Introduction
Subcutaneous panniculitis-like T-cell lymphoma
(SPTCL) is a cytotoxic cutaneous T-cell lymphoma of predominantly
α/β T-cell origin, which has an excellent prognosis, particularly
when there is no association with haemophagocytic syndrome (HPS)
(1,2).
The incidence of SPTCL is <1% of all non-Hodgkin lymphoma cases.
SPTCL is most common in young adults with a median age of 36 years
(range, 9–79 years) and has a female predominance with a male to
female ratio of 0.5 (2). Patients
with SPTCL typically present with multiple subcutaneous nodules in
the extremities and trunk, and are often treated with
doxorubicin-based chemotherapy and radiotherapy (3). In SPTCL without an association with HPS,
the first treatment to be considered should be systemic steroids or
other immunosuppressive agents (1,2). CNS
involvement occurs in ~5% of all systemic lymphomas, although its
rate varies depending on the histology and stage of the lymphoma
(4,5).
The rate of CNS involvement is undoubtedly higher in other
lymphomas such as aggressive non-Hodgkin's lymphoma (NHL) (30–50%)
(6). Regarding cutaneous T-cell
lymphoma, a limited number of studies have reported of CNS
involvement. CNS involvement in cutaneous T-cell lymphoma is fatal,
and no consensus is currently available regarding optimal
treatment. Therefore, the present study aimed to investigate and
discuss optimal treatment strategies.
Case report
A 27-year-old male was admitted to The First
Affiliated Hospital of Zhengzhou University (Zhengzhou, Henan,
China) on September 1, 2008. The patient presented with
erythematous nodules on the left buttock and left inguinal lymph
node enlargement that had been apparent for 9 months. The patient
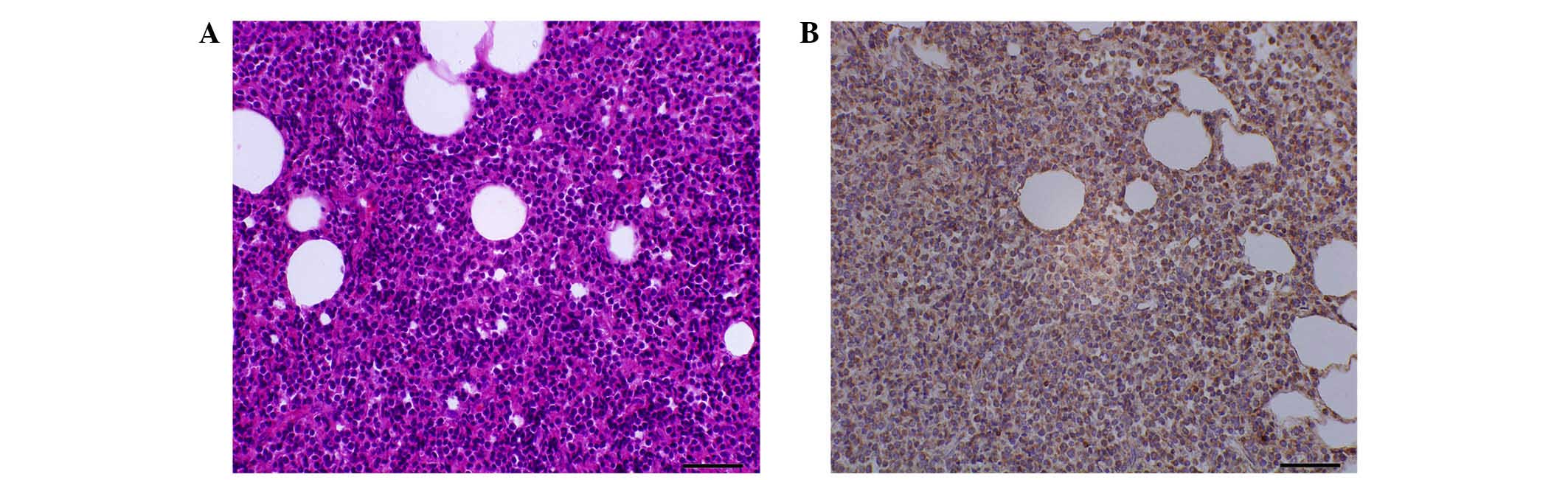
was diagnosed with SPTCL based on a skin biopsy (×400
magnification; Fig. 1A) according to
the World Health Organization European Organization for Research
and Treatment of Cancer classification of primary cutaneous
lymphomas (7). The biopsy revealed
lobular panniculitis with massive atypical lymphoid infiltrates, as
well as inflammatory infiltration of the subcutaneous fat and
histiocytes. The rimming of fat cells by atypical T lymphocytes in
the subcutaneous fat was also visible, consistent with a diagnosis
of SPTCL. The surgical specimen was fixed in 4% formalin, embedded
in paraffin and stained with hematoxylin and eosin. Microscopic
analysis identified a number of medium-sized lymphocytes
infiltrating fatty lobules mixed with histiocytes.
