Introduction
Ovarian cancer is the leading cause of mortality due
to gynecological malignancy in recent years (1). Although the diagnosis and treatments of
ovarian cancer are constantly under development, the prognosis of
the patients remains poor, which is due to the biological behavior
of the tumor (2). Ovarian cancer
cells are able to migrate and invade into the peritoneal/pelvic
cavity. Therefore, clarification of the mechanisms of cell
migration and invasion is important to identify novel therapy
methods for ovarian cancer treatment.
Blood supply is essential for tumors metastasis
(3). Previous studies have suggested
that the development of tumor microcirculation appears to be a
rate-limiting step for metastasis (3–5). In 1999,
Maniotis et al (6) identified
a novel phenomenon in the biology of tumor vascularization known as
vasculogenic mimicry (VM). VM is the unique ability of highly
aggressive tumor cells, but not of poorly aggressive cells, to
mimic the presence and function of endothelial cells and form
matrix-rich networks de novo, which are able to convey blood
plasma and red blood cells (6–8). In VM,
the blood vessels wall is lined exclusively by tumor cells, without
the participation of endothelial cells (9,10). As a
secondary circulation system, VM has been recognized as an
important form of vasculogenic structure in solid tumors, including
melanoma, breast cancer, hepatocellular carcinoma, prostate cancer,
clear cell renal cell carcinoma, glioblastoma, gastric
adenocarcinoma, colorectal cancer and gestational choriocarcinoma
(6,11–19). In
ovarian cancer, VM has been correlated with poor prognosis, low
survival and increased risk of cancer recurrence (6). However, the mechanisms involved in the
generation of VM in ovarian cancer remain obscure.
Human chorionic gonadotropin (HCG), a hormone of
trophoblastic origin, was considered to be a proangiogenic factor
(20). HCG increases uterine arterial
blood flow and stimulates angiogenesis in the ovary by stimulating
the proliferation of vascular endothelial cells and the expression
of vascular endothelial growth factor (VEGF) (21–23). HCG
induces neovascularization in placenta during pregnancy (24). In ovarian cancer, the HCG protein and
its receptor, luteinizing hormone receptor (LHR), exhibited
positive expression (25). HCG is
also ‘ectopically’ expressed in numerous malignant tumors such as
endometrial carcinoma and ovarian, testicular and breast cancer
(26–30). A previous study by the present authors
demonstrated that HCG promotes VM formation in three-dimensional
cultures of OVCAR-3 cells, and anoxia could promote VM formation
via activation of hypoxia-inducible factor-1 (31). Therefore, the present authors
hypothesize that HCG may be important in the development of VM in
ovarian cancer.
To further investigate the role of chorionic
gonadotropin, beta polypeptide 5 (CGB5, which is the fifth subunit
of β-HCG and was identified as the key part of HCG) in ovarian
cancer VM formation in vivo, a tumor formation model in
vivo was constructed in the present study, and the effect of
HCG binding to its receptor in VM formation was investigated in
vivo. The results demonstrated the role of CGB5 in VM formation
in vivo, and support a novel approach for ovarian cancer
therapy.
Materials and methods
Cell lines and cell culture
The human epithelial ovarian cancer cell line
OVCAR-3 and the human choriocarcinoma cell line BeWo were purchased
from the American Type Culture Collection (Manassas, VA, USA).
OVCAR-3 cells were cultured at 37°C and 5% CO2, in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.). BeWo cells were cultured in F-12 medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 15% FBS.
CGB5 expression and small interfering
RNA (siRNA) lentivirus production and infection
Human CGB complementary DNA (cDNA; accession no.
NM_033043.1) was obtained from a human fetal ovary library, and
subcloned into the pLenti6/entre-EGFP vector (Invitrogen; Thermo
Fisher Scientific, Inc.), which led to pLenti/EGFP-CGB5. The siRNA
template sequence 5′-AGCAGCAACAGCAGCAGCCTC-3′, which targeted CGB5,
was constructed into the pcDNA™6.2-GW/EmGFP-miR vector
(Invitrogen; Thermo Fisher Scientific, Inc.), and the construct was
named pGFP-CGB5_siR. A total of 3 µg pLenti/EGFP-CGB5 vector,
pGFP-CGB5_siR or pLenti6/entre-EGFP (which served as a control) and
9 µg ViraPower™ Packaging Mix (Invitrogen; Thermo Fisher
Scientific, Inc.) were used to co-transfect 5×106 293FT
cells (Thermo Fisher Scientific, Inc.) with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according the
manufacturer's protocol. After transfection for 48 h, the
supernatant containing the lentiviruses was harvested, centrifuged
and stored at −80°C. For infection, the cells were seeded at 1×105
cells/well into a 6-well plate and cultured overnight, and then
lentiviruses were added. After 24 h of infection, 10 ng blastin
(Invitrogen; Thermo Fisher Scientific, Inc.) was added to the cells
to obtain a stable cell clone, and the cells were then sorted by
flow cytometry.
Animals and experiments
BALB/c female nude mice were purchased from the
laboratory animal center of the Chinese Academy of Sciences
(Beijing, China). Mice that were 4–6 weeks old and had an average
body weight of 18–20 g were used in the study. The experimental
procedures were performed according to the standards established by
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (32).
For tumor formation in vivo, 36 mice were randomly divided
into 6 groups, and injected with normal OVCAR-3 cells, control
vector-transfected OVCAR-3 cells, CGB5-overexpressing OVCAR-3
cells, CGB5-knockdown OVCAR-3 cells or BeWo cells (6 mice/group).
Tumors were allowed to grow for 2–6 weeks after tumor inoculation,
and the mice were then sacrificed by cervical decapitation. Next,
tumors were subjected to histopathological examination,
transmission electron microscopy observation and reverse
transcription-polymerase chain reaction (RT-PCR) analysis.
