Introduction
With the rapid development of the worldwide economy,
the structure of people's diets has changed in recent years
(1). Colorectal cancer (CRC) has
become one of the most common malignant tumors of the digestive
tract in China, and is the major cause of gastrointestinal
cancer-associated mortality (2). One
reason for the high frequency of tumor metastasis is that the
majority of CRC patients are diagnosed at an advanced stage
(3). Malignant CRCs often possess the
characteristics of rapid progression and invasion, which contribute
to local and liver metastases and can result in a poor prognosis
(4,5).
MicroRNAs (miRs) are known to be involved in a variety of
physiological processes, including cell survival, growth and
metabolism (6). Studies have
demonstrated that miRs are involved in the regulation of metastasis
in various types of cancer (7,8). Several
types of miR have been found to be important for the process of
tumor metastasis, suggesting that miRs may be used as a potential
transfer marker (9,10).
The twist-induced miR, miR-10b, has been previously
found to promote tumor metastasis in cancer (11). One study indicated that homeobox D10
(HOXD10) is the target gene of miR-10b and is closely associated
with the inhibition of cell migration and invasion (12). The expression level of HOXD10 was
found to decrease during the malignant progression of cancer
(13). The ras homolog family member
C (RhoC) protein is a member of the Ras superfamily of guanine
triphosphate (GTP)-binding proteins. Numerous studies have
indicated that tumor metastasis is associated with an abnormal AKT
signaling pathway, as AKT mediates a variety of basic cellular
processes, including the resistance of cells to apoptosis and cell
cycle effects (14). Although certain
studies have reported a potential association between miR-10b and
CRC (15,16), the effect of miR-10b on CRC metastasis
and the associated molecular mechanism remain elusive.
The purpose of the present study was to investigate
the expression of miR-10b in CRC patients, to evaluate the effect
of miR-10b on CRC invasion and migration and to determine whether
miR-10b expression is mediated by HOXD10 targeting RhoC. The
results of the present study showed that the overexpression of
miR-10b in patients with CRC was associated with a decreased level
of HOXD10 protein, which was accompanied by the increased
expression of RhoC. These results suggested that miR-10b may be
important in the migration and invasion of CRC.
Materials and methods
Clinical summary
A total of 126 primary CRC samples were collected
consecutively during surgery from patients with histologically
confirmed CRC, who underwent surgical resections at the Department
of General Surgery, Yangpu Hospital, Tongji University School of
Medicine (Shanghai, China) between January 2012 and January 2014.
Adjacent healthy tissues were also taken from these patients with
CRC to form the adjacent non-tumor tissue group. Additionally, 126
healthy people, who had undergone physical examinations in the same
hospital, were used as a control group. Tissue Specimens were
obtained from colonoscopy, and were immediately frozen in liquid
nitrogen and stored at −80° C until further processing. Only CRC
patients that had not received any preoperative radio or
chemotherapy were enrolled in the present study. All patients were
staged according to the International Union Against Cancer of CRC
TNM Staging system (2010; 7th edition) for CRC (17). The present study was conducted in
accordance with the Declaration of Helsinki (18) and with approval from the Ethics
Committee of Yangpu Hospital, Tongji University School of Medicine.
Written informed consent was obtained from all participants.
Sample treatment
Adjacent non-cancerous tissues were carefully
dissected from the surrounding benign tissues by a certified
pathologist, and the margin was confirmed through
immunohistochemistry by two independent pathologists at the
Department of Pathology, Yangpu Hospital, Tongji University School
of Medicine. The surrounding non-tumor tissues were used as control
for each tumor tissue. All samples were immediately frozen in
liquid nitrogen following surgical removal until required.
RNA extraction and reverse
transcription (RT)-quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from the resected tissues
for the messenger RNA (mRNA) expression assay using
TRIzol® reagent (Gibco; Thermo Fisher Scientific, Inc.
Waltham, MA, USA). Lysed samples were bound to a silica-based
filter and treated with DNase I (20,000 units; Invitrogen; Thermo
Fisher Scientific, Inc.) RT-PCR was performed using the Reverse
Transcriptase kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's instructions. After complementary DNA was
obtained, RT-PCR was performed using the SYBR Green PCR reagent kit
(Thermo Fisher Scientific, Inc.). qPCR was performed using an
initial denaturation step at 95°C for 5 min, then 40 cycles of
amplification, consisting of denaturation at 95°C for 15 sec,
annealing at 60°C for 30 sec and elongation at 72°C for 30 sec on a
7500 Fast Real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). All 20-µl reactions were performed in
triplicate. 5S ribosomal RNA (rRNA) was used as the internal
control. The primer sequences were as follows: miR-10b, forward,
5′-TACCCTGTAGAACCGAATTTG-3′ and reverse,
5′-AACTGGTGTCGTGGAGTCGGC-3′; primers used for detection of HOXD10,
forward, 5′-GACATGGGGACCTATGGAATGC-3′ and reverse,
5′-TGGTGGTTCACTTCTCTTTTGG-3′; and 5S rRNA, forward,
5′-CCATACCACCTGGAAACGC-3′ and reverse, 5′-TACTAACCGAGCCCGACCCT-3′
(Jrdun Biotechnology, Shanghai, China). Data analysis for miR
expression was performed using the 2−ΔΔCq method
(19).
