Introduction
Endometrial cancer is the most common invasive
gynaecological malignancy in developed countries, with >280,000
cases annually (1). There is no
effective screening test for early detection and, furthermore, a
lack of curative therapies. Alarmingly, the incidence is
increasing, particularly in women of reproductive age (2). Mortality is primarily associated with
advanced or recurrent disease. Although currently available radio-,
brachy- or chemotherapies may achieve a transient treatment
response, the median survival time for these women is <1 year
(3).
Type I ‘endometrioid’ carcinoma is the most common
type of endometrial cancer, accounting for ~85% of cases (4). It is often preceded by endometrial
hyperplasia, a proliferative process within the endometrial glands
that leads to an increase in the glandular-stromal ratio. This
process is commonly associated with unopposed estrogen stimulation
and may also be due to specific genetic alterations (4). Type I endometrial tumours are staged
according to the guidelines of the International Federation of
Gynaecology and Obstetrics (FIGO) (5). Tumour grade (G) is based histologically
on the extent to which the cancer forms glands that display similar
morphology to normal endometrium, and also on metastatic behavior:
the extent to which the cancer invades the uterine corpus and the
surrounding peritoneum (4). While it
is considered that endocrine, genetic and inflammatory factors
contribute to its initiation and progression (4), the precise etiology and molecular basis
of endometrial cancer are poorly understood. Once the critical
regulators are discovered, targeted and more effective treatment
options may be developed.
Fibulin-5, also known as DANCE [developmental
arteries and neural crest epidermal growth factor (EGF)-like] or
EVEC (embryonic vascular EGF-like repeat-containing), is a member
of the fibulin family, which is characterised by calcium-binding
EGF-like repeats and a globular carboxyl-terminal fibulin-type
structure (6). The fibulin family
comprises six family members (fibulins 1–6), which are commonly
expressed extracellular matrix (ECM) proteins localised to the
basement membrane, stroma and ECM fibers (7). Functionally, fibulin-5 regulates
cell-to-cell and cell-to-matrix communication; it also alters ECM
structure and functions in tumourigenesis (6,8). Tumour
progression and metastases can depend largely on varying extents of
cancer cell proliferative, invasive and/or migratory phenotypes,
which also involves the adhesion and de-adhesion of cells.
Fibulin-5 inhibits cancer cell proliferation and
invasion in several tumour types (9)
and its expression is frequently reduced in a number of types of
human cancer, including hepatocellular (10), breast (9), ovarian (9), colon (9),
prostate (11), bladder (12) and lung (13) cancers, suggesting a potential tumour
suppressor role. However, certain studies have demonstrated a
pro-tumourigenic role: Fibulin-5 has been shown to enhance the
malignancy of human fibrosarcoma cells (9) and mammary epithelial cells, where it
initiates the epithelial-mesenchymal transition (EMT) (14). Therefore, the precise function of
fibulin-5 in tumourigenesis differs between different cancer
types.
The role of fibulin-5 in human endometrial cancer
has never been investigated. We hypothesised that fibulin-5
expression may be reduced in human endometrial cancer, as in other
epithelial malignancies. The present study aimed to determine the
expression and localisation of fibulin-5 in human endometrial
cancer across G1-3 tumours. Furthermore, the effect of fibulin-5
transient knockdown on Ishikawa endometrial epithelial cancer cell
adhesion, proliferation, invasion and migration was determined
using the xCELLigence real-time system in vitro.
Materials and methods
Participants and patient samples
Endometrial cancer tissue biopsies (n=10 per grade)
were collected from postmenopausal women undergoing total abdominal
hysterectomy for endometrial carcinoma at the Monash Medical Centre
(Melbourne, Australia) (Table I). The
Human Ethics Committee approved the research project and informed
consent was obtained from each patient. Tumours were graded
histologically by a specialist gynaecological pathologist according
to the guidelines of FIGO (2009), as described previously (15). Proliferative-phase endometrium (n=10)
was collected at curettage from women between days 7 and 13 of
their menstrual cycle who were scheduled for tubal ligation as a
non-tumour control group. A pathologist declared no obvious
endometrial pathology. Women had no steroid treatment or other
medication for ≥2 months prior to tissue collection. Written
informed consent was obtained from each patient and the study was
approved by the Southern Health Human Research and Ethics
committee. Biopsies were fixed in 4% neutral-buffered formalin
overnight prior to paraffin embedding.
 | Table I.Clinical characteristics of the
included patients (n=30). |
Table I.
Clinical characteristics of the
included patients (n=30).