Hematoxylin and eosin (H&E)
staining and cluster of differentiation (CD)34-periodic acid-Schiff
(PAS) dual staining
The tumor xenograft specimens were fixed in 10%
neutral buffered formalin and paraffin-embedded. Paraffin-embedded
specimens were cut into serial 6-µm sections to be placed on
slides. The sections were rehydrated through graded alcohols into
water. Endogenous peroxidase was blocked with 3% hydrogen peroxide
in 50% methanol for 10 min at room temperature. Upon rehydration,
the sections were washed with phosphate-buffered saline (PBS) for
15 min and then pre-treated with 0.01 mol/l citrate acid buffer (pH
6.0) for 20 min at 100°C in a microwave oven. After rinsing with
PBS, slides were incubated with primary antibodies against CD34
(sc-74499, Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:100
dilution) overnight. After washing in PBS for three times, the
slides were incubated with peroxidase-conjugated donkey anti-rabbit
antibody (P0448, Dako North America, Inc., Carpinteria, CA, USA;
1:100 dilution) for 30 min. The reactions were visualized with
diaminobenzidine (EnVision; Dako North America, Inc.) and
counterstained with hematoxylin (EnVision; Dako North America,
Inc). The CD34-immunohistochemically stained sections were further
stained with PAS, followed by counterstaining with hematoxylin.
These double-stained sections were used for observing microvascular
and VM structures. Quantification of VM was performed as follows:
VM channels and endothelium-dependent vessels in the
H&E-stained sections were counted under a microscope using ×400
magnification, while CD34-PAS dual staining sections were viewed at
×400 magnification. The channels defined as VM were lined by
PAS-positive material, with red cells in the center of the
channels, but were not lined by CD34-positive endothelial cells.
The mean numbers of VM in 10 areas were calculated as the VM
channel density for each section.
Transmission electron microscopy
observation
Fresh tumor xenograft tissues (0.5-mm3)
were fixed in cold 2.5% glutaraldehyde in 0.1 mol/l sodium
cacodylate buffer and post-fixed in a solution of 1% osmium
tetroxide, then dehydrated and embedded in a standard manner
(33). The specimens were then
embedded, sectioned and stained by routine means for analysis with
a transmission electron microscope (34).
Immunohistochemical staining
The paraffin sections were deparaffinized and
rehydrated according to the method described above. After rinsing
with PBS, the slides were incubated overnight with primary
antibodies against Ki-67 (mouse monoclonal; sc-23900, Santa Cruz
Biotechnology, Inc.; 1:100 dilution), VEGF (sc-7269, mouse
monoclonal; Santa Cruz Biotechnology, Inc., 1:50), factor VIII
(goat polyclonal; sc-33584, Santa Cruz Biotechnology, Inc.; 1:100
dilution), β-HCG (sc-7822, mouse monoclonal; Santa Cruz
Biotechnology, Inc.; 1:100 dilution), LHR (sc-293165, mouse
monoclonal; Santa Cruz Biotechnology, Inc.; 1:100 dilution) and
CD31 (rabbit polyclonal; sc-8306, Santa Cruz Biotechnology, Inc.;
1:100 dilution). After washing in PBS for three times, the slides
were incubated with peroxidase-conjugated donkey anti-mouse or
anti-rabbit antibody (P0447 and P0448, respectively, Dako North
America, Inc.; both 1:100 dilution) for 30 min. Negative controls
were prepared by replacing the primary antibody with Tris-buffered
saline. The reactions were visualized by diaminobenzidine and
counterstained with hematoxylin.
RNA isolation and RT-PCR
Total RNA was isolated from the tumor xenograft
tissues using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.).
First-strand cDNA was synthesized from 20 µl total RNA using
oligo(dT)18 primers and reverse transcriptase (Promega Corporation,
Madison, WI, USA). RT-PCR was conducted using PCR Master Mix
(Promega Corporation) with an initial denaturing step at 94°C for 5
min, followed by 20 cycles at 94°C for 45 sec, 58°C for 45 sec and
72°C for 45 sec, and a further extension at 72°C for 10 min.
Glyceraldehyde 3-phosphate dehydrogenase was used as an internal
control. The sequences of the primers are indicated in Table I.
 | Table I.Sequences of the primers used in
reverse transcription-polymerase chain reaction. |
Table I.
Sequences of the primers used in
reverse transcription-polymerase chain reaction.
| Gene name | Accession no. | Sequence
(5′-3′) |
|---|
| CGB5 | NM_033043.1 | Forward:
CTACTGCCCCACCATGACC |
|
|
| Reverse:
ATGGACTCGAAGCGCACATC |
| CD31 | NM_000442.4 | Forward:
ATTGCAGTGGTTATCATCGGAGTG |
|
|
| Reverse:
CTCGTTGTTGGAGTTCAGAAGTGG |
| Factor VIII | NM_000132.3 | Forward:
ATCGAGGGTCTCGGGGATGCG |
|
|
| Reverse:
TGCGAAGAGTGCTGCGAATGCT |
| LHR | NM_000610.3 | Forward:
TGGCTGCTGTAAACGTCGGG |
|
|
| Reverse:
GGAGAGCTGTACCTTGACAGTG |
| GAPDH | NM_001256799.1 | Forward:
AAGGTGAAGGTCGGAGTC |
|
|
| Reverse:
GAAGATGGTGATGGGATTTC |
Detection of secreted β-HCG by
enzyme-linked immunosorbent assay (ELISA)
The concentrations of β-HCG released in the
supernatants of the cell cultures were measured by specific human
β-HCG ELISA, according to the manufacturer's protocol (BD
Pharmingen, San Diego, CA, USA). Briefly, cells in 100 µl medium
were seeded at 5×103 cells/well onto 96-well plates, and
transfected with different reagents, as described earlier. At the
appropriate time, 100 µl supernatants were harvested for ELISA.