Immunohistochemical analysis revealed that the skin lesions were
positive for cluster of differentiation (CD)3, CD43, CD99,
leukocyte common antigen and B-cell lymphoma-2, and negative for
CD20, CD79a, CD45RO, paired box protein Pax-5, CD10, CD23, CD4 and
CD8. The immunohistochemical staining was positive for T-cell
receptor-βF1 (×400 magnification; Fig.
1B).
Physical examination confirmed erythematous nodules
on the left buttock and left inguinal lymph node enlargement.
Laboratory assessments were conducted and revealed the following:
White blood cell (WBC) count, 8.34×109/l (normal range,
4–10×109/l); absolute neutrophil count,
4.76×109/l; (normal range, 2–7.7×109/l);
haemoglobin, 142 g/l (normal range, 110–160 g/l); and platelet
count, 198×109/l (normal range,
100–300×109/l). The results of the liver function
assessments were identified to be elevated as follows: alanine
aminotransferase, 159 U/l; aspartate aminotransferase, 71 U/l;
serum total bilirubin, 11.6 µmol/l; direct bilirubin, 3.9 µmol/l;
indirect bilirubin, 8 µmol/l; and albumin, 51.1 g/l. The level of
lactate dehydrogenase (LDH) was 251 IU/l and the β2-microglobulin
level was 1.76 mg/l. Renal function parameters were within normal
ranges, with a blood urea nitrogen level of 6.4 mmol/l and a serum
creatinine level of 65 µmol/l. Bone marrow aspirate and biopsy
showed no evidence of lymphoma. Computed tomography scans revealed
mediastinal and axillary lymph node enlargement. Based on the
clinical presentation and imaging findings, the patient was staged
as T3bN2M0 according to the tumour-node-metastasis classification
system for primary cutaneous lymphomas other than mycosis fungoides
and Sézary syndrome (8). There was no
relevant personal or familial medical history. The patient was
immunocompetent and human immunodeficiency virus-negative.
The patient was treated with 4 cycles of the
cyclophosphamide, epirubicin, vincristine and prednisolone (CHOP)
regimen (750 mg/m2 cyclophosphamide by infusion, day 1;
50 mg/m2 doxorubicin by infusion, day 1; 1.4
mg/m2 vincristine by infusion, day 1; 100
mg/m2 prednisolone taken orally, days 1, 2, 3, 4 and 5)
from September 5, 2008 to November 18, 2008, which resulted in
partial remission for 3 months. The patient subsequently received 4
cycles of the etoposide, methylprednisolone, cytarabine and
cisplatin regimen (40 mg/m2 etoposide by infusion, days
1, 2, 3 and 4; 500 mg methylprednisolone by infusion, days 1, 2, 3
and 4; 2,000 mg/m2 cytarabine, day 5; 25
mg/m2 cisplatin, days 1, 2, 3 and 4) from December 2,
2008 to February 3, 2009, which resulted in complete remission for
8 months. The patient then underwent radiation therapy (36 Gy in 20
fractions; 1.8 Gy per fraction) targeted to the cutaneous tumours
for 4 weeks from February 10, 2009 to March 10, 2009.