| Patient no. | Age, years | Menopausal
status | Cancer grade | % MI |
|---|
| 1 | 65 | Post | 1 | 0 |
| 2 | 56 | Post | 1 | 29 |
| 3 | 84 | Post | 1 | 80 |
| 4 | 34 | UK | 1 | 0 |
| 5 | 78 | Post | 1 | 4 |
| 6 | 55 | Post | 1 | 0 |
| 7 | 51 | UK | 1 | 0 |
| 8 | 77 | Post | 1 | 21 |
| 9 | 48 | UK | 1 | 0 |
| 10 | 78 | Post | 1 | 12 |
| 11 | 73 | Post | 2 | 38 |
| 12 | 52 | UK | 2 | UK |
| 13 | 60 | Post | 2 | 18 |
| 14 | 88 | Post | 2 | 73 |
| 15 | 63 | Post | 2 | 100 |
| 16 | 54 | UK | 2 | 38 |
| 17 | 61 | Post | 2 | UK |
| 18 | 60 | Post | 2 | 27 |
| 19 | 75 | Post | 2 | 73 |
| 20 | 71 | Post | 2 | 49 |
| 21 | 54 | Post | 3 | 38 |
| 22 | 59 | Post | 3 | 33 |
| 23 | 77 | Post | 3 | 25 |
| 24 | 59 | UK | 3 | UK |
| 25 | 68 | Post | 3 | 13 |
| 26 | 55 | UK | 3 | 81 |
| 27 | 64 | Post | 3 | 49 |
| 28 | 62 | Post | 3 | 52 |
| 29 | 71 | Post | 3 | 19 |
| 30 | 66 | Post | 3 | UK |
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
RNA from endometrial cancer or benign endometrium
whole tissue was obtained from the Victorian Cancer Biobank
(Melbourne, Australia). To assess the RNA yield, purity and
concentration, 2 µl was analysed using a Nanodrop spectrophotometer
(ND-1000; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
RT-qPCR was performed as previously described (16). Total RNA (250 ng) was reverse
transcribed using Superscript III RNA polymerase, random primers,
RNase inhibitor (RNaseOUT), deoxynucleoside triphosphates and
First-Strand buffer (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol and amplified by PCR.
RT-qPCR analyses were performed on the Applied Biosystems 7500HT
Fast Block Real Time PCR system (Thermo Fisher Scientific, Inc.) in
triplicate (final reaction volume, 10 µl) in 384-well Micro Optical
plates with the 2X Fast-Start SYBR Green Master Mix containing ROX
passive reference dye (Roche Diagnostics, Indianapolis, IN, USA)
and 400 nM primers. The primer sequences were as follows: Fibulin-5
forward, 5′-AGCAGGATCGAAGGGTTTTT-′3; fibulin-5 reverse,
5′-TGGGTTTGGGAAGACAGAAC-′3; 18s forward,
5′-GATCCATTGGAGGGCAAGTCT-′3; and 18s reverse,
5′-CCAAGATCCAACTACGAGCTT-′3 (Sigma-Aldrich, Castle Hill,
Australia). The PCR cycling conditions were as follows: 95°C for 10
min; followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Relative expression levels were calculated by the comparative
quantification cycle (ΔΔCq) method (17), with 18s ribosomal RNA serving as the
endogenous control for normalisation. Products were sequenced to
confirm specificity.
Immunohistochemistry
Formalin-fixed human endometrial cancer or
proliferative-phase endometrial sections (4 µm) were dewaxed in
Histosol (Chem-Supply, Gillman, Australia; 2×10 min) and rehydrated
in ethanol, and antigen retrieval was performed in 0.01 M sodium
citrate (pH 6) prior to quenching of endogenous peroxidase activity
with 3% hydrogen peroxide in methanol for 10 min. Non-specific
binding was blocked with 10% normal goat serum and 2% normal human
serum (Sigma-Aldrich, St. Louis, MO, USA), in Tris-buffered saline
for 30 min. A polyclonal rabbit anti-human fibulin-5 antibody
(#HPA000848; Sigma-Aldrich; dilution, 1:100) was applied overnight
at 4°C. Negative control isotype rabbit IgG (Dako, Glostrup,
Denmark; 0.19 µg/ml) was included for every tissue section.
Antibody localisation was detected by sequential application of
biotinylated goat anti-rabbit IgG (#BA-1000; Vector Laboratories,
Inc., Burlingame, CA, USA; dilution, 1:200) for 30 min, followed by
the Vectastain Elite ABC kit (#PK-6100; Vector Laboratories, Inc.)
for 30 min. Peroxidase activity was visualised by the application
of DakoCytomation diaminobenzidine substrate (Dako). Tissues were
counterstained with Harris hematoxylin (Sigma-Aldrich) and mounted.
Staining intensities in the epithelial and stromal compartments
were scored from 0 (no staining) to 3 (intense staining) by two
independent, blinded assessors.
PCR and gel electrophoresis
Total RNA from human endometrial cancer cells
[Ishikawa (provided by Dr M. Nishida, Tsukuba University, Tochigi,
Japan), HEC1A and AN3CA cells (ATCC, Manassas, VA, USA),
representative of human endometrial cancer G1-3, respectively] and
primary human proliferative-phase endometrial epithelial cells
(which were isolated as described previously) (16), was isolated using the TRI Reagent RNA
isolation system (Sigma-Aldrich) following the manufacturer's
protocol. All samples were treated with DNase I (Ambion DNAfree;
Thermo Fisher Scientific, Inc.) and concentrations were quantified
using the NanoDrop 1000. Total RNA (250 ng) was reverse transcribed
using the Superscript III First-Strand Synthesis System
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. PCR was performed using a Veriti Thermal
Cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
GoTaq Green Master Mix 2X (Promega Corporation, Madison, WI, USA)
according to the manufacturer's instructions. cDNA was analysed for
fibulin-5 and 18s (sequences as described for RT-qPCR) using
reaction conditions as follows: Initial denaturation at 95°C for 3
mins; 30 cycles of denaturation at 95°C for 30 sec, annealing at
60°C for 30 sec and extension at 72°C for 1 min; and a final
extension at 72°C for 10 min. The PCR products were run on a 1.5%
agarose gel with an Invitrogen 1,000-bp DNA ladder (Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) to determine gene
expression.