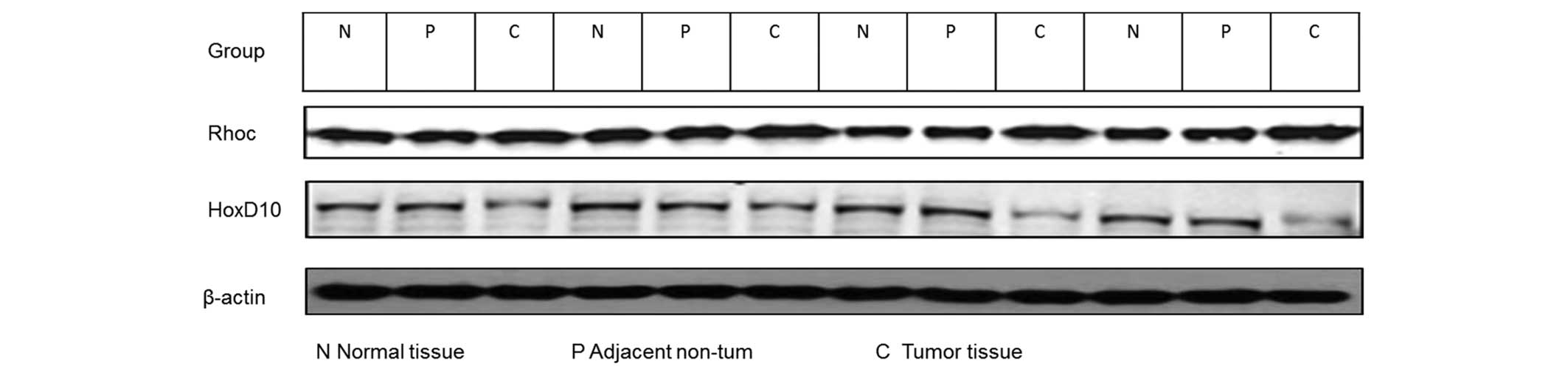
Western blot analysis
Cold radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) was used to
extract proteins from solubilized tissues. Protein assay reagents
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used to
determine the protein concentration. Equal amounts of protein was
then separated using a NuPAGE 4–12% Bis-Tris gradient gel
(Invitrogen; Thermo Fisher Scientific, Inc.) and transferred to a
polyvinylidene fluoride membrane. The membrane was incubated with
primary polyclonal goat RhoC (dilution, 1:200; catalog no.,
sc-12116), polyclonal goat HOXD10 (dilution, 1:200; catalog no.,
sc-33004) and polyclonal goat β-actin (dilution, 1:10,000; catalog
no., sc-1616) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibodies overnight at 4°C, blocked with 2% non-fat milk in
phosphate-buffered saline (PBS)/Tween-20, and then incubated at
room temperature with 5 mg anti-goat IgG horseradish peroxidase
(HRP)-conjugated antibodies (dilution, 1:10,000; catalog no.,
AS09605; Santa Cruz Biotechnology, Inc.) for 45 min. An enhanced
chemiluminescence reaction (Invitrogen; Thermo Fisher Scientific,
Inc.) was performed for 40 min. The relative densities of proteins
was quantified with Image J software v.1.48u (National Institutes
of Health, Bethesda, MD, USA).
Immunohistochemistry
Tumor or matched normal tissues were fixed in 4%
paraformaldehyde, paraffin embedded, and sliced into 4 µm sections.
The primary antibody used for HOXD10 immunostaining was a mouse
monoclonal anti-HOXD10 antibody (dilution, 1:50; Santa Cruz
Biotechnology, Inc.). The sections were incubated with 5 mg
HRP-conjugated anti-mouse IgG polyclonal secondary antibody
(dilution, 1:10,000; catalog no., NB910-95603; Abcam) for 45 min,
and the reaction products were visualized by immersing the sections
in 0.03% diaminobenzidine solution containing 2 mM hydrogen
peroxide for 1–5 min. Nuclei were lightly stained with Mayer's
hematoxylin. The bound antibodies were detected with a
biotin-streptavidin-peroxidase system (Vector Laboratories, Inc.,
Burlingame, CA, USA), and 3,3′-diaminobenzidine hydrochloride
(Sigma-Aldrich, St. Louis, MO, USA) was used as the chromogen. Two
independent pathologists from the Department of Pathology, Yangpu
Hospital, Tongji University School of Medicine, performed the
quantitative assessment of immunohistochemical staining. Staining
in nuclei was graded as: 0, no evidence of immunoreactive cells; 1,
the proportion of immunoreactive cells is <20%; 2, the
proportion of immunoreactive cells is 20–70%; or 3, the proportion
of immunoreactive cells is >70%. Immunohistochemical reactivity
was evaluated and classified into 4 groups: i) Negative (−); ii)
weakly positive (+); iii) moderately positive (++); or iv) strongly
positive (+++).
