Introduction
Cancer cells can adopt two main migration
mechanisms, the mesenchymal movement and the amoeboid one, mainly
depending on cellular morphology, cytoskeletal organization,
cell-extracellular matrix (ECM) interactions and ECM degradation
(1,2).
The mesenchymal movement is generally used by cells of mesenchymal
origin with an elongated morphology and actin organized in stress
fibres, and requires the activation of the Rac signaling pathway
and ECM-degrading proteases such as matrix metalloproteinases
(MMPs) (1). Cells using the amoeboid
movement mostly have a round shape and cortical actin bundles. In
these cells, actomyosin contraction, which is driven by myosin
phosphorylation by Rho-associated kinase (ROCK), is the principal
propulsive force, which allows the cells to squeeze through the ECM
(3).
In tumor cells of epithelial origin, the shift to
the mesenchymal phenotype through the epithelial-mesenchymal
transition (EMT) process is associated with the acquisition of the
mesenchymal movement, together with high migration and metastatic
capacities (4). The EMT can be driven
by the expression of different transcription factors, including
Snail (which is encoded by the Snail gene) (5). Snail activation is associated with
E-cadherin downregulation, adherent junction destabilization,
cellular polarization and increased MMP expression (5).
By contrast, the adoption of the amoeboid movement
has been described in tumor cells of mesenchymal origin upon
inhibition of MMP activity or integrin function (6,7). In
addition, variations in p53, p27 or ephrin type-A receptor 2
expression have been reported to lead to a mesenchymal-amoeboid
transition (MAT) in sarcoma and melanoma cells (8–10).
The present authors previously reported the
occurrence of spontaneous MAT in human telomerase-immortalized
fibroblasts, named cen3tel, which underwent malignant
transformation during in vitro propagation (11). During the acquisition of the
tumorigenic phenotype, a transition from the typical elongated
shape of human fibroblasts with actin organized in fibres to a
roundish shape with cortical actin bundles was observed (11). In addition, the invasion of
tumorigenic cells relied on ROCK activity, as demonstrated by their
decreased invasion capacity upon treatment with a ROCK inhibitor,
but not after exposure to an MMP inhibitor (11). At the molecular level, MAT was
associated with a reduced expression of the ROCK-1 cellular
inhibitor Round (Rnd)3 (also known as RhoE) (11). Exogenous Rnd3 expression led to a
reduction in cells' in vitro invasion and in vivo
metastasis formation, indicating that Rnd3 levels participate in
controlling cellular invasiveness (11).
In the present study, cells undergoing MAT were also
demonstrated to be characterized by Snail downregulation,
and Snail exogenous expression induced a shift towards an
MMP-dependent migration mechanism, further indicating a role for
Snail in the modulation of neoplastic cells' movement.
Materials and methods
Cell lines, cell culture, transfection
and plasmids
The cen3tel cellular system has been previously
described (11,12). Briefly, it was derived from primary
cen3 fibroblasts by infection with a human telomerase reverse
transcriptase (hTERT)-containing retrovirus (13). Telomerase-expressing cells were
propagated in culture to population doubling (PD) ~1,000, and a
gradual acquisition of the neoplastic phenotype was observed
(11,12). In the present study, cen3tel cells at
five stages of propagation were used, from cells behaving as normal
fibroblasts (early cen3tel cells) to cells at the fifth stage,
which were tumorigenic and metastatic in nude mice (phase III
tumorigenic cells, PD ~1,000). Mid cen3tel cells represented cells
at the early phases of transformation, which were
anchorage-independent but not tumorigenic. Cells from tumorigenic
phases I–III induced tumors in mice with decreasing latency time
(11,12). Phase III tumorigenic cells were used
as recipient for the transfection with Snail-encoding plasmids.