Cell culture and cytokine treatments
in Ishikawa cells
Ishikawa cells were kindly provided by Dr M. Nishida
(Tsukuba University, Tochigi, Japan) and cultured in Dulbecco's
modified Eagle's medium (DMEM) with 10% foetal calf serum (FCS).
Confluent cells were serum starved for 24 h and treated with
recombinant human transforming growth factor-β (TGF-β) (#240-B-010;
R&D Systems, Inc., Minneapolis, MN, USA; 1 or 10 ng/ml) or
phosphate-buffered saline control. To assess the potential of TGF-β
to induce fibulin-5 mRNA expression, cells were collected after 6 h
of treatment, RNA was extracted and RT-qPCR analysis was performed
as described. To determine the downstream intracellular target of
fibulin-5, confluent cells were serum starved for 24 h prior to
treatment with 0.1 or 0.5 µg/ml recombinant human fibulin-5
(#3095-FB-025; R&D Systems, Inc.) (based on pilot studies) for
10 min. Cell lysates were collected using lysis buffer [50 mM
Tris-base, 150 mM NaCl, 2 mM ethylenediaminetetraacetic acid, 2 mM
ethylene glycol tetraacetic acid, 25 mM NaF and 25 mM
β-glycerolphosphate (pH 7.5)] containing protease inhibitor (2
µl/ml) (Thermo Fisher Scientific, Inc.) for western blot.
Small interfering RNA (siRNA)
transfection of Ishikawa cells
Ishikawa cells were cultured to 70% confluence and
transfected with commercially generated and validated ON-TARGETplus
SMARTpool siRNA (GE Healthcare Dharmacon, Inc., Lafayette, CO, USA)
that targeted either fibulin-5 (FBN5 siRNA) or no specific sequence
as a scrambled (Scr) control. Delivery was performed using
Invitrogen Lipofectamine RNAiMAX Transfection Reagent (Thermo
Fisher Scientific, Inc.) according to manufacturer's instructions.
Cells were transfected for 72 h prior to RNA collection to test for
transfection efficiency or prior to beginning the functional
experiments, as described previously (18).
xCELLigence real-time cell functional
studies in Ishikawa cells
Experiments were conducted using the xCELLigence
Real-Time Cell Analysis (RTCA) DP instrument (Roche Applied
Science; ACEA Biosciences, Inc., San Diego, CA, USA), which was
placed in a humidified incubator maintained at 37°C with 95% air/5%
CO2. For adhesion and proliferation, cells were seeded
in an E-plate 96 at 10,000 cells/well in 5% FCS DMEM, and the plate
was monitored once every 10 min for 6 h (for adhesion), then once
every hour for a total of 72 h (for proliferation). Cell migration
and invasion were assessed using CIM-plate 16 (ACEA Biosciences,
Inc.) with 8 mm pores. To measure cell invasion, wells were coated
on the upper surface of the transwell with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA; dilution, 1:10). To measure
migration, the same protocol was used on an uncoated plate. Cells
were seeded into the upper chamber at 10,000/well in 5% FCS DMEM
medium, and 10% FCS DMEM medium was added to the lower chamber. The
CIM-plate 16 was monitored every 30 min for 72 h total (18). Data was calculated using RTCA software
version 1.2, supplied with the instrument (ACEA Biosciences, Inc.),
and exported for statistical analysis.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blot analysis
SDS-PAGE and western blot analysis to detect
phosphorylated (p) and total extracellular signal-regulated kinases
(ERK) 1/2 were performed as previously described (19), using monoclonal rabbit IgG antibodies
against human phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (#4370)
and p44/42 MAPK (Erk1/2) (#4695), both purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA)].