Statistics
Data are presented as the mean ± standard deviation.
Statistical tests were performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). The Student's t-test (two-tailed) was used make
comparisons between two groups, the correlation between HOXD10 and
RhoC was assessed using Spearman's rank correlation coefficient and
differences between two groups for immunohistochemistry were
analyzed using the Mann-Whitney U test. P<0.05 were considered
to indicate a statistically significant difference.
Results
Patient characteristics
A total of 126 patients with CRC, who had not
previously received preoperative radiotherapy or chemotherapy, were
enrolled in the present study. All patients underwent surgical
resection at the Department of General Surgery, Yangpu Hospital,
Shanghai Tongji University School of Medicine. All postoperative
samples were confirmed as CRC through histopathological evaluation.
The study consisted of 74 male and 52 female patients, of which 46
patients possessed tumors that were >5 cm in diameter and 80
possessed tumors of ≤5 cm in diameter. Good, moderate and poor
tumor differentiation was detected in 49, 69 and 8 cases,
respectively. In total, 42 patients exhibited N1 lymph node
metastasis and 18 patients exhibited N2 lymph node metastasis. A
total of 19 cases were in stage I, 45 cases were in stage II and 62
cases were in stage III of disease. The characteristics of the
enrolled patients are listed in Table
I.
 | Table I.Patient characteristics (n=126). |
Table I.
Patient characteristics (n=126).
| Characteristic | No. of patients
(%) | P-value |
|---|
| Tumor site |
| 0.043 |
|
Colon | 74 (58.7) |
|
|
Rectum | 52 (41.3) |
|
| Liver
metastases | 3 (2.4) |
|
|
Multifocal | 1 (0.7) |
|
| Age, years |
| 0.763 |
| ≥50 | 64 (50.8) |
|
|
<50 | 62 (49.2) |
|
| Size of tumor |
| 0.013 |
| ≤5 cm
diameter | 80 (63.5) |
|
| >5 cm
diameter | 46 (36.5) |
|
| Gender |
| 0.435 |
| Male | 74 (58.7) |
|
|
Female | 52 (41.3) |
|
| Tumor
classification |
| 0.023 |
| T1 | 9 (7.1) |
|
| T2 | 31 (24.6) |
|
| T3 | 81 (64.3) |
|
| T4 | 5 (4.0) |
|
| Lymph node
status |
| 0.016 |
| N0 | 66 (52.4) |
|
| N1 | 42 (33.3) |
|
| N2 | 18 (14.3) |
|
| Histological
differentiation |
| 0.018 |