Statistical analysis
All experiments were repeated ≥3 times. All
numerical data were presented as the mean ± standard deviation.
Data were analyzed using the two-tailed t test. P<0.05
was considered to indicate a statistically significant
difference.
Results
CGB5 promotes tumor xenografts
formation in vivo
To clarify if CGB5 affected VM formation and tumor
development in vivo, the CGB5-expression vector and the
siRNA vector targeting CGB5 were transfected into OVCAR-3 cells.
Stable expression cell lines were obtained, and the expression of
CGB5 at the messenger RNA (mRNA) level was effectively upregulated
and downregulated, respectively, in OVCAR-3 cells, as detected by
RT-PCR (Fig. 1A and B). Secreted
β-HCG was measured in the supernatant of OVCAR-3 cells (Fig. 1C). Xenografts were established using
these cells, which were resuspended at a density of
1×107 cells/ml, and 200 µl of this cell suspension was
injected subcutaneously into nude mice. BeWo cells with high
expression of β-HCG were used as a positive control (Fig. 1D). The tumors appeared gradually in
the subcutaneous area of the right armpit of the mice following
inoculation. After 2–6 weeks, tumors had grown to an average size
of 1–2 cm3. The tumor formation rates of the nude mouse
xenografts were 100.0% (6/6) for CGB5-overexpressing OVCAR-3 and
BeWo cells, 83.3% (5/6) for normal or control vector-transfected
OVCAR-3 cells and 33.3% (2/6) for CGB5-knockdown OVCAR-3 cells. The
rates of tumor growth were also different, with tumors derived from
BeWo and CGB5-overexpressing OVCAR-3 injected cells growing faster
than tumors derived from CGB5-knockdown OVCAR-3 cells (Fig. 1E). In addition, the cell proliferation
marker Ki-67 was detected by immunohistochemical staining, thus
supporting such phenomenon (Fig. 1F).
These data indicated that CGB5 may promote cell proliferation and
tumor growth in ovarian cancer.
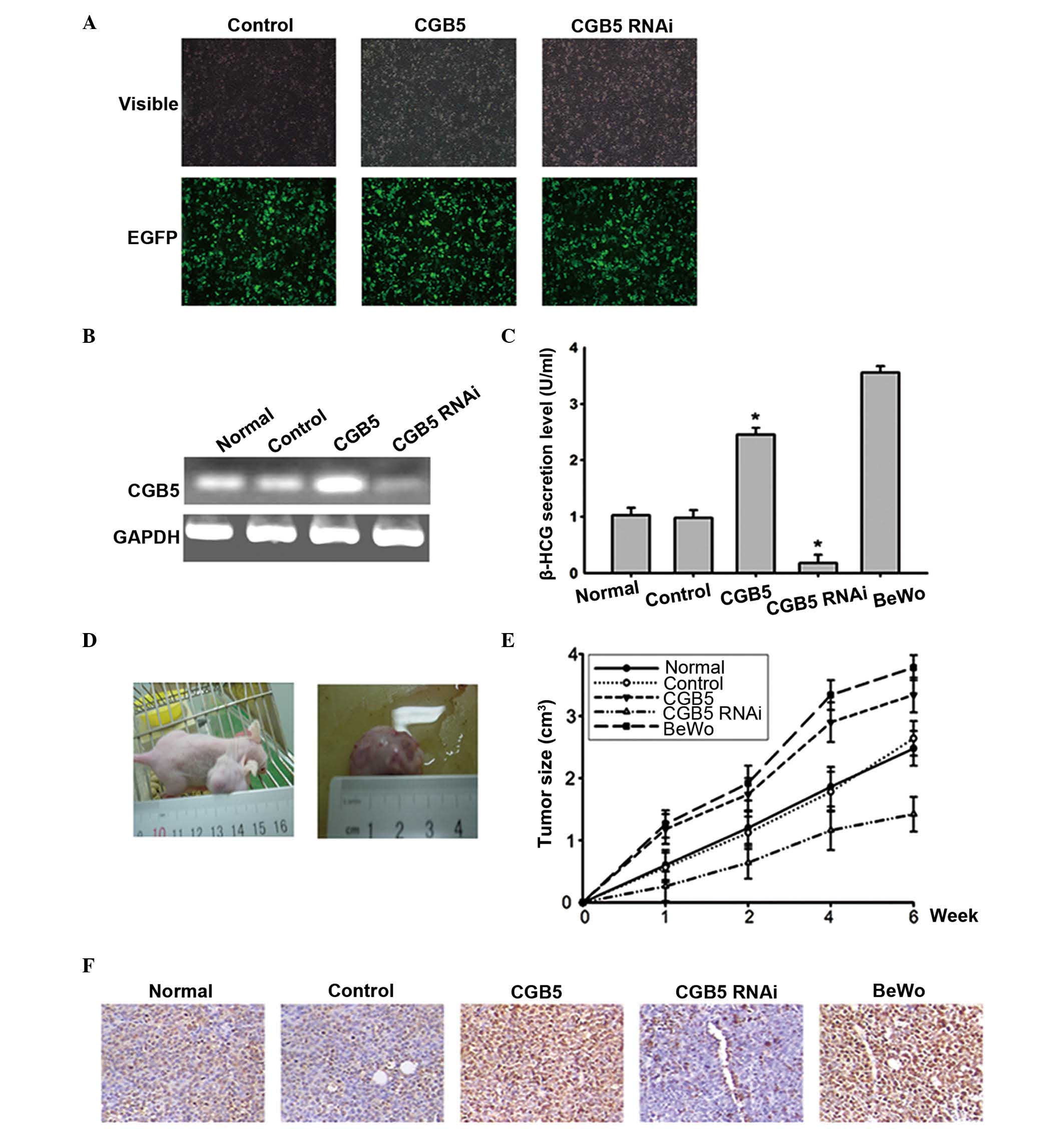 | Figure 1.Effect of CGB5 on tumor xenografts
formation in vivo. (A) Lentivirus vector expressing CGB5 and
small interfering RNA vector were infected in OVCAR-3 cells. EGFP
expression demonstrated that the above vectors were stably infected
in these cells. Control, pLenti6/entre-EGFP-infected cells; CGB5,
CGB5-overexpressing cells; and CGB5 RNA interference,
CGB5-knockdown cells. The BeWo cell line, which secrets high levels
of β-HCG, was used as a positive control. Magnification, ×40. (B)
Reverse transcription-polymerase chain reaction was used to
determine the messenger RNA levels of CGB5 in the stably
transfected cells. (C) The supernatant of these stably transfected
cells was collected, and the β-HCG levels were detected by
enzyme-linked immunosorbent assay. *P<0.05 vs. normal cells. (D)
Cells were injected subcutaneously into the nude mice, and the
tumors appeared gradually in the subcutaneous area of the right
armpit of the mice at 2–6 weeks post-inoculation. (E) The speed of
tumor growth was measured at 1, 2, 4 and 6 weeks after injection.