Statistical analysis
Statistical analysis was conducted using GraphPad
Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA,
USA) and data assessed by Student's t-test for two groups. Multiple
groups were compared using a one-way analysis of variance with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Fibulin-5 mRNA and protein are
downregulated in human endometrial cancer
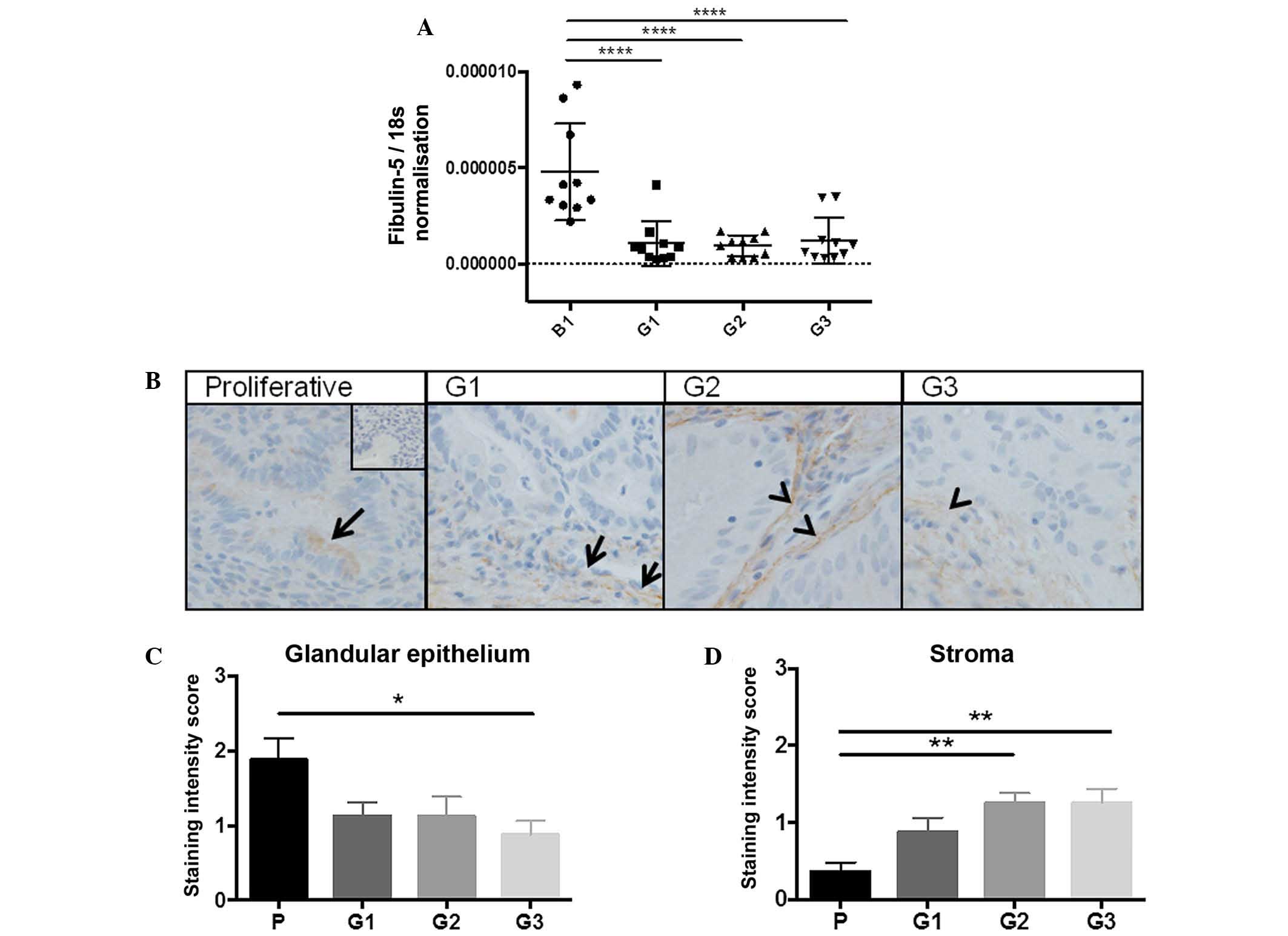
RT-qPCR was performed to measure fibulin-5 mRNA
levels in whole tissue from G1-3 endometrial tumours compared with
benign endometrium. When fibulin-5 expression was normalised to
18s, a significant reduction in gene expression was observed across
all endometrial cancer grades compared to the benign group (all
P<0.0001; n=10/group) (Fig. 1A).
Tumour tissues are heterogeneous and gene expression data does not
provide information on which cell types produce fibulin-5.
Therefore, fibulin-5 was immunolocalised in G1-3 endometrial tumour
or proliferative-phase endometrial tissues. Fibulin-5 localised to
the glandular epithelium in proliferative-phase endometrium, with
little to no staining in the stroma (Fig.
1B). In G1 well-differentiated tumour tissues, fibulin-5
localised to the tumour epithelial and stromal cells, although
staining was sporadic throughout the tissue. By comparison in G2
moderately differentiated and G3 poorly differentiated tumours,
fibulin-5 almost exclusively localised to the tumour stroma, with
some weak staining in the epithelial compartment. When staining
intensity was scored, there was a significant reduction in
epithelial staining between proliferative endometrium and G3
tumours (P=0.0121; n=10/group) (Fig.
1C) and a significant increase in staining in the tumour stroma
in G2 and G3 compared to proliferative phase endometrium (P=0.0010
and P=0.0060, respectively; n=10/group) (Fig. 1D).