| Well | 49 (38.9) |
|
|
Moderate | 69 (54.8) |
|
| Poor | 8 (6.3) |
|
| Stage |
| 0.007 |
| I | 19 (15.1) |
|
| II | 45 (35.7) |
|
| III | 62 (49.2) |
|
| HOXD10 staining |
| 0.025 |
| Strong
(>1+) | 43 (34.1) |
|
| Weak
(≤1+) | 83 (65.9) |
|
| RhoC staining |
| 0.014 |
| Strong
(>1+) | 75 (59.5) |
|
| Weak
(≤1+) | 51 (40.5) |
|
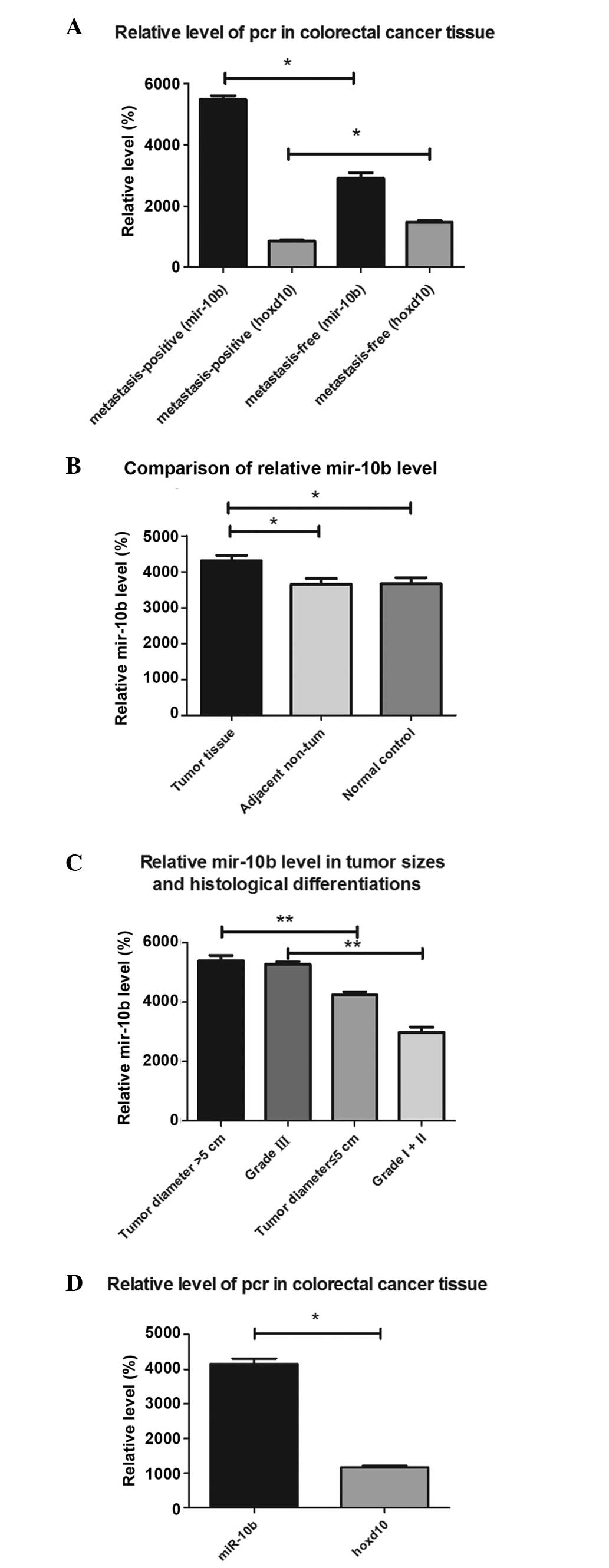
miR-10b is highly expressed in primary
CRC samples
In order to explore the potential role of miR-10b in
CRC development, the expression of miR-10b was investigated in
tumor specimens obtained from 126 CRC patients. The patients were
classified into two groups, according to the presence or absence of
lymph node metastasis; significant differences were identified in
the mean expression levels of miR-10b and HOXD10 between the two
groups (P=0.001 and P=0.005). The results of the RT-qPCR indicated
that the mean expression level of miR-10b in tumor tissues with
positive lymph node metastasis was 1.8-fold greater compared with
tumor tissues without lymph node metastasis. In addition, the mean
expression level of HOXD10 in tumor tissues with positive lymph
node metastasis was 0.58-fold greater compared with tumor tissue
without lymph node metastasis (Fig.
1A). Significant differences in the mean miR-10b expression
level were also indicated between the CRC tissue group and the
healthy individuals and adjacent tissues (P=0.012 and P=0.001;
Fig. 1B), while there was no
statistically significant difference between the adjacent non-tumor
tissue group and the normal control tissue group (P=0.986). There
was no significant difference in the expression levels of miR-10b
with respect to age and gender (P=0.156). The results showed that
there were significant differences in the expression of miR-10b in
cases of CRC with varying tumor diameter and histological
differentiation (P=0.013 and P=0.018). A significant difference in
miR-10b expression was also indicated between stage I+II and stage
III tumors (P=0.007; Fig. 1C). There
were significant differences in miR-10b expression between cases
with liver metastasis and those with multifocal metastases
(P=0.004). A statistically significant difference was identified
between the level of miR-10b and HOXD10 expression (P=0.009;