(F) The cell proliferation marker Ki-67 was detected by
immunohistochemical analysis. Magnification, ×400. CGB5, chorionic
gonadotropin, beta polypeptide 5; GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; EGFP, enhanced green fluorescent protein; RNAi, RNA
interference; HCG, human chorionic gonadotropin. |
The effect of CGB5 on VM formation in
tumor xenografts
H&E staining and CD34-PAS dual staining were
used to observe the morphology characteristic of VM in tumor
xenografts. Based on H&E staining, spaces filled with red blood
cells, which were completely surrounded by cancer cells, were
observed (Fig. 2A a-e). Furthermore,
staining of the endothelial cell marker CD34 was used to identify
the endothelium in cancer tissue sections, while PAS staining was
used to determine the basement membrane of tumor blood vessels
(Fig. 2A f-j). The channel consisted
of tumor cells that were positive for PAS and negative for CD34.
Following statical analysis in 10 fields, the average numbers of
blood supply patterns in tumors were defined as the number of VM in
one section. Compared with the normal cells group, VM channels in
xenografts were significantly increased in CGB5-overexpressing
OVCAR-3 cells and in the BeWo cell group, while they were
significantly reduced in the CGB5-knockdown OVCAR-3 cells group
(Fig. 2B). There were cavities in the
central structure of tumor nests, as clearly analyzed by
transmission electron microscopy. Single, double, or multiple red
blood cells-containing cavities structures could be observed in the
choriocarcinoma specimens, according to their cell morphology. The
number of cavities was much higher in CGB5-overexpressing tumor