Cells were grown and transfected as previously
described (11). Snail-encoding
plasmids, p green fluorescent protein (GFP)-Snail-wild-type (wt)
and pGFP-Snail-6SA, were obtained from Addgene Inc. (Cambridge, MA,
USA). The plasmid pGPF-Snail-6SA contains the complementary DNA
(cDNA) for a Snail protein in which the codons encoding for serine
97, 101, 108, 112, 116 and 120 were mutated to encode for an
alanine. These aminoacid changes make the protein more stable, thus
preventing its phosphorylation by glycogen synthase kinase 3 beta
and subsequent proteasomal degradation (14). Clones isolated after transfection with
an empty vector were named C1 and C2.
Motility and invasion assays
Cell invasiveness was assayed using modified Boyden
chambers (Neuro Probe, Inc., Gaithersburg, MD, USA) with
polycarbonate polyvinylpyrrolidone-free nuclepore filters (8-mm
pore size) as previously described (11). The ROCK inhibitor Y27632 (cat.
#688000; Calbiochem; Merck Millipore, Darmstadt, Germany) or the
MMP inhibitor Ro 28–2653 (kindly provided by Dr H. W. Krell; Roche
Diagnostics GmbH, Mannheim, Germany) were used in the invasion
assays to inhibit the ameboid or the mesenchymal movement,
respectively.
Western blot analysis
Whole-cell lysates were prepared using
co-immunoprecipitation (Co-IP) or Laemmli buffer as previously
described (11). The anti-Snail
antibody (dilution 1:500; clone H-130; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was used on extracts prepared with the CoIP
buffer. The anti-fibronectin antibody (dilution 1:5,000; clone
LN-6; Sigma-Aldrich, St. Louis, MO, USA) and the anti-vimentin
antibody (dilution 1:2,000; clone S-20; Santa Cruz Biotechnology,
Inc.) were used on extracts prepared with the Laemmli buffer.
Microarray analysis
All the details of the microarray analysis and the
results obtained are described in Ostano et al (12).
Animal models and ethics
statement
To investigate the tumorigenic potential of
Snail-expressing clones, 107 cells were injected
subcutaneously into severe combined immunodeficiency (SCID) mice
(Harlan Italy S.r.l., Milan, Italy). For the study, 7-week-old
female SCID mice were used, which were housed at 24°C in
individually ventilated cages with light/dark cycles of 12 h. Mice
were provided with sterile food and water ad libitum and
manipulated in aseptic conditions. After injection, mice were
monitored 2 or 3 times/week to assess tumor appearance and growth.
To investigate their metastatic ability, 2×106 cells
were injected intravenously in SCID mice. Mice were monitored daily
and sacrificed at the appearance of distress symptoms. Animals were
autopsied, and in order to evaluate metastatic foci, tissues were
collected and stored in Bouin's solution.
Procedures involving animals and their care were
conducted in conformity with the following laws, regulations and
policies governing the care and use of laboratory animals: Italian
Governing Law [D.lgs 26/2014, authorisation no. 19/2008-A, issued
on March 6, 2008 by the Ministry of Health of Italy (Rome, Italy)];
Mario Negri Institute for Pharmacological Research (Milan, Italy)
Institutional Regulations and Policies providing internal
authorisation for persons conducting animal experiments (Quality
Management System Certificate-UNI EN ISO 9001:2008-Reg. No 8576-A);
National Institutes of Health Guide for the Care and Use of
Laboratory Animals (2011 edition); European Union directives and
guidelines (EEC Council Directive 2010/63/UE); and the Guidelines
for the Welfare and Use of Animals in Cancer Research (15).
The animal experiments conducted in the present
study have been reviewed and approved by the Animal Care and Use
Committee of the Mario Negri Institute for Pharmacological
Research, which includes members ad hoc for ethical issues
(approval ID Frap1). Animals were housed in the animal care
facilities of the Mario Negri Institute for Pharmacological
Research, which meet international standards and are regularly
checked by a certified veterinarian who is responsible for health
monitoring, animal welfare supervision, and experimental protocols
and procedures revision.