Fig. 1D). These data suggest that
miR-10b may be involved in the process of CRC metastasis. In
addition, an inverse correlation was identified between miR-10b and
HOXD10 expression.
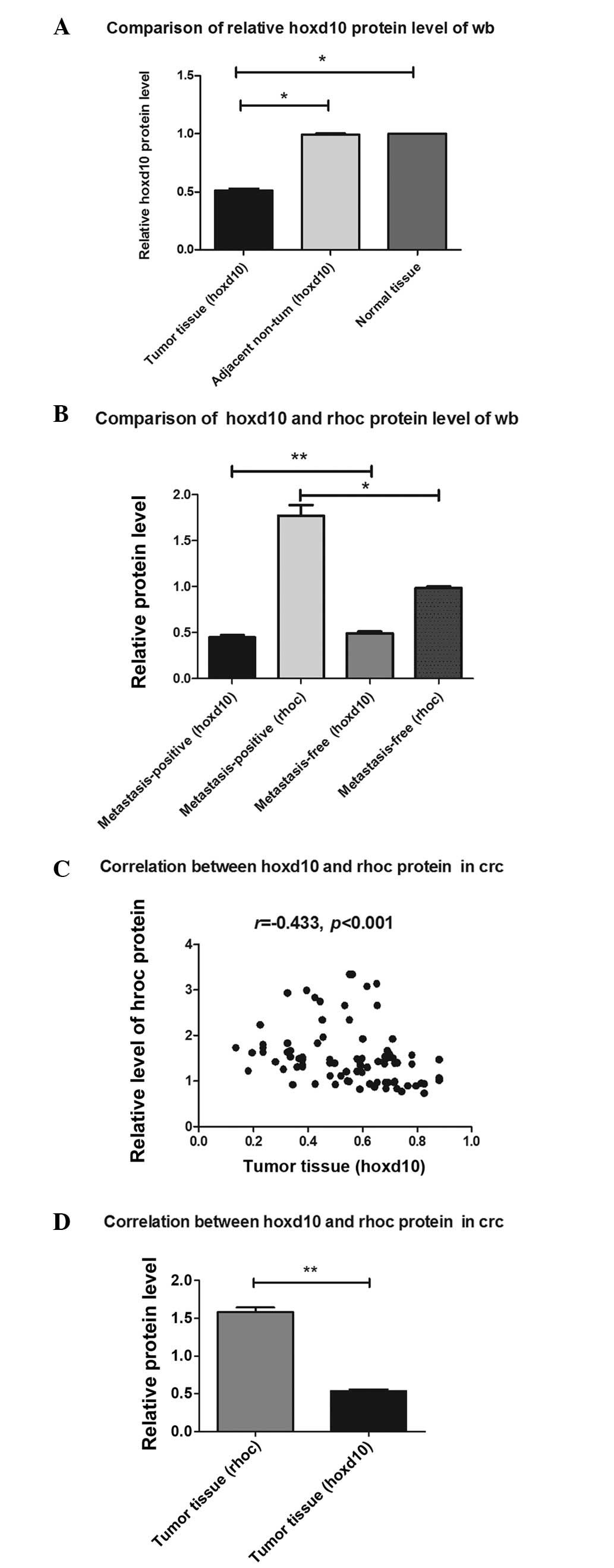
Western blot analysis of protein
expression of HOXD10 in CRC samples
Since the decreased expression of HOXD10 protein has
been shown to enhance the invasiveness and metastasis of tumors
(20), the present study aimed to
determine whether miR-10b would inhibit the translation of HOXD10
mRNA, thereby affecting the expression of downstream targets of
RhoC. As shown in Fig. 2, there were
significant differences in the mean level of HOXD10 expression
between the CRC tissue group and the adjacent non-tumor or normal
control tissue group (P=0.001; Fig.
2A), while there was no statistical significance between the
adjacent non-tumor tissue group and the normal control tissue group
(P=0.490). RhoC protein levels were dramatically upregulated in
tumor tissues with positive lymph node metastasis compared with
tissues with no lymph node metastasis (P<0.01). There were also
significant differences in HOXD10 protein level between the CRC
samples with positive lymph node metastasis and the CRC samples
without positive lymph node metastasis (P=0.014) (Fig. 2B). As shown in Figs. 2C and 3,
there was a significant inverse correlation between HOXD10 protein
level and protein level (r=−0.433; P<0.001). There was
statistically significant difference between RhoC protein level and
HOXD10 protein level in the CRC tissue group (P=0.002; Fig. 2D).
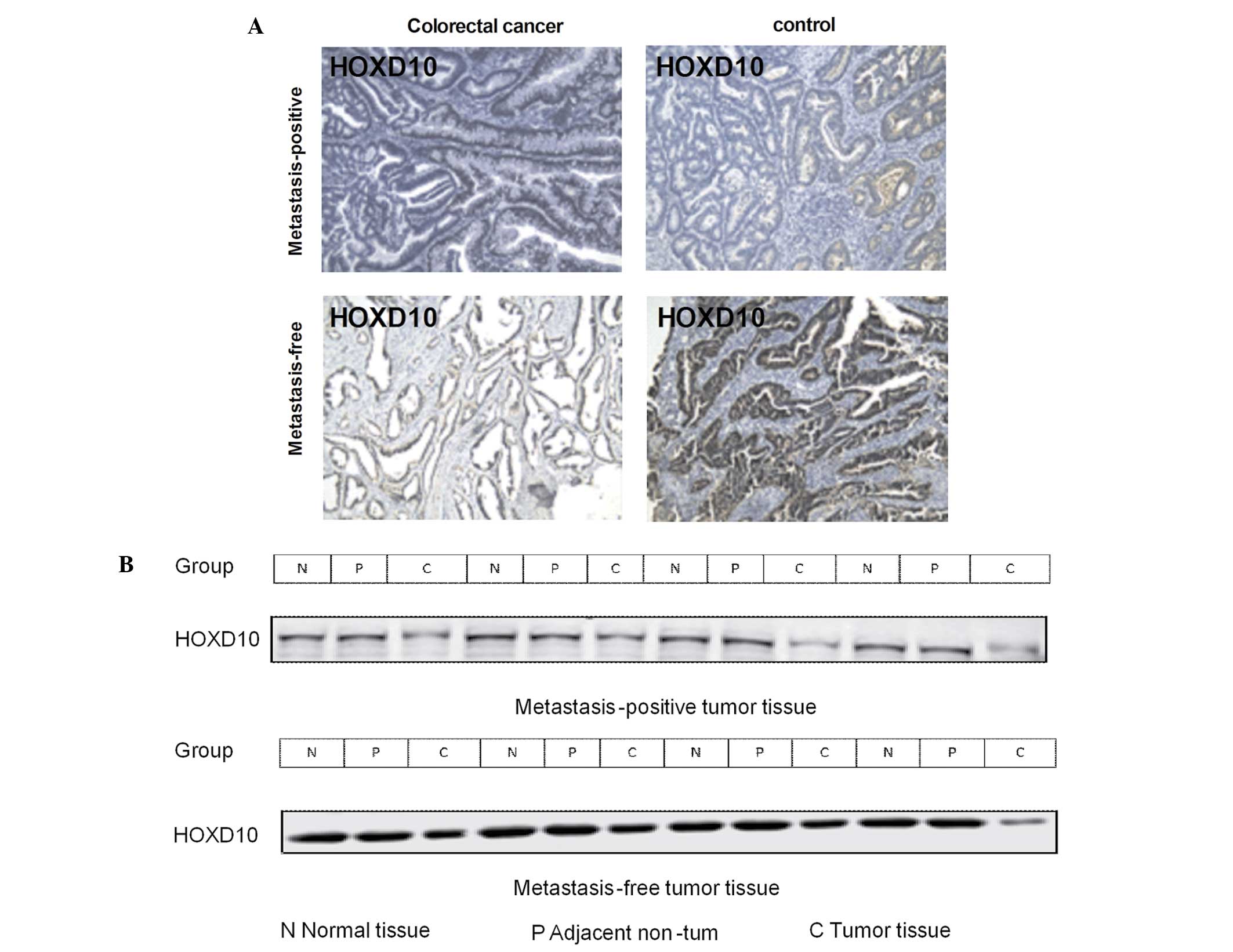
Difference in protein expression of
HOXD10 between metastasis-positive and metastasis-free samples
The results of the western blot analysis indicated
that HOXD10 expression in tumor tissues from the
metastasis-positive group were decreased compared with in
metastasis-free tissues (Fig. 2B). To
confirm the result of western blot analysis, immunohistochemistry
was also performed to detect the expression of HOXD10. HOXD10
immunoexpression was found to be attenuated in metastasis-positive
tissues compared with metastasis-free tissues (Fig. 4A). A significant inverse correlation