nests than in normal ovarian cancer specimens (Fig. 2C). These results indicated that HCG
may promote the process of VM formation in ovarian cancer.
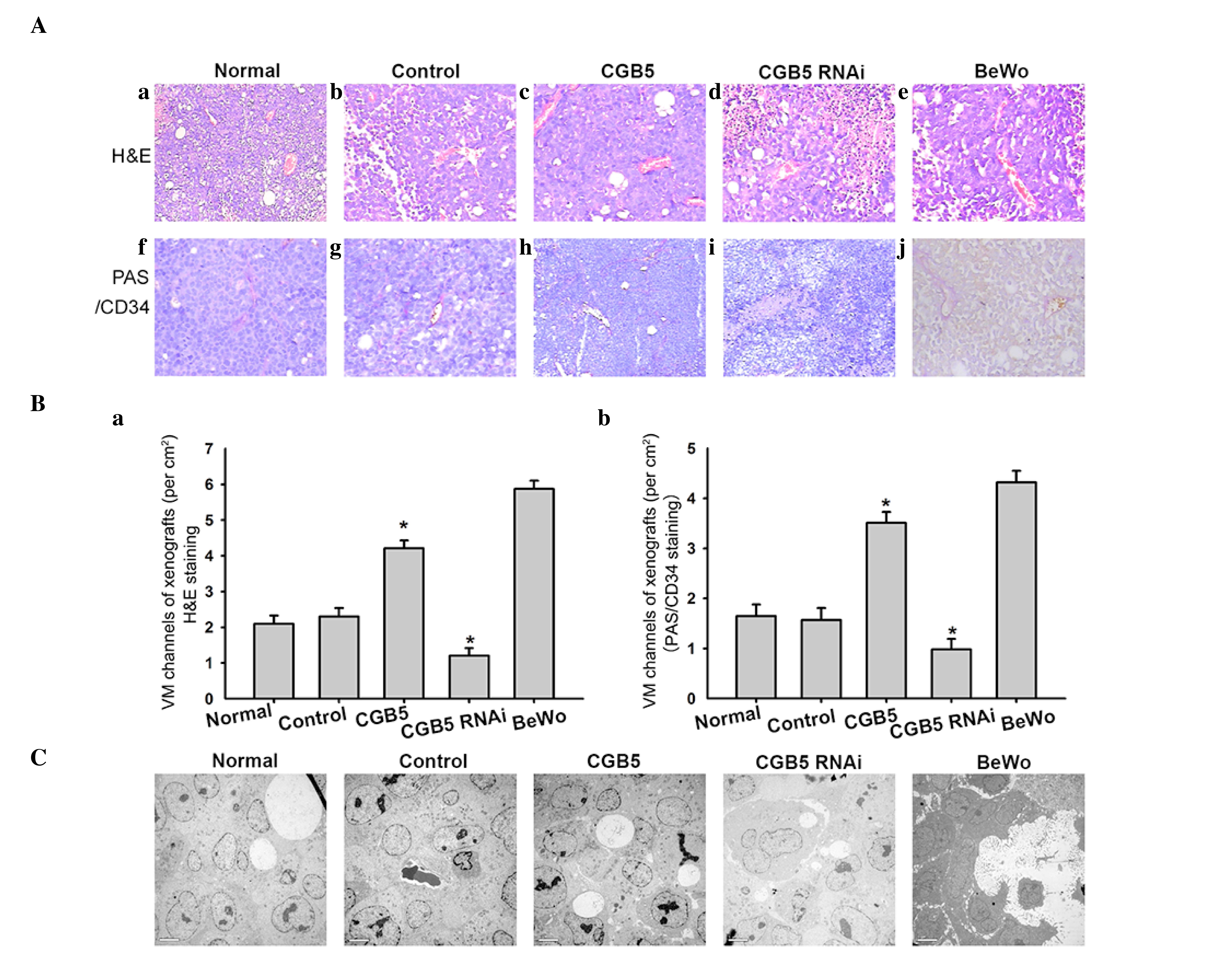 | Figure 2.Effect of CGB5 on VM formation by
H&E and CD34-PAS staining. (A) After tumor growth to 1–2 cm3,
the tumors were collected and cut into serial 6-µm sections. (a-e)
H&E staining was used to detect VM formation. (f-j) PAS
staining was used to identify matrix-associated vascular channels,
while CD34 staining was used to identify the endothelium in the
cancer tissue sections. PAS-positive and CD34-negative channels
were considered as VM. (B) VM quantification was performed as
follows: (a) VM channels and endothelium-dependent vessels in the
H&E-stained sections were counted using ×400 magnification, and
(b) CD34-PAS dual staining sections were viewed at ×400
magnification. *P<0.05 vs. normal cells. (C) Transmission
electron microscopy examination. CGB5, chorionic gonadotropin, beta
polypeptide 5; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
RNAi, RNA interference; HCG, human chorionic gonadotropin; VM,
vasculogenic mimicry; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff. |
CGB5 promotes vascular marker
expression in tumor xenografts
To further confirm VM formation in the tumor
xenografts, the vascular markers CD31, VEGF and factor VIII were
detected. The mRNA levels of CD31, VEGF and factor VIII were
increased in the CGB5-overexpressing cell group, whereas they were
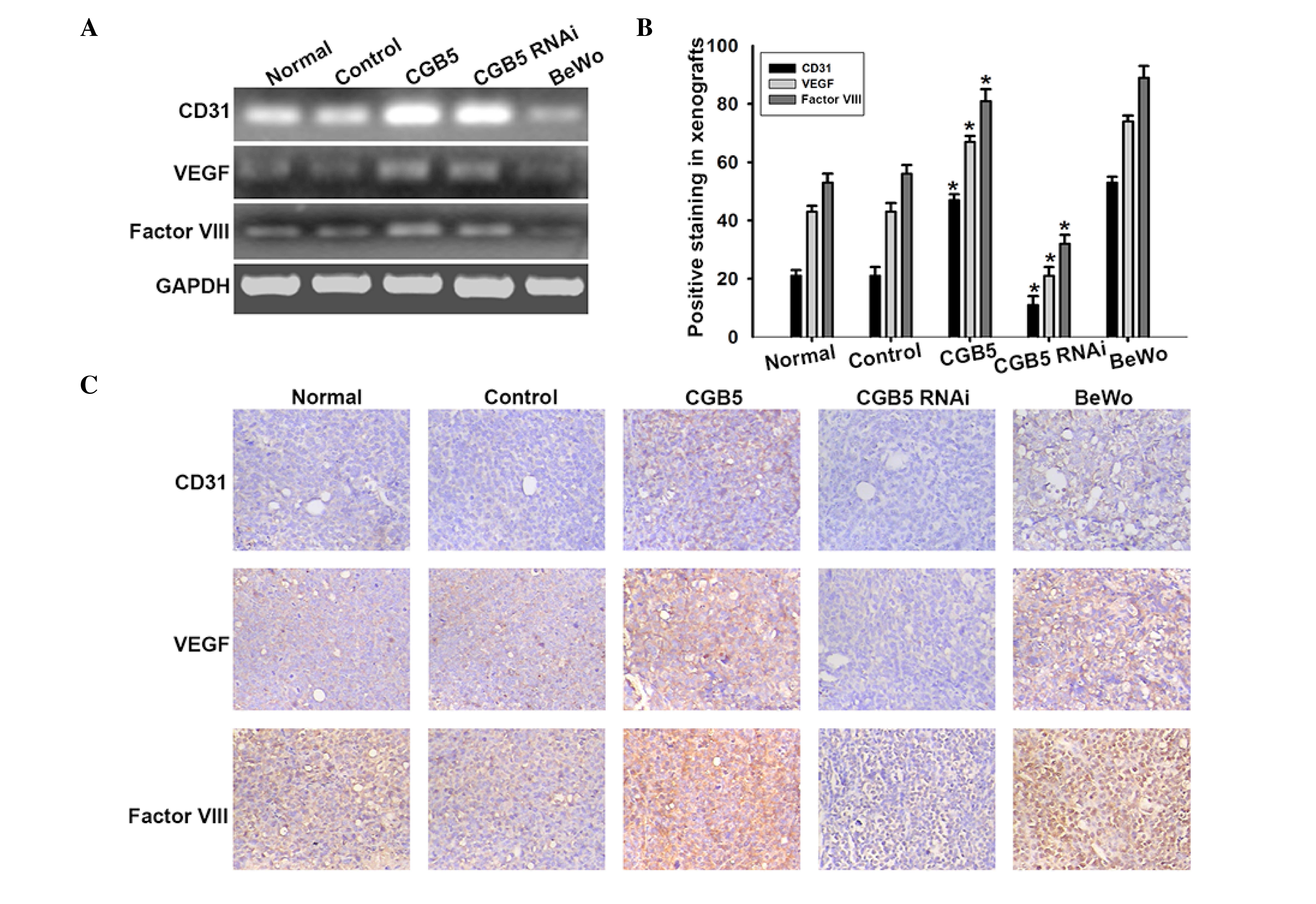
decreased in the CGB5-knockdown cell group (Fig. 3A). The results of immunohistochemical
staining of CD31, VEGF and factor VIII exhibited the same tendency
as the mRNA results (Fig. 3B).
