibeau F, Antoine M, et al: Ki67 expression and docetaxel efficacy
in patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2809–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes - dealing with the diversity of breast cancer: Highlights
of the St. Gallen International Expert Consensus on the Primary
Therapy of Early Breast Cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pritchard KI, Shepherd LE, O'Malley FP,
Andrulis IL, Tu D, Bramwell VH and Levine MN: National Cancer
Institute of Canada Clinical Trials Group: HER2 and responsiveness
of breast cancer to adjuvant chemotherapy. N Engl J Med.
354:2103–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG): Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
An overview of the randomized trials. Lancet. 365:1687–1717. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Swain SM, Jeong JH, Geyer CE Jr,
Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J,
Vogel VG, Erban JK, et al: Longer therapy, iatrogenic amenorrhea
and survival in early breast cancer. N Engl J Med. 362:2053–2065.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alba E, Calvo L, Albanell J, De la Haba
JR, Arcusa Lanza A, Chacon JI, Sanchez-Rovira P, Plazaola A, Lopez
Garcia-Asenjo JA, Bermejo B, et al: GEICAM: Chemotherapy (CT) and
hormonotherapy (HT) as neoadjuvant treatment in luminal breast
cancer patients: Results from the GEICAM/2006-03, a multicenter,
randomized, phase-II study. Ann Oncol. 23:3069–3074. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakamoto G, Inaji H, Akiyama F, Haga S,
Hiraoka M, Inai K, Iwase T, Kobayashi S, Sakamoto G, Sano M, et al:
Japanese Breast Cancer Society: General rules for clinical and
pathological recording of breast cancer 2005. Breast Cancer.
12(Suppl): S1–S27. 2005.PubMed/NCBI
|
|
15
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: American Society of Clinical Oncology;
College of American Pathologists: Recommendations for human
epidermal growth factor receptor 2 testing in breast cancer:
American Society of Clinical Oncology/College of American
Pathologists clinical practice guideline update. Arch Pathol Lab
Med. 138:241–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foulkes WD, Reis-Filho JS and Narod SA:
Tumor size and survival in breast cancer - a reappraisal. Nat Rev
Clin Oncol. 7:348–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amir E, Miller N, Geddie W, Freedman O,
Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G,
Laupacis A, et al: Prospective study evaluating the impact of
tissue confirmation of metastatic disease in patients with breast
cancer. J Clin Oncol. 30:587–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marty M, Cognetti F, Maraninchi D, Snyder
R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Antón A, Lluch A,
et al: Randomized phase II trial of the efficacy and safety of
trastuzumab combined with docetaxel in patients with human
epidermal growth factor receptor 2-positive metastatic breast
cancer administered as first-line treatment: The M77001 study
group. J Clin Oncol. 23:4265–4274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gabriel N. Hortobagyi: Drug therapy:
Treatment of breast cancer. N Engl J Med. 339:974–984. 1998.
View Article : Google Scholar : PubMed/NCBI
|