in the protein expression of HOXD10 was also observed in
metastasis-positive tissues compared with metastasis-free tissues
(Fig. 4B). These data suggest that
HOXD10 may be involved in the process of CRC metastasis.
Discussion
The HOXD10 gene encodes transcription factors and
exerts functions mainly through the activation or inhibition of
downstream target genes (21). HOXD10
was previously considered to be a major factor in the negative
regulation of tumor metastasis, as the overexpression of miR-10b
was hypothesized to increase RhoC and AKT signaling by targeting
HOXD10, thus facilitating invasion in tumors (22). The present study measured the
expression of miR-10b, HOXD10 and RhoC in healthy control subjects
and CRC patients. miR-10b was found to be highly expressed in CRC
tissues, accompanied by the increased expression of RhoC.
Consistent with previous findings, that miR-10b could drive tumor
metastasis (23–25), the present study found that increased
miR-10b also contributed to metastasis in CRC. In addition,
increased levels of miR-10b were detected in poorly-differentiated
CRC tissues, indicating a strong capability for metastasis. HOXD10
expression was found to be attenuated in lymph node
metastasis-positive CRC tissues, compared with metastasis-free CRC.
These results suggest that miR-10b could reduce the threshold of
metastasis in CRC as the positive regulator, which was inversely
associated to HOXD10.
There were significant differences in the mean level
of miR-10b expression between the tumor and the adjacent non-tumor
tissues (P<0.05), while there was no significant difference
between the adjacent non-tumor and normal CRC tissue (P>0.05;
Fig. 1). There were significant
differences in miR-10b level between CRC cases of various stages,
tumor diameter, lymph node metastasis status and differentiation.
Furthermore, there was a statistically significant difference
between the multi- and mono-focal cases (Table I). miR-10b was previously observed to
be abnormally expressed in several tumor types, including glioma,
pancreatic adenocarcinoma and glioblastoma (26–28). The
current findings, together with the findings of previous studies,
suggest the involvement of miR-10b in the metastatic behavior of
CRC (29,30).