Results
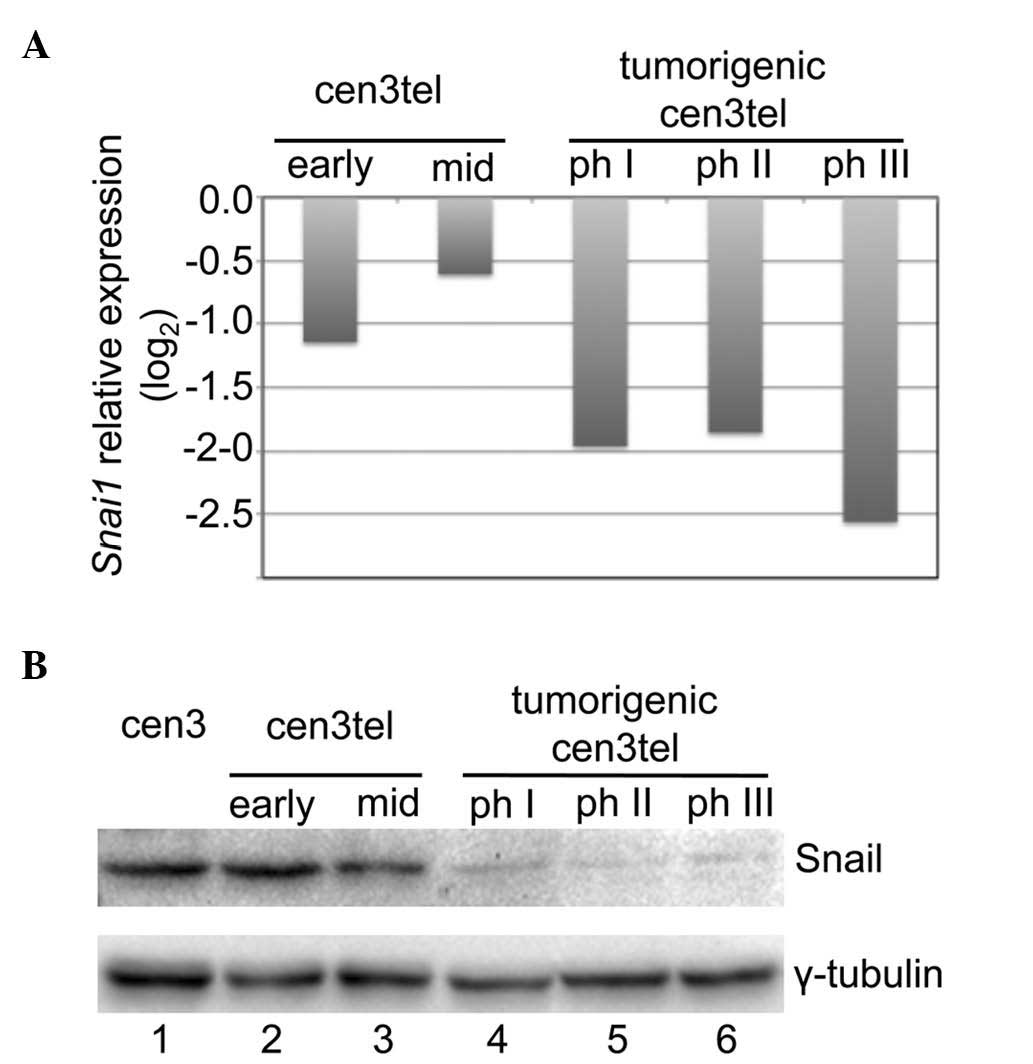
Microarray analysis on cen3tel cells representing
five different phases of the malignant transformation process
exhibited a decrease in Snail expression in cen3tel
tumorigenic cells compared with early and mid cen3tel cells
(Fig. 1A). The decreased Snail
expression with the transformation process was confirmed at the
protein level by western blotting (Fig.
1B).