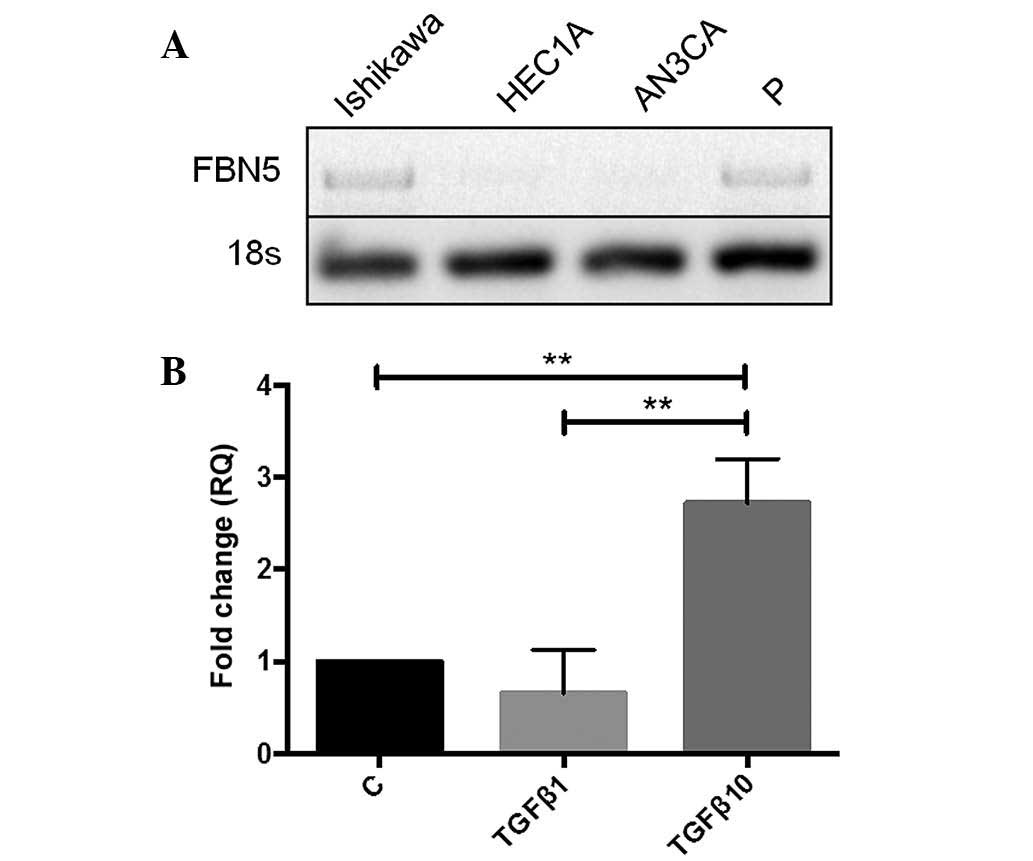
Fibulin-5 mRNA is downregulated in
endometrial cancer cell lines with increasing grade
To perform functional studies, the gene expression
of fibulin-5 in human endometrial cancer cell lines was determined.
G1-derived Ishikawa cells and proliferative-phase endometrial
epithelial cells expressed fibulin-5, whilst G2-derived HEC1A and
G3-derived AN3CA cells did not (Fig.
2A). Given this, Ishikawa cells were used for all functional
studies.
TGF-β induces fibulin-5 expression in
Ishikawa endometrial epithelial cancer cells
To investigate the regulation of fibulin-5 in human
endometrial epithelial cancer cells, Ishikawa cells were treated
with TGF-β, which is associated with EMT and known to regulate
fibulin-5 in other tumour types (14). A significant 2.8-fold increase in
fibulin-5 gene expression following treatment with 10 ng/ml TGF-β
was observed compared to the control and the lower concentration of
TGF-β (1 ng/ml) after 6 h (P=0.0062 and P=0.0074, respectively;
n=3/group) (Fig. 2B).
Fibulin-5 siRNA knockdown increases
Ishikawa cell adhesion and proliferation
Ishikawa cells were the only cell line tested that
expressed fibulin-5. To mimic the loss of fibulin-5 gene expression
observed in endometrial cancer in women, fibulin-5 gene expression
was transiently silenced using siRNA. This led to a significant 77%
reduction in transcript levels in FBN5 siRNA-treated Ishikawa cells
compared to the Scr control (P=0.0037; n=3/group) (Fig. 3A). Subsequently, the adhesion,
proliferation, invasion and migration functions of Scr- or FBN5
siRNA-treated Ishikawa cells were tested on the xCELLigence RTCA
system. At 6 h, FBN5 siRNA-treated Ishikawa cells adhered
significantly more than the Scr control group (P=0.0475; n=3/group)
(Fig. 3B), and by 72 h, there was a
significant increase in FBN5 siRNA-treated Ishikawa cell
proliferation vs. the Scr control (P=0.0384; n=3/group) (Fig. 3C). At 72 h there was an increasing
trend in both invasion and migration in FBN5 siRNA-treated cells
compared to the control group (P=0.0936 and P=0.1205, respectively)
(Fig. 3D and E).