The present study found significantly greater
concentrations of miR-10b in CRC tissues compared with in adjacent
non-tumor tissues and normal tissues from healthy controls. The
concentrations of miR-10b increased in line with a later clinical
stage and lymph node metastasis. In addition, miR-10b expression in
high-grade CRC with multifocal lesions and liver metastases was
significantly increased compared with CRC patients with a single
lesion and those without liver metastasis. These findings indicate
that miR-10b may be involved in the invasion and migration of CRC
and may provide a novel therapeutic target. In the present study,
miR-10b expression levels were elevated in metastasis-positive CRC
specimens compared with metastasis-free tumor tissues, and this
elevation was accompanied with the downregulation of HOXD10. HOXD10
has been previously found to repress the expression of genes
involved in tumor metastasis, including RhoC (12). miR-10b overexpression may increase
RhoC expression, indicating that increased RhoC contributed to
miR-10b-induced invasiveness, as the target gene of HOXD10. In
addition, the expression of miR-10b was greater in
poorly-differentiated CRC tissues compared with well-differentiated
ones. Spearman's rank correlation coefficient revealed that there
was a strong negative correlation between HOXD10 and RhoC protein
levels (Fig. 2C). The results of the
present study showed that the overexpression of miR-10b increased
RhoC expression by targeting HOXD10, thus facilitating the invasion
and metastasis of CRC.
In conclusion, the present study suggests that
increased miR-10b levels are associated with the degree of
metastasis in CRC patients. The increased expression of RhoC and
the downregulation of HOXD10 were correlated with high expression
levels of miR-10b. These results may elucidate the underlying
mechanisms and provide a novel therapeutic target for inhibiting
the invasion and metastasis of CRC.
Acknowledgements
The present study was supported by grants from the
Youth Issues of Shanghai Municipal Health Bureau of China,
Shanghai, China (grant no., 20124y155).
References
|
1
|
Dai J, Zou T, Wang L, Zhang Y and Liu Y:
Investigation of the interaction between quercetin and human serum
albumin by multiple spectra, electrochemical impedance spectra and
molecular modeling. Luminescence. 29:1154–1161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhong L, Zhang X and Covasa M: Emerging
roles of lactic acid bacteria in protection against colorectal
cancer. World J Gastroenterol. 20:7878–7886. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dueland S, Hagness M, Line PD, Guren TK,
Tveit KM and Foss A: Is liver transplantation an option in
colorectal cancer patients with nonresectable liver metastases and
progression on all lines of standard chemotherapy? Ann Surg Oncol.
22:2195–2200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cleven AH, Derks S, Draht MX, Smits KM,
Melotte V, Van Neste L, Tournier B, Jooste V, Chapusot C,
Weijenberg MP, et al: CHFR promoter methylation indicates poor
prognosis in stage II microsatellite stable colorectal cancer. Clin
Cancer Res. 20:3261–3271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Xi Q, Wang Q and Wei P:
Downregulation of microRNA-100 correlates with tumor progression
and poor prognosis in colorectal cancer. Med Oncol. 31:2352014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adams SV, Newcomb PA, Burnett-Hartman AN,
Wurscher MA, Mandelson M, Upton MP, Zhu LC, Potter JD and Makar KW:
Rare circulating microRNAs as biomarkers of colorectal neoplasia.
PLoS One. 9:e1086682014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drusco A, Nuovo GJ, Zanesi N, Di Leva G,
Pichiorri F, Volinia S, Fernandez C, Antenucci A, Costinean S,
Bottoni A, et al: MicroRNA profiles discriminate among colon cancer
metastasis. PLoS One. 9:e966702014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang G, Li J, Sun B, Li S, Lü L, Wang Y,
Chen B and Xiao Z: Deep sequencing reveals complex mechanisms of
microRNA deregulation in colorectal cancer. Int J Oncol.
45:603–610. 2014.PubMed/NCBI
|
|
9
|
Phua LC, Chue XP, Koh PK, Cheah PY, Chan
EC and Ho HK: Global fecal microRNA profiling in the identification
of biomarkers for colorectal cancer screening among Asians. Oncol
Rep. 32:97–104. 2014.PubMed/NCBI
|
|
10
|
Yin J, Bai Z, Song J, Yang Y, Wang J, Han
W, Zhang J, Meng H, Ma X, Yang Y, et al: Differential expression of
serum miR-126, miR-141 and miR-21 as novel biomarkers for early
detection of liver metastasis in colorectal cancer. Chin J Cancer
Res. 26:95–103. 2014.PubMed/NCBI
|
|
11
|
Haque I, Banerjee S, Mehta S, De A,
Majumder M, Mayo MS, Kambhampati S, Campbell DR and Banerjee SK:
Cysteine-rich 61-connective tissue growth factor-nephroblastoma
overexpressed 5 (CCN5)/Wnt-1-induced signaling protein-2 (WISP-2)
regulates microRNA-10b via hypoxia-inducible factor-1α-TWIST
signaling networks in human breast cancer cells. J Biol Chem.
286:43475–43485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Z, Zhu J, Cao H, Ren H and Fang X:
MiR-10b promotes cell invasion through RhoC-AKT signaling pathway
by targeting HOXD10 in gastric cancer. Int J Oncol. 40:1553–1560.
2012.PubMed/NCBI
|
|
13
|
Yu X, Li Z, Shen J, Wu WK, Liang J, Weng X
and Qiu G: MicroRNA-10b promotes nucleus pulposus cell
proliferation through RhoC-Akt pathway by targeting HOXD10 in
intervetebral disc degeneration. PLoS One. 8:e830802013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bu Q, Tang HM, Tan J, Hu X and Wang DW:
Expression of RhoC and ROCK-1 and their effects on MAPK and Akt
proteins in prostate carcinoma. Zhonghua Zhong Liu Za Zhi.