At 6 months after the completion of treatment, the
patient developed a progressive headache, nausea, vomiting,
numbness of limbs and blurry vision. During admission, this
clinical status rapidly declined, and the patient collapsed into a
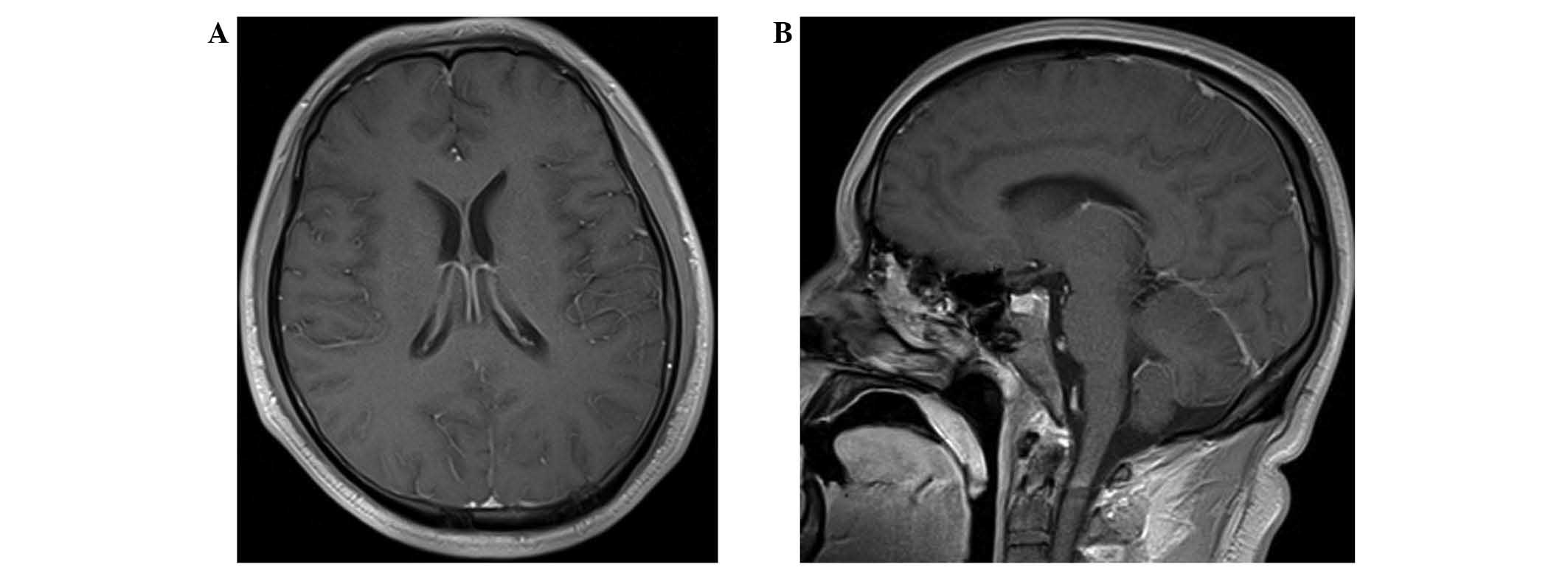
coma. A magnetic resonance imaging (MRI) study of the brain with
gadolinium contrast showed no apparent changes to the brain
parenchyma and leptomeninges, or to the eyes (Fig. 2). A stereotactic biopsy was not
performed as there were no significant lesions. Cerebral spinal
fluid (CSF) analysis revealed the following: WBC count,
0.002×109/l; glucose concentration, 2.6 mmol/l; elevated
protein, 1,034 mg/l; chloride, 120 mmol/l; and LDH, 63 U/l, without
the presence of atypical lymphocytes. Flow cytometry studies of the
CSF were not performed. Another bone marrow aspirate and biopsy
showed no evidence of apparent lymphoma cells. Based on the
clinical manifestations and a history of SPTCL, the patient was
diagnosed with SPTCL with CNS involvement and atypical MRI
features.