To test whether Snail decreased expression could
play a role in modulating the switch from the mesenchymal movement
to the amoeboid motility observed in cen3tel tumorigenic cells,
Snail was exogenously expressed in phase III tumorigenic cells. For
that purpose, cen3tel cells PD ~1,000 were transfected either with
a plasmid containing the cDNA for the human wt Snail
(pGFP-Snail-wt), or a plasmid containing the cDNA for a mutated
Snail isoform (pGFP-Snail-6SA), which is more stable. Transfected
cells were selected using G418 (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA); resistant clones were isolated and the
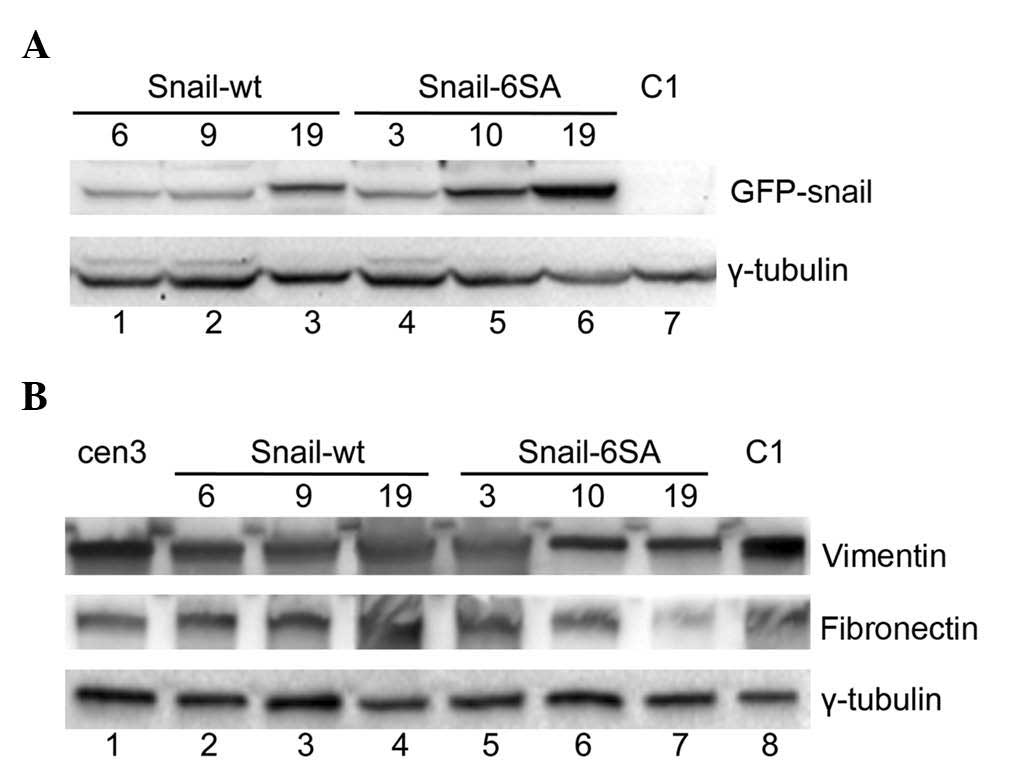
expression of the GFP-Snail protein was confirmed by fluorescence
microscopy (data not shown). Three clones expressing the wt protein
(named Snail-wt 6, 9 and 19), and three clones expressing the
modified protein (named Snail-6SA 3, 10 and 19) were selected for
further investigations. By western blotting with an anti-Snail
antibody, Snail expression was analyzed in the clones. As indicated
in Fig. 2A, no signal corresponding
to the recombinant GFP-Snail protein was observed in the
mock-transfected C1 clone (lane 7), as expected, while bands of
variable intensities were observed in all other clones. In
particular, the highest levels of Snail expression were observed in
clones Snail-6SA 10 and 19 (lanes 5 and 6) and in clone Snail-wt 19
(lane 3).
In epithelial tumors undergoing EMT, Snail
expression is associated with an increased expression of
mesenchymal markers, including vimentin and fibronectin (5). In cen3tel cells, exogenous Snail
expression did not positively control the levels of either vimentin
or fibronectin (Fig. 2B).
Furthermore, it did not induce a change in the organization of the
actin cytoskeleton, with actin organized in cortical rings in the
clones as in the parental and mock-transfected cells (data not
shown).