Compared with normal cells, the expression of vascular markers was
higher in CGB5-overexpressing OVCAR-3 and BeWo cells. By contrast,
the expression of these vascular markers was weak and focal in the
CGB5-knockdown OVCAR-3 cell group (Fig.
3C).
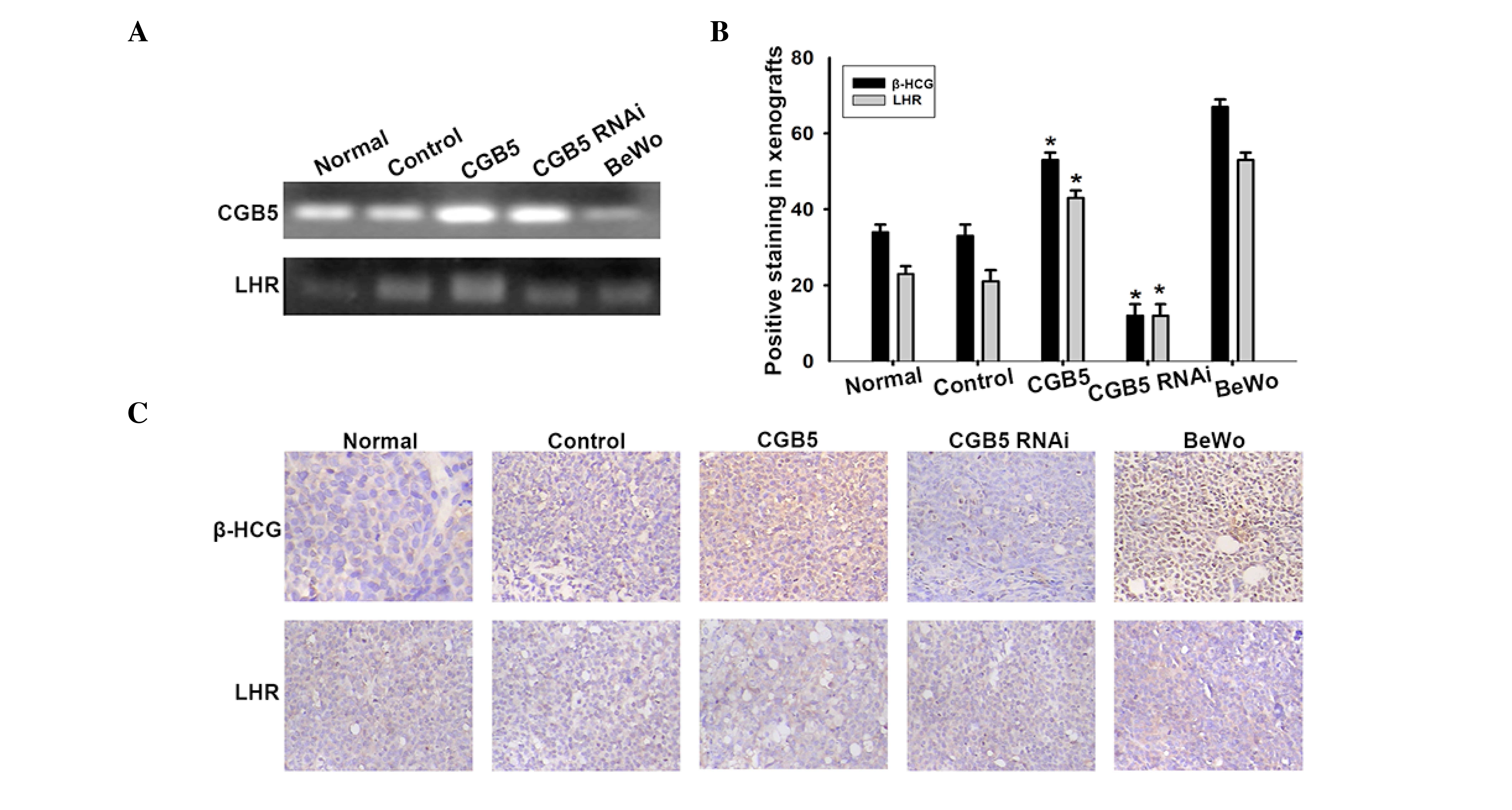
Change in LHR expression in tumor
xenografts
Previous studies indicated that CGB5 may promote VM
and tumor formation in vitro (31). However, the effect of CGB5 in the
pathway mediated by LHR binding remains unknown. In the present
study, the mRNA expression levels of LHR changed following the same
tendency than CGB5 (Fig. 4A).
Furthermore, immunochemistry demonstrated that LHR expression was
increased in the CGB5-overexpressing OVCAR-3 cell group, while it
was downregulated in the CGB5-knockdown OVCAR-3 cell group
(Fig. 4B and C). These data indicated
that CGB5 promotes VM formation in ovarian carcinoma via activation
of LHR signal transduction.
Discussion
HCG, which is primarily produced by the placenta,
was considered to be a proangiogenic factor in recent reports
(35). In the present study, CGB5
(the fifth subunit of β-HCG, which was identified as the key part
of HCG) was used to investigate the role of HCG in VM formation.