The patient was administered 6 cycles of a
fotemustine, teniposide and dexamethasone (FTD) regimen (100
mg/m2 fotemustine by 1-h infusion on day 1; 60
mg/m2 teniposide by >0.5-h infusion on days 2, 3 and
4; 18 mg/m2 dexamethasone by 1-h infusion on days 1, 2,
3, 4 and 5; and 12 mg methotrexate, 50 mg cytosine arabinoside plus
5 mg dexamethasone intrathecally on days 2 and 7) for 4 months from
October 2009 to February 2010. This resulted in complete resolution
of all neurological symptoms. Within 3 weeks of the initial
chemotherapy cycle, all the patient's symptoms had improved
significantly. During 78 months of follow-up, the patient
maintained a sustained remission status with no recurrence and
returned to work without any neurological disorders. However, 7
months after the completion of treatment, the patient presented
with bilateral osteonecrosis of the femoral heads, which was
suspected to have developed as a result of SPTCL treatment. The
patient subsequently underwent hip replacement surgery. Follow-up
is scheduled every 6 months for 10 years post-treatment. At
present, the overall survival time is 91 months and the patient is
not currently receiving any further treatment.
Discussion
The present study suggested that SPTCL patients who
receive a CHOP (−like) regimen as first-line treatment may
subsequently relapse or experience recurrence. The reason for this
finding is not yet clear. In the present case, although the patient
showed a CR to initial treatment, CNS relapse occurred 12 months
after the diagnosis, and within 6 months of the completion of
treatment without systemic disease. Therefore, it is important to
recognize that not all patients with α/β T-cell lymphomas have an
excellent prognosis. To the best of our knowledge, the present
study is the first report of SPTCL with CNS involvement with
long-term remission.
The reported incidence of CNS involvement in SPTCL
is extremely rare, as this lymphoma belongs to an indolent subtype
and frequently involves the subcutaneous tissues. A study by
Bernstein et al (9) of
patients with aggressive non-Hodgkin's lymphoma found that the
majority of cases of CNS relapse occurred during, or shortly after,
chemotherapy completion, indicating that such patients may already
possess subclinical CNS disease at the time of diagnosis, and a
small number of lymphoma cells may have existed in the CNS at
presentation. The risk factors for CNS involvement in SPTCL are not
well studied.
Although the systemic treatment of NHL has improved,
the development of an effective and tolerable treatment for CNS
disease is clinically difficult due to the poor penetration of the
drug into the CNS and its inability to cross the blood-brain
barrier. The relapse of systemic lymphoma within the CNS is
generally correlated with a poor prognosis, with few long-term
survivors following treatment with conventional therapy (10). Observations suggest that high-dose
methotrexate (HD-MTX) and whole-brain radiotherapy (WBRT) are
sufficient to treat CNS lymphoma. However, the optimal regimen for
HD-MTX has not been firmly defined (11). Despite the fact that the patients who
undergo high-dose chemotherapy and autologous stem cell transplant
have improved outcomes, the majority of patients with CNS relapse
of systemic lymphoma will not be candidates for such an aggressive
approach. So, it is highly likely that therapeutic outcomes have
now reached a plateau and that further innovations are urgently
required to facilitate treatment of CNS lymphomas, particularly for
the aging population, among whom a significant proportion cannot
tolerate high-dose chemotherapy and/or WBRT (12–14).