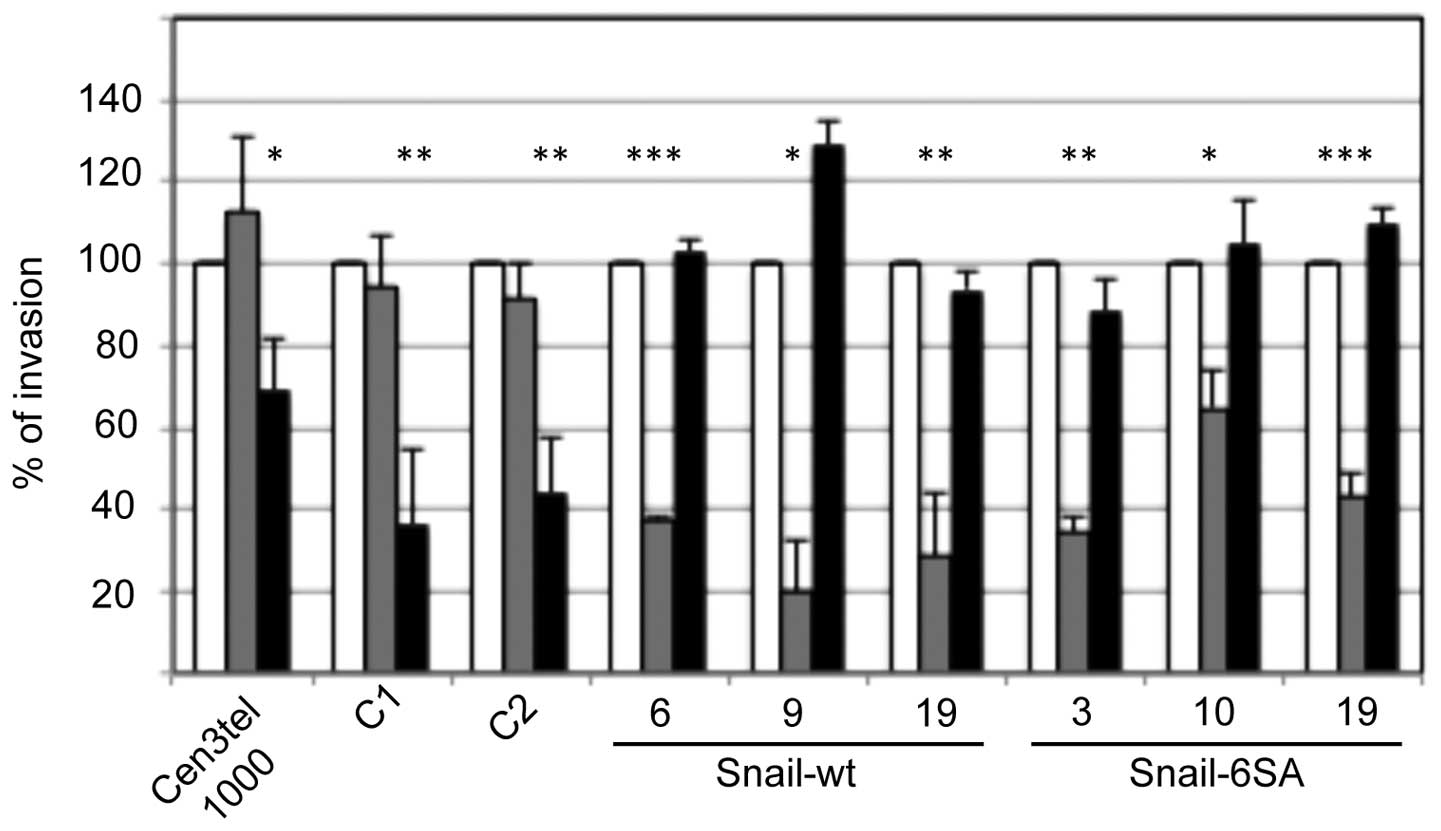
Whether Snail expression could play a role in
determining the type of movement adopted by cen3tel cells was next
tested by analyzing the invasion capacity of the Snail-expressing
clones in the presence of the ROCK inhibitor Y27632 or the MMP
inhibitor Ro 28–2653. As shown in Fig.