The current study identified that CGB5 may increase tumor growth,
VM formation and LHR expression. These data indicated that, in
ovarian carcinoma, CGB5 was the promoter of VM formation and tumor
growth via the activation of LHR signal transduction.
HCG, a heterodimeric glycoprotein hormone, is
composed of a single α-subunit and a target-specific β-subunit,
which is encoded by a multigene cluster composed of six homologous
sequences (β1, β2, β3, β5, β7 and β8) (24,36,37);
however, only the heterodimers containing β5, β3 or β8 are
transcriptionally active (37,38). Since
the expression of the β5-subunit is the highest of all subunits
(37), the present study selected
CGB5 as the potential target gene in VM formation. It has been
reported that HCG can increase uterine arterial blood flow and
stimulate angiogenesis in the ovary by stimulating vascular
endothelial cell proliferation and VEGF expression (21–22). HCG
can also prevent luteolysis by maintaining luteal blood flow
(31). In early pregnancy, HCG
expression is associated with endometrial angiostimulation, since
HCG increases blood supply and alters the uterine vasculature via
vasodilatation, thus increasing the permeability, development and
maturation of newly formed blood vessels (25). It has been reported that HCG exerts
its effect possibly by interacting with its LHR receptor (39). The present study identified that CGB5
overexpression promoted tumor growth and formation, which was
accompanied by an increase in LHR expression.
VM describes the unique ability of highly aggressive
tumor cells to mimic the presence and function of endothelial
cells, thus forming matrix-rich networks de novo, which
could convey blood plasma and red blood cells (6–8). Based on
H&E staining, channels which PAS-positive material and red
cells in the center of the channels but CD34-negative endothelial
cells may be identified as VM in vivo. The present study
suggested that CGB5 may promote OVCAR-3 cells tumor growth and VM
formation in tumor xenografts. It has been reported that certain
ovarian cancer cells may differentiate into endothelial-like cells.
To further identify the formation of VM, vascular markers,
including CD31, factor VIII and VEGF, were also detected in OVCAR-3
cells in vivo. The expression of CD31, factor VIII and VEGF
followed the same tendency as the levels of CGB5 in these
cells.
In conclusion, ovarian cancer is a silent killer,
and the present results provide clearly in vivo evidence
that HCG is important in the formation of VM. Therefore, it is
possible to speculate that the use of anti-HCG therapeutic
targeting may provide a novel opportunity to circumvent tumors that
express HCG, such as ovarian cancer and choriocarcinoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
30801226).
References
|
1
|
Zhang B, Barekati Z, Kohler C, Radpour R,
Asadollahi R, Holzgreve W and Zhong XY: Proteomics and biomarkers
for ovarian cancer diagnosis. Ann Clin Lab Sci. 40:218–225.
2010.PubMed/NCBI
|
|
2
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang S, Zhang D and Sun B: Vasculogenic
mimicry: Current status and future prospects. Cancer Lett.
254:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vartanian AA, Burova OS, Stepanova EV,
Baryshnikov AY and Lichinitser MR: Melanoma vasculogenic mimicry is
strongly related to reactive oxygen species level. Melanoma Res.
17:370–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Folkman J: Fundamental concepts of the
angiogenic process. Curr Mol Med. 3:643–651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun W, Fan YZ, Zhang WZ and Ge CY: A pilot
histomorphology and hemodynamic of vasculogenic mimicry in
gallbladder carcinomas in vivo and in vitro. J Exp Clin Cancer Res.
30:462011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hess AR, Seftor EA, Seftor RE and Hendrix
MJ: Phosphoinositide 3-kinase regulates membrane Type 1-matrix
metalloproteinase (MMP) and MMP-2 activity during melanoma cell
vasculogenic mimicry. Cancer Res. 63:4757–4762. 2003.PubMed/NCBI
|
|
9
|
Sun B, Zhang D, Zhang S, Zhang W, Guo H
and Zhao X: Hypoxia influences vasculogenic mimicry channel
formation and tumor invasion-related protein expression in
melanoma. Cancer Lett. 249:188–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sood AK, Seftor EA, Fletcher MS, Gardner
LM, Heidger PM, Buller RE, Seftor RE and Hendrix MJ: Molecular
determinants of ovarian cancer plasticity. Am J Pathol.
158:1279–1288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shirakawa K, Wakasugi H, Heike Y, Watanabe
I, Yamada S, Saito K and Konishi F: Vasculogenic mimicry and
pseudo-comedo formation in breast cancer. Int J Cancer. 99:821–828.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guzman G, Cotler SJ, Lin AY, Maniotis AJ
and Folberg R: A pilot study of vasculogenic mimicry
immunohistochemical expression in hepatocellular carcinoma. Arch
Pathol Lab Med. 131:1776–1781. 2007.PubMed/NCBI
|
|
13
|
Sharma N, Seftor RE, Seftor EA, Gruman LM,
Heidger PM Jr, Cohen MB, Lubaroff DM and Hendrix MJ: Prostatic
tumor cell plasticity involves cooperative interactions of distinct
phenotypic subpopulations: Role in vasculogenic mimicry. Prostate.
50:189–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vartanian AA, Stepanova EV, Gutorov SL,
Solomko ESh, Grigorieva IN, Sokolova IN, Baryshnikov AY and
Lichinitser MR: Prognostic significance of periodic
acid-Schiff-positive patterns in clear cell renal cell carcinoma.