The success of treatment depends on effective
CNS-directed therapy. In the present study, an FTD regimen was
selected based on the pharmacokinetic properties of the drugs and
dose levels that were aimed at delivering effective chemotherapy to
the CNS. The patient received the FTD regimen and subsequently
achieved a CR, with long-term survival. A study by Wu et al
demonstrated that the FTD regimen can be effective in primary and
secondary CNS lymphomas (15).
Fotemustine is known to penetrate into the CNS, and has been
investigated previously in a number of brain tumours (16). Teniposide was included in this regimen
as it is more lipophilic to cross the blood-brain barrier and is
also eliminated at a slower rate (17). Glucocorticoids (prednisone and
dexamethasone) play an essential role in the treatment of systemic
lymphoma. Clinical studies have shown that dexamethasone has a
longer half-life and greater CNS penetration. However, the use of
glucocorticoid in CNS lymphoma is controversial due to
steroid-induced diagnostic delay. Even if CNS lymphoma is
confirmed, steroid use should be tapered off as quickly as possible
(11). Moreover, dexamethasone may
cause numerous adverse effects, including infection, bone fracture,
osteonecrosis, mood and behavioural problems, and myopathy
(18). The patient in the present
study developed bilateral osteonecrosis of the femoral heads due to
the use of dexamethasone. However, treatment with such
glucocorticoids can produce rapid symptomatic improvement (11); the FTD regimen has an acceptable
toxicity profile and could be the template for such a regimen.
Therefore, the relative efficacy of dexamethasone is dose-dependent
and must be carefully weighed against toxicity, and research is
required to optimize supportive care to prevent and manage
glucocorticoid toxicities. Intrathecal chemotherapy has been
historically used and was recommended for high-risk lymphoma
patients. However, Deng et al (19) reported that its effectiveness in
diffuse large B-cell lymphoma patients is uncertain.
In conclusion, involvement of the CNS in SPTCL is a
rare complication and is associated with a poor prognosis. To the
best of our knowledge, this is the first case report of secondary
CNS SPTCL with long-term remission. The study indicates that the
FTD regimen can be effective in SPTCL with CNS involvement.
References
|
1
|
Go RS and Wester SM: Immunophenotypic and
molecular features, clinical outcomes, treatments, and prognostic
factors associated with subcutaneous panniculitis-like T-cell
lymphoma: A systematic analysis of 156 patients reported in the
literature. Cancer. 101:1404–1413. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willemze R, Jansen PM, Cerroni L, Berti E,
Santucci M, Assaf C, Canninga-van Dijk MR, Carlotti A, Geerts ML,
Hahtola S, et al: Subcutaneous panniculitis-like T-cell lymphoma:
Definition, classification and prognostic factors: An EORTC
cutaneous lymphoma group study of 83 cases. Blood. 111:838–845.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parveen Z and Thompson K: Subcutaneous
panniculitis-like T-cell lymphoma: redefinition of diagnostic
criteria in the recent World Health Organization-European
Organization for Research and Treatment of Cancer classification
for cutaneous lymphomas. Arch Pathol Lab Med. 133:303–308.
2009.PubMed/NCBI
|
|
4
|
van Besien K, Ha CS, Murphy S, McLaughlin
P, Rodriguez A, Amin K, Forman A, Romaguera J, Hagemeister F,
Younes A, et al: Risk factors, treatment, and outcome of central
nervous system recurrence in adults with intermediate-grade and
immunoblastic lymphoma. Blood. 91:1178–1184. 1998.PubMed/NCBI
|
|
5
|
MacKintosh FR, Colby TV, Podolsky WJ,
Burke JS, Hoppe RT, Rosenfelt FP, Rosenberg SA and Kaplan HS:
Central nervous system involvement in non-Hodgkin's lymphoma: An
analysis of 105 cases. Cancer. 49:586–595. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colocci N, Glantz M and Recht L:
Prevention and treatment of central nervous system involvement by
non-Hodgkin's lymphoma: A review of the literature. Semin Neurol.