3, the two inhibitors had an opposite effect on
Snail-expressing clones compared with parental and mock-transfected
cells. In fact, while in control and mock-transfected clones (C1
and C2), invasiveness was decreased by Y27632 but was not affected
by Ro 28–2653, as expected, in all Snail-expressing clones,
invasiveness was clearly decreased upon exposure to Ro 28–2653 but
was not reduced by Y27632, indicating a switch from a
ROCK-dependent movement to a protease-dependent motility.
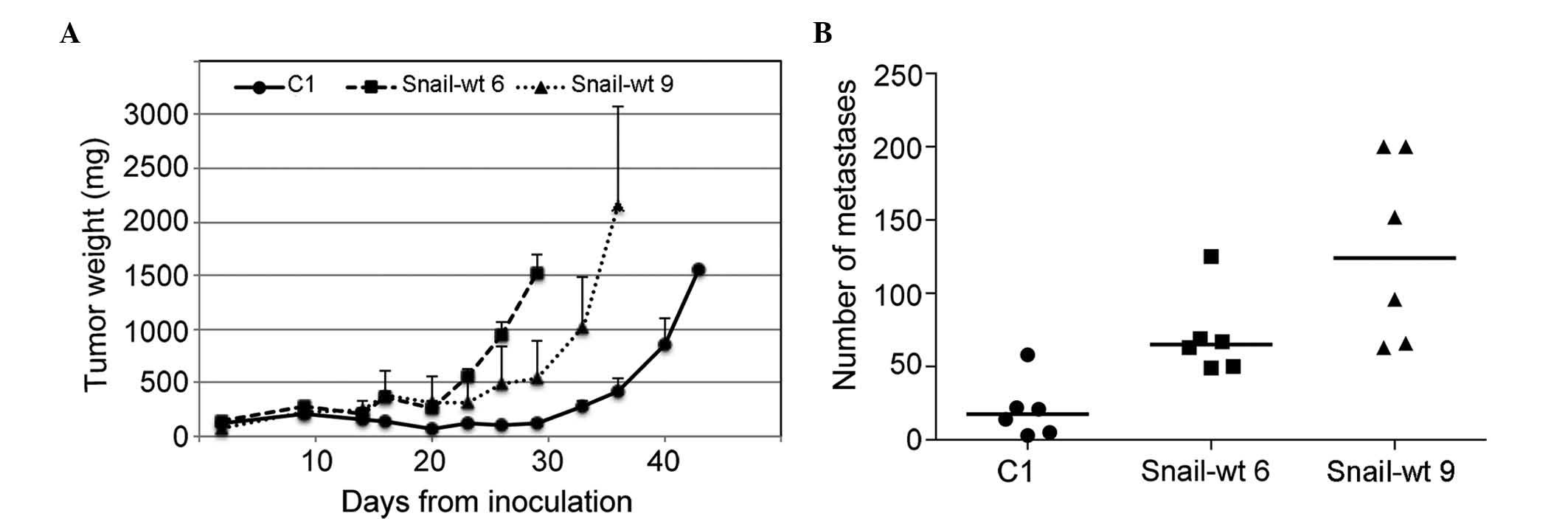
Finally, the in vivo tumorigenicity and
metastatic potential of mock-transfected cells and two Snail
clones, Snail-wt 6 and 9, were tested by inoculating cells either
subcutaneously or into the tail vein of SCID mice. It was observed
that both Snail clones had a faster rate of tumor growth than
control cells (Fig. 4A), and
generated a greater number of lung metastases compared with
mock-transfected cells (Fig. 4B). The
size of the metastases was not increased in Snail-expressing
clones, with 45% of metastases being >1 mm in mock-transfected
cells, compared with only 25 and 14% in the Snail-wt 6 and 9
clones, respectively.