Can J Urol. 16:4726–4732. 2009.PubMed/NCBI
|
|
15
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Gu Y, Zhang Z, Zhang S, Zhang D,
Saleem AF, Zhao X and Sun B: Vasculogenic mimicry: A new prognostic
sign of gastric adenocarcinoma. Pathol Oncol Res. 16:259–266. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baeten CI, Hillen F, Pauwels P, de Bruine
AP and Baeten CG: Prognostic role of vasculogenic mimicry in
colorectal cancer. Dis Colon Rectum. 52:2028–2035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shih IeM: Trophoblastic vasculogenic
mimicry in gestational choriocarcinoma. Mod Pathol. 24:646–652.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sood AK, Fletcher MS, Zahn CM, Gruman LM,
Coffin JE, Seftor EA and Hendrix MJ: The clinical significance of
tumor cell-lined vasculature in ovarian carcinoma: Implications for
anti-vasculogenic therapy. Cancer Biol Ther. 1:661–664. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zygmunt M, Herr F, Keller-Schoenwetter S,
Kunzi-Rapp K, Münstedt K, Rao CV, Lang U and Preissner KT:
Characterization of human chorionic gonadotropin as a novel
angiogenic factor. J Clin Endocrinol Metab. 87:5290–5296. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Toth P, Li X, Rao CV, Lincoln SR,
Sanfilippo JS, Spinnato JA II and Yussman MA: Expression of
functional human chorionic gonadotropin/human luteinizing hormone
receptor gene in human uterine arteries. J Clin Endocrinol Metab.
79:307–315. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wulff C, Dickson SE, Duncan WC and Fraser
HM: Angiogenesis in the human corpus luteum: Simulated early
pregnancy by HCG treatment is associated with both angiogenesis and
vessel stabilization. Hum Reprod. 16:2515–2524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berndt S, Perrier D'Hauterive S, Blacher
S, Péqueux C, Lorquet S, Munaut C, Applanat M, Hervé MA, Lamandé N,
Corvol P, et al: Angiogenic activity of human chorionic
gonadotropin through LH receptor activation on endothelial and
epithelial cells of the endometrium. FASEB J. 20:2630–2632. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michel RM, Aguilar JL and Arrieta O: Human
chorionic gonadotropin as an angiogenic factor in breast cancer
during pregnancy. Med Hypotheses. 68:1035–1040. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szajnik M, Nowak-Markwitz E, Szczepański
MJ and Spaczyński M: Assessment of expression of luteinizing
hormone (LH)/human chorionic gonadotropin (hCG) receptor (LH/hCGR)
and hCG protein in ovarian cancer tissues. Ginekol Pol. 78:939–943.
2007.(In Polish). PubMed/NCBI
|
|
26
|
Muller CY and Cole LA: The quagmire of hCG
and hCG testing in gynecologic oncology. Gynecol Oncol.
112:663–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lempiäinen A, Stenman UH, Blomqvist C and
Hotakainen K: Free beta-subunit of human chorionic gonadotropin in
serum is a diagnostically sensitive marker of seminomatous
testicular cancer. Clin Chem. 54:1840–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iles RK, Delves PJ and Butler SA: Does hCG
or hCGβ play a role in cancer cell biology? Mol Cell Endocrinol.
329:62–70. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Daniel KD, Kim GY, Vassiliou CC,
Jalali-Yazdi F, Langer R and Cima MJ: Multi-reservoir device for
detecting a soluble cancer biomarker. Lab Chip. 7:1288–1293. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jankowska AG, Andrusiewicz M, Fischer N
and Warchol PJ: Expression of hCG and GnRHs and their receptors in
endometrial carcinoma and hyperplasia. Int J Gynecol Cancer.
20:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su M, Wei W, Xu X, Wang X, Chen C, Su L
and Zhang Y: Role of hCG in vasculogenic mimicry in OVCAR-3 ovarian
cancer cell line. Int J Gynecol Cancer. 21:1366–1374. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kavak S, Ayaz L and Emre M: Effects of
rosiglitazone with insulin combination therapy on oxidative stress
and lipid profile in left ventricular muscles of diabetic rats. Exp
Diabetes Res. 2012:9056832012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Newton PT, Staines KA, Spevak L, Boskey
AL, Teixeira CC, Macrae VE, Canfield AE and Farquharson C:
Chondrogenic ATDC5 cells: An optimised model for rapid and
physiological matrix mineralisation. Int J Mol Med. 30:1187–1193.
2012.PubMed/NCBI
|
|
34
|
Scimeca M, Orlandi A, Terrenato I,
Bischetti S and Bonanno E: Assessment of metal contaminants in
non-small cell lung cancer by EDX microanalysis. Eur J Histochem.
58:24032014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brouillet S, Hoffmann P, Chauvet S,
Salomon A, Chamboredon S, Sergent F, Benharouga M, Feige JJ and
Alfaidy N: Revisiting the role of hCG: new regulation of the
angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci.
69:1537–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamada AL, Nakabayashi K, Sato A, Kiyoshi
K, Takamatsu Y, Laoag-Fernandez JB, Ohara N and Maruo T:
Transfection of antisense chorionic gonadotropin beta gene into
choriocarcinoma cells suppresses the cell proliferation and induces
apoptosis. J Clin Endocrinol Metab. 90:4873–4879. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bo M and Boime I: Identification of the
transcriptionally active genes of the chorionic gonadotropin beta
gene cluster in vivo. J Biol Chem. 267:3179–3184. 1992.PubMed/NCBI
|
|
38
|
Talmadge K, Boorstein WR, Vamvakopoulos
NC, Gething MJ and Fiddes JC: Only three of the seven human
chorionic gonadotropin beta subunit genes can be expressed in the
placenta. Nucleic Acids Res. 12:8415–8436. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meng XL, Rennert OM and Chan WY: Human
chorionic gonadotropin induces neuronal differentiation of PC12
cells through activation of stably expressed
lutropin/choriogonadotropin receptor. Endocrinology. 148:5865–5873.
2007. View Article : Google Scholar : PubMed/NCBI
|