24:395–404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willemze R, Jaffe ES, Burg G, Cerroni L,
Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL,
Duncan LM, et al: WHO-EORTC classification for cutaneous lymphomas.
Blood. 105:3768–3785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim YH, Willemze R, Pimpinelli N,
Whittaker S, Olsen EA, Ranki A, Dummer R and Hoppe RT: ISCL and the
EORTC: TNM classification system for primary cutaneous lymphomas
other than mycosis fungoides and Sezary syndrome: A proposal of the
international society for cutaneous lymphomas (ISCL) and the
cutaneous lymphoma task force of the European organization of
research and treatment of cancer (EORTC). Blood. 110:479–484. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bernstein SH, Unger JM, Leblanc M,
Friedberg J, Miller TP and Fisher RI: Natural history of CNS
relapse in patients with aggressive non-Hodgkin's lymphoma: A
20-year follow-up analysis of SWOG 8516 - the Southwest Oncology
Group. J Clin Oncol. 27:114–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hollender A, Kvaloy S, Lote K, Nome O and
Holte H: Prognostic factors in 140 adult patients with
non-Hodgkin's lymphoma with systemic central nervous system (CNS)
involvement. A single centre analysis. Eur J Cancer. 36:1762–1768.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rubenstein JL, Gupta NK, Mannis GN,
Lamarre AK and Treseler P: How I treat CNS lymphomas. Blood.
122:2318–2330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kasenda B, Schorb E, Fritsch K, Finke J
and Illerhaus G: Prognosis after high-dose chemotherapy followed by
autologous stem-cell transplantation as first-line treatment in
primary CNS lymphoma - a long-term follow-up study. Ann Oncol.
26:608–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omuro A, Correa DD, DeAngelis LM,
Moskowitz CH, Matasar MJ, Kaley TJ, Gavrilovic IT, Nolan C,
Pentsova E, Grommes CC, et al: R-MPV followed by high-dose
chemotherapy with TBC and autologous stem-cell transplant for newly
diagnosed primary CNS lymphoma. Blood. 125:1403–1410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen YB, Batchelor T, Li S, Hochberg E,
Brezina M, Jones S, Del Rio C, Curtis M, Ballen KK, Barnes J, et
al: Phase 2 trial of high-dose rituximab with high-dose cytarabine
mobilization therapy and high-dose thiotepa, busulfan, and
cyclophosphamide autologous stem cell transplantation in patients
with central nervous system involvement by non-Hodgkin lymphoma.
Cancer. 121:226–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu JJ, Wang XH, Li L, Li X, Zhang L, Sun
ZC, Fu XR, Ma W, Chang Y, Zhang XD, et al: Fotemustine, teniposide
and dexamethasone in treating patients with CNS lymphoma. Asian Pac
J Cancer Prev. 15:4733–4738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vassal G, Boland I, Terrier-Lacombe MJ,
Watson AJ, Margison GP, Venuat AM, Morizet J, Parker F, Lacroix C,
Lellouch-Tubiana A, et al: Activity of fotemustine in
medulloblastoma and malignant glioma xenografts in relation to
O6-alkylguanine-DNA alkyltransferase and alkylpurine-DNA
N-glycosylase activity. Clin Cancer Res. 4:463–468. 1998.PubMed/NCBI
|
|
17
|
Muggia FM: Teniposide: Overview of its
therapeutic potential in adult cancers. Cancer Chemother Pharmacol.
34(Suppl): S127–S133. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inaba H and Pui CH: Glucocorticoid use in
acute lymphoblastic leukaemia. Lancet Oncol. 11:1096–1106. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng L, Song Y, Zhu J, Zheng W, Wang X,
Xie Y, Lin N, Tu M, Ping L, Ying Z, et al: Secondary central
nervous system involvement in 599 patients with diffuse large
B-cell lymphoma: Are there any changes in the rituximab era? Int J
Hematol. 98:664–671. 2013. View Article : Google Scholar : PubMed/NCBI
|