Discussion
Cancer cells' capacity to transition from a type of
movement to another is a large problem in metastasis fighting
(1). In the present study, it has
been demonstrated that human fibroblasts undergoing malignant
transformation can adopt the amoeboid movement and switch back to
an MMP-dependent movement after the induction of Snail
expression.
In the cen3tel cellular system, a reduction in Snail
expression was observed during in vitro propagation. In
cells at the initial phases after hTERT immortalization, Snail
levels were similar to those observed in primary fibroblasts.
However, when cells became tumorigenic, Snail was downregulated.
Despite the low Snail levels, tumorigenic cells neither displayed
variations in the expression of mesenchymal markers, including
vimentin and fibronectin [lanes 1 and 9 in Fig. 2B and Belgiovine et al (16)], nor started expressing epithelial
markers such as cytokeratins (16).
In the clones expressing either the wt or the mutated Snail
protein, vimentin and fibronectin were expressed at variable
levels, but not at levels higher than those in the control cells,
indicating that, in cells of mesenchymal origin, the expression of
these proteins does not depend on Snail. This supports the
hypothesis that Snail is important for the induction of the
mesenchymal phenotype, but not for its maintenance (17).
By contrast, Snail levels appeared to be important
for the determination of the type of movement used by cancer cells.
In fact, exogenous Snail expression in tumorigenic cells induced a
switch from a ROCK-dependent movement to a protease-dependent one.
In the present study, no differences were observed among clones
depending on the levels of Snail or the type of exogenous protein
expressed. Exogenous Snail could promote the mesenchymal movement
by positively regulating MMP expression and by stimulating
invadopodia formation and local matrix degradation through Twist
induction (18–20).
In Snail-expressing clones, invasion was insensitive
to the ROCK inhibitor Y27632, suggesting that the mesenchymal
movement prevails over the amoeboid one. During the transformation
process, Snail downregulation may be a pre-requisite for the
establishment of the amoeboid movement, which could be induced by
low Rnd3 levels and consequent high ROCK activity. This hypothesis
is in agreement with the data by Taddei et al (21), indicating that MAT can be engaged by
melanoma cells only if EMT is partially suppressed. In tumorigenic
cen3tel cells, the mesenchymal phenotype could be partially
suppressed upon Snail downregulation. Indeed, in tumorigenic cells,
the loss of both the elongated mesenchymal shape and the actin
organization in stress fibres was observed.
When injected into the tail vein of
immunocompromised mice, Snail-expressing clones exhibited a
significantly greater capacity to form lung metastasis than control
cells, with an increase in the number, but not in the size, of
metastases, suggesting that the protease-dependent movement makes
cells more efficient in extravasation than parental cells, which
rely on the amoeboid movement. Although it cannot be excluded that
the higher growth rate observed in the Snail-expressing tumors
compared with that of the tumours induced by mock-transfected cells
could facilitate lung colonization, and thus contribute to the
higher frequency of metastasis formation, the present results
suggest a more aggressive behavior of Snail-expressing cells.
In conclusion, the present results confirm the role
of Snail in directing the mesenchymal movement and the
aggressive/metastatic behavior of mesenchymal tumor cells.
Furthermore, the present findings highlight the plasticity of tumor
cells in adapting their movement in response to changes in gene
expression.
Glossary
Abbreviations
Abbreviations:
|
MMP
|
matrix metalloproteinase
|
|
ROCK
|
Rho-associated kinase
|
|
EMT
|
epithelial-mesenchymal transition
|
|
MAT
|
mesenchymal-amoeboid transition
|
|
Rnd
|
Round
|
|
PD
|
population density.
|
References
|
1
|
Sahai E: Mechanisms of cancer cell
invasion. Curr Opin Genet Dev. 15:87–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedl P and Wolf K: Plasticity of cell
migration: A multiscale tuning model. J Cell Biol. 188:11–19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rath N and Olson MF: Rho-associated
kinases in tumorigenesis: Re-considering ROCK inhibition for cancer
therapy. EMBO Rep. 13:900–908. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wolf K, Mazo I, Leung H, Engelke K, von
Andrian UH, Deryugina EI, Strongin AY, Bröcker EB and Friedl P:
Compensation mechanism in tumor cell migration:
Mesenchymal-amoeboid transition after blocking of pericellular
proteolysis. J Cell Biol. 160:267–277. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carragher NO, Walker SM, Scott Carragher
LA, Harris F, Sawyer TK, Brunton VG, Ozanne BW and Frame MC:
Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid
modes of tumour cell invasion: A link to integrin function.