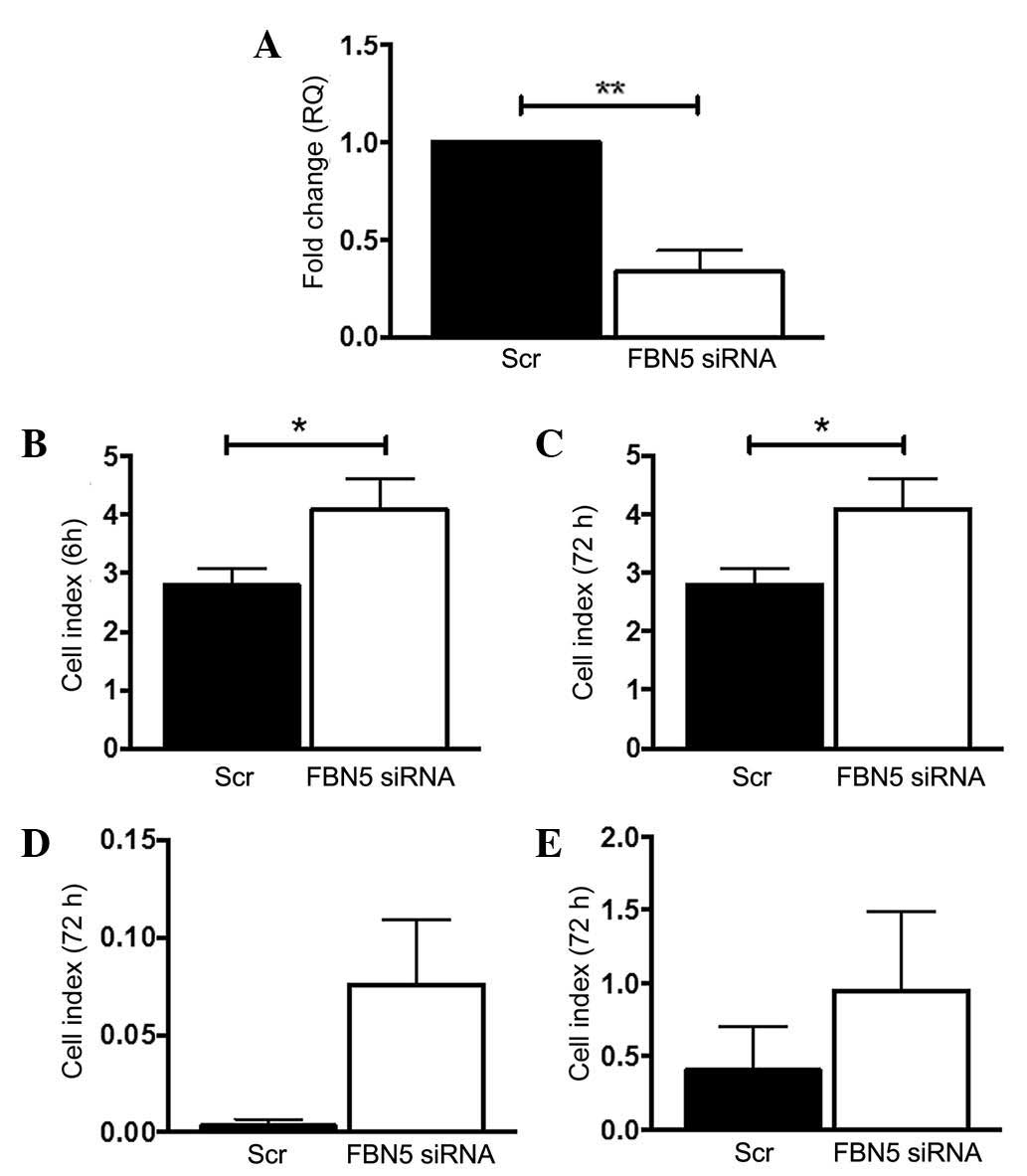 | Figure 3.Fibulin-5 silencing by siRNA alters
endometrial cancer cell function. (A) Knockdown of fibulin-5 was
confirmed by reverse transcription-quantitative polymerase chain
reaction, normalised to 18s, expressed as fold-change (RQ) from the
control. The effects of Scr control or FBN5 siRNA treatment on
Ishikawa cell (B) adhesion, (C) proliferation, (D) invasion and (E)
migration were determined using the xCELLigence Real-Time Cell
Analysis system, which measures electrical impedance caused by cell
attachment and spreading, expressed as the Cell Index. Experiments
were repeated in three cell passages in triplicate wells. Data are
presented as the mean ± standard error of the mean. *P<0.05 and
**P<0.01 (Students t-test); n=3/group. siRNA, small
interfering RNA; RQ, relative quantitation; Scr, scrambled
sequence; FBN5, fibulin-5. |
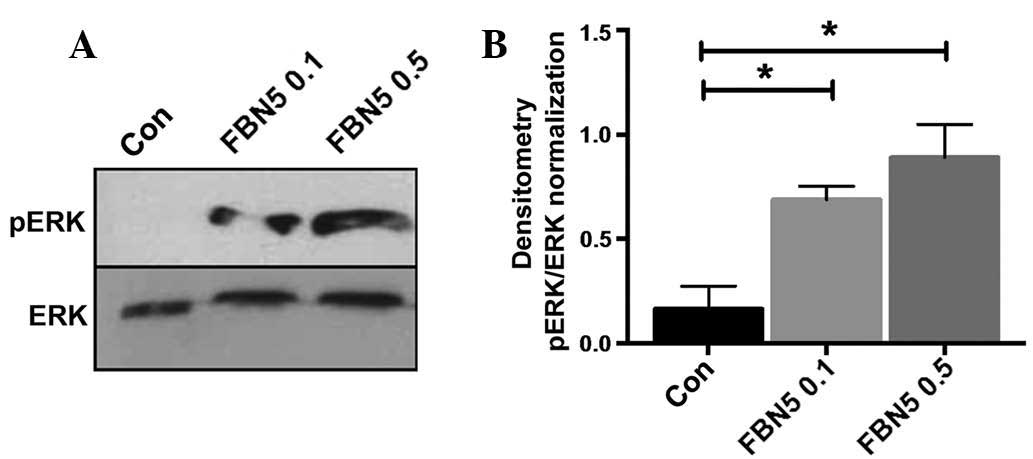
Fibulin-5 activates ERK in Ishikawa
cells
To determine how fibulin-5 may act downstream to
exert these functional changes, the effect of recombinant human
fibulin-5 on ERK activation in Ishikawa cells was assessed. ERK was
not phosphorylated at basal levels in Ishikawa cells (Fig. 4A). Recombinant fibulin-5 treatment
activated pERK in a dose-dependent manner at 0.1 and 0.5 µg/ml
(P=0.0440 and P=0.0132, respectively; n=3) (Fig. 4A and B).