33:202–206. 2011.(In Chinese). PubMed/NCBI
|
|
15
|
Nishida N, Yamashita S, Mimori K, Sudo T,
Tanaka F, Shibata K, Yamamoto H, Ishii H, Doki Y and Mori M:
MicroRNA-10b is a prognostic indicator in colorectal cancer and
confers resistance to the chemotherapeutic agent 5-fluorouracil in
colorectal cancer cells. Ann Surg Oncol. 19:3065–3071. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YF, Li Z, Zhao XH, Zuo XM, Zhang Y,
Xiao YH, Li J and Peng ZH: MicroRNA-10b is upregulated and has an
invasive role in colorectal cancer through enhanced Rhoc
expression. Oncol Rep. 33:1275–1283. 2015.PubMed/NCBI
|
|
17
|
von Winterfeld M, Hoffmeister M,
Ingold-Heppner B, Jansen L, Tao S, Herpel E, Schirmacher P, Dietel
M, Chang-Claude J, Autschbach F, et al: Frequency of
therapy-relevant staging shifts in colorectal cancer through the
introduction of pN1c in the 7th TNM edition. Eur J Cancer.
50:2958–2965. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rickham PP: Human experimentation. Code of
ethics of the World Medical Association. Declaration of Helsinki.
Br Med J. 2:1771964. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Chen S, Xue M, Zhong J, Wang X,
Gan L, Lam EK, Liu X, Zhang J, Zhou T, et al: Homeobox D10 gene, a
candidate tumor suppressor, is downregulated through promoter
hypermethylation and associated with gastric carcinogenesis. Mol
Med. 18:389–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao H, Li H, Yu G, Xiao W, Hu J, Tang K,
Zeng J, He W, Zeng G, Ye Z and Xu H: MicroRNA-10b promotes
migration and invasion through KLF4 and HOXD10 in human bladder
cancer. Oncol Rep. 31:1832–1838. 2014.PubMed/NCBI
|
|
21
|
Xue M, Fang Y, Sun G, Zhuo W, Zhong J,
Qian C, Wang L, Wang L, Si J and Chen S: IGFBP3, a transcriptional
target of homeobox D10, is correlated with the prognosis of gastric
cancer. PLoS One. 8:e814232013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Cai F, Zhang B, Barekati Z and
Zhong XY: The level of circulating miRNA-10b and miRNA-373 in
detecting lymph node metastasis of breast cancer: Potential
biomarkers. Tumour Biol. 34:455–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Severino P, Brüggemann H, Andreghetto FM,
Camps C, Klingbeil Mde F, de Pereira WO, Soares RM, Moyses R,
Wünsch-Filho V, Mathor MB, et al: MicroRNA expression profile in
head and neck cancer: HOX-cluster embedded microRNA-196a and
microRNA-10b dysregulation implicated in cell proliferation. BMC
Cancer. 13:5332013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yigit MV, Ghosh SK, Kumar M, Petkova V,
Kavishwar A, Moore A and Medarova Z: Context-dependent differences
in miR-10b breast oncogenesis can be targeted for the prevention
and arrest of lymph node metastasis. Oncogene. 32:1530–1538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guessous F, Alvarado-Velez M,
Marcinkiewicz L, Zhang Y, Kim J, Heister S, Kefas B, Godlewski J,
Schiff D, Purow B and Abounader R: Oncogenic effects of miR-10b in
glioblastoma stem cells. J Neurooncol. 112:153–163. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karsy M, Arslan E and Moy F: Current
progress on understanding MicroRNAs in glioblastoma multiforme.
Genes Cancer. 3:3–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue Y, Abou Tayoun AN, Abo KM, Pipas JM,
Gordon SR, Gardner TB, Barth RJ Jr, Suriawinata AA and Tsongalis
GJ: MicroRNAs as diagnostic markers for pancreatic ductal
adenocarcinoma and its precursor, pancreatic intraepithelial
neoplasm. Cancer Genet. 206:217–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moghimi-Dehkordi B and Safaee A: An
overview of colorectal cancer survival rates and prognosis in Asia.
World J Gastrointest Oncol. 4:71–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarkar S, Mukherjee R, Paira SK, Roy B,
Banerjee S and Mukherjee SK: Profile of colorectal cancer in
Eastern India. J Indian Med Assoc. 110:901–903. 2012.PubMed/NCBI
|
|
30
|
Wong MC, Lam TY, Tsoi KK, Chan VC, Hirai
HW, Ching JY and Sung JJ: Predictors of advanced colorectal
neoplasia for colorectal cancer screening. Am J Prev Med.
46:433–439. 2014. View Article : Google Scholar : PubMed/NCBI
|