Oncogene. 25:5726–5740. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gadea G, de Toledo M, Anguille C and Roux
P: Loss of p53 promotes RhoA-ROCK-dependent cell migration and
invasion in 3D matrices. J Cell Biol. 178:23–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berton S, Belletti B, Wolf K, Canzonieri
V, Lovat F, Vecchione A, Colombatti A, Friedl P and Baldassarre G:
The tumor suppressor functions of p27(kip1) include control of
mesenchymal/amoeboid transition. Mol Cell Biol. 29:5031–5045. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parri M, Taddei ML, Bianchini F, Calorini
L and Chiarugi P: EphA2 reexpression prompts invasion of melanoma
cells shifting from mesenchymal to amoeboid-like motility style.
Cancer Res. 69:2072–2081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belgiovine C, Frapolli R, Bonezzi K,
Chiodi I, Favero F, Mello-Grand M, Dei Tos AP, Giulotto E,
Taraboletti G, D'Incalci M and Mondello C: Reduced expression of
the ROCK inhibitor Rnd3 is associated with increased invasiveness
and metastatic potential in mesenchymal tumor cells. PLoS One.
5:e141542010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ostano P, Bione S, Belgiovine C, Chiodi I,
Ghimenti C, Scovassi AI, Chiorino G and Mondello C: Cross-analysis
of gene and miRNA genome-wide expression profiles in human
fibroblasts at different stages of transformation. OMICS. 16:24–36.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mondello C, Chiesa M, Rebuzzini P, Zongaro
S, Verri A, Colombo T, Giulotto E, D'incalci M, Franceschi C and
Nuzzo F: Karyotype instability and anchorage-independent growth in
telomerase-immortalized fibroblasts from two centenarian
individuals. Biochem Biophys Res Commun. 308:914–921. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al: Committee of the National Cancer Research
Institute: Guidelines for the welfare and use of animals in cancer
research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Belgiovine C, Chiodi I and Mondello C:
Relocalization of cell adhesion molecules during neoplastic
transformation of human fibroblasts. Int J Oncol. 39:1199–1204.
2011.PubMed/NCBI
|
|
17
|
Rowe RG, Li XY, Hu Y, Saunders TL,
Virtanen I, de Garcia Herreros A, Becker KF, Ingvarsen S, Engelholm
LH, Bommer GT, et al: Mesenchymal cells reactivate Snail1
expression to drive three-dimensional invasion programs. J Cell
Biol. 184:399–408. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miyoshi A, Kitajima Y, Kido S, Shimonishi
T, Matsuyama S, Kitahara K and Miyazaki K: Snail accelerates cancer
invasion by upregulating MMP expression and is associated with poor
prognosis of hepatocellular carcinoma. Br J Cancer. 92:252–258.
2005.PubMed/NCBI
|
|
19
|
Ota I, Li XY, Hu Y and Weiss SJ: Induction
of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration
program in cancer cells by Snail1. Proc Natl Acad Sci USA.
106:20318–20323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eckert MA, Lwin TM, Chang AT, Kim J, Danis
E, Ohno-Machado L and Yang J: Twist1-induced invadopodia formation
promotes tumor metastasis. Cancer Cell. 19:372–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taddei ML, Giannoni E, Morandi A, Ippolito
L, Ramazzotti M, Callari M, Gandellini P and Chiarugi P:
Mesenchymal to amoeboid transition is associated with stem-like
features of melanoma cells. Cell Commun Signal. 12:242014.
View Article : Google Scholar : PubMed/NCBI
|