Discussion
The current study is the first to identify and
determine a functional role for fibulin-5 in endometrial cancer.
The findings demonstrated that fibulin-5 gene expression is
downregulated in human type I endometrial cancer tissue G1-3
compared with benign endometrium. To determine which cell types
express fibulin-5, immunohistochemistry was performed, revealing a
shift from epithelial to stromal protein immunolocalisation with
increasing tumour grade in women. In vitro, loss of
fibulin-5 function promoted Ishikawa endometrial epithelial cancer
cell adhesion and proliferation and led to a trend in increased
cell invasion and migration, suggesting that loss of fibulin-5 may
promote endometrial cancer progression in women. These findings
support previous studies in which fibulin-5 was found to be
downregulated in other cancer types, including breast, ovarian,
colon (9), hepatocellular (10), prostate (11), bladder (12) and lung (13) cancers. However, this contrasts with
findings in human fibrosarcoma cells (9) and malignant mammary epithelial cells in
breast cancer (14), suggesting that
the actions of this ECM protein are tissue-dependent.
The current study is also the first to identify
fibulin-5 in the cycling human endometrium. From the present
findings, the localisation pattern of fibulin-5 is predominantly
cytoplasmic in the glandular epithelium in normal endometrium, with
very minimal production in the stroma. In contrast to normal
endometrium, fibulin-5 localisation in endometrial cancer tissue
transitioned from the endometrial epithelium to the stromal
compartment with increasing tumour grade, suggesting a potential
role for this protein in EMT. EMT is a critical process in cancer
metastasis, during which epithelial cells acquire phenotypes of
motile fibroblasts (20). While the
functional role of fibulin-5 in endometrial cancer stromal cells
remains to be determined, its elevated staining intensity in the
tumour stroma with increasing tumour grade suggests it may act to
facilitate tumour progression by acting on the local tumour
environment (21). In other tumour
types, including lung and liver, findings suggest a mechanism in
tumour fibroblasts whereby fibulin-5 suppresses metastasis
formation by inhibiting production of matrix metalloproteinase 9
and reducing the invasive behavior of fibroblasts (21).
In the present study, fibulin-5 gene silencing
resulted in increased Ishikawa cell adhesion. Given the functional
role of fibulin-5 as an adhesion molecule via binding of its RGD
motif to a number of integrins, including α4β1, α5β1, α9β1, αvβ3,
and αvβ3 (22), this suggests that
fibulin-5 may mediate the adhesion phase of invasion via integrin
binding. Fibulin-5 knockdown also resulted in increased Ishikawa
cell proliferation, supporting previous findings in malignant
epithelial cells (9) and suggesting
that it may play an antiproliferative role in human endometrial
cancer.
A trend in increased Ishikawa cell invasion and
migration was also observed. These cells are derived from G1
endometrial cancer, which is not a highly invasive or migratory
tumour type (23); this may account
for the low baseline levels of invasion and migration, and
consequently non-significant functional results in the current
study. This model is, however, advantageous for investigating the
transition from normal to malignant phenotypes early in endometrial
cancer development. To investigate these specific functions, it
would therefore be interesting to overexpress fibulin-5 in G2 or G3
cancer cells (otherwise lacking fibulin-5 gene expression). This
may allow more precise determination of the effect of fibulin-5 on
invasion and migration and could elucidate whether fibulin-5 may
have an anti-tumourigenic effect and have any therapeutic potential
for endometrial cancers.
To determine what may regulate fibulin-5 expression
in human endometrial epithelial cancer cells, Ishikawa cells were
exposed to TGF-β, a cytokine that is known to regulate fibulin-5 in
other cell types (14). TGF-β
upregulated fibulin-5 gene expression in Ishikawa endometrial
cancer epithelial cells. Although, this was not investigated at the
protein level, it is of interest, given that TGF-β1 levels are
dramatically downregulated with increasing endometrioid endometrial
cancer grade in women, as compared with normal endometrium
(24). This could potentially
explain, at least partially, the mechanism by which endometrial
cancers lose fibulin-5 expression with increasing tumour grade.
However, to investigate whether the expression of fibulin-5 is
correlated with the level of TGF-β1, it would be of interest to
examine the expression of TGF-β1 and fibulin-5 in the same cohort
of clinical specimens.
Finally, the current study investigated the
downstream signaling of fibulin-5 by examining ERK activation. The
ERK signaling pathway is regulated by phosphorylation and
dephosphorylation by specific kinases (25). The findings revealed no or low ERK
activation at basal levels in Ishikawa cells. By contrast, ERK was
activated in a dose-dependent manner in response to fibulin-5,
suggesting that it may act functionally via this pathway in this
cell type. In support, a previous study demonstrated that fibulin-5
suppressed lung cancer epithelial cell proliferation via activated
ERK signalling (9).
In summary, the present study demonstrated that the
ECM protein fibulin-5 is regulated by TGF-β in human endometrial
epithelial cancer cells and is downregulated in the epithelial
compartment in human type I endometrioid endometrial cancer.
Functionally, the loss of fibulin-5 gene expression in endometrial
epithelial cancer cells enhanced adhesion and proliferation of
Ishikawa cells in vitro, potentially mediated by suppressed
ERK activation, suggesting that loss of fibulin-5
expression/function could be pro-tumourigenic in women.
Acknowledgements
The authors would like to acknowledge the support of
the Victorian Government's Operational Infrastructure Support
Program and the Australian Government National Health and Medical
Research Council (NHMRC) Independent Research Institute
Infrastructure Support Scheme. Professor Eva Dimitriadis was
supported by an NHMRC Fellowship (#550905). Miss Amy Winship was
supported by an Australian Postgraduate Award.
The authors also wish to acknowledge the technical
support of Dr Michelle Van Sinderen (Centre for Reproductive
Health, The Hudson Institute of Medical Research, Melbourne,
Australia). The authors are grateful to all of the women who
donated samples, and to the research nurse, Sister Judi Hocking
(Centre for Reproductive Health, The Hudson Institute of Medical
Research).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soliman PT, Oh JC, Schmeler KM, Sun CC,
Slomovitz BM, Gershenson DM, Burke TW and Lu KH: Risk factors for
young premenopausal women with endometrial cancer. Obstet Gynecol.
105:575–580. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elit L and Hirte H: Current status and
future innovations of hormonal agents, chemotherapy and
investigational agents in endometrial cancer. Curr Opin Obstet
Gynecol. 14:67–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Di Cristofano A and Ellenson LH:
Endometrial Carcinoma. Annu Rev Pathol. 2:57–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Albig AR and Schiemann WP: Fibulin-5
function during tumorigenesis. Future Oncol. 1:23–35. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: Physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagisawa H, Schluterman MK and Brekken
RA: Fibulin-5, an integrin-binding matricellular protein: Its
function in development and disease. J Cell Commun Signal.
3:337–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schiemann WP, Blobe GC, Kalume DE, Pandey
A and Lodish HF: Context-specific effects of fibulin-5 (DANCE/EVEC)
on cell proliferation, motility, and invasion. Fibulin-5 is induced
by transforming growth factor-beta and affects protein kinase
cascades. J Biol Chem. 277:27367–27377. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y
and Liu Q: Fibulin-5 inhibits hepatocellular carcinoma cell
migration and invasion by down-regulating matrix
metalloproteinase-7 expression. BMC Cancer. 14:9382014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wlazlinski A, Engers R, Hoffmann MJ, Hader
C, Jung V, Müller M and Schulz WA: Downregulation of several
fibulin genes in prostate cancer. Prostate. 67:1770–1780. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu Z, Ai Q, Xu H, Ma X, Li HZ, Shi TP,
Wang C, Gong DJ and Zhang X: Fibulin-5 is down-regulated in
urothelial carcinoma of bladder and inhibits growth and invasion of
human bladder cancer cell line 5637. Urol Oncol. 29:430–435. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue W, Sun Q, Landreneau R, Wu C,
Siegfried JM, Yu J and Zhang L: Fibulin-5 suppresses lung cancer
invasion by inhibiting matrix metalloproteinase-7 expression.
Cancer Res. 69:6339–6346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YH, Albig AR, Regner M, Schiemann BJ
and Schiemann WP: Fibulin-5 initiates epithelial-mesenchymal
transition (EMT) and enhances EMT induced by TGF-beta in mammary
epithelial cells via a MMP-dependent mechanism. Carcinogenesis.
29:2243–2251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yap J, Salamonsen LA, Jobling T, Nicholls
PK and Dimitriadis E: Interleukin 11 is upregulated in uterine
lavage and endometrial cancer cells in women with endometrial
carcinoma. Reprod Biol Endocrinol. 8:632010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuman C, Menkhorst EM, Rombauts LJ, Holden
S, Webster D, Bilandzic M, Osianlis T and Dimitriadis E:
Preimplantation human blastocysts release factors that
differentially alter human endometrial epithelial cell adhesion and
gene expression relative to IVF success. Hum Reprod. 28:1161–1171.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Van Sinderen M, Cuman C, Winship A,
Menkhorst E and Dimitriadis E: The chrondroitin sulfate
proteoglycan (CSPG4) regulates human trophoblast function.
Placenta. 34:907–912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lay V, Yap J, Sonderegger S and
Dimitriadis E: Interleukin 11 regulates endometrial cancer cell
adhesion and migration via STAT3. Int J Oncol. 41:759–764.
2012.PubMed/NCBI
|
|
20
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Møller HD, Ralfkjær U, Cremers N, Frankel
M, Pedersen RT, Klingelhöfer J, Yanagisawa H, Grigorian M, Guldberg
P, Sleeman J, et al: Role of fibulin-5 in metastatic organ
colonization. Mol Cancer Res. 9:553–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y,
Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, et
al: Fibulin-5/DANCE is essential for elastogenesis in vivo.
Nature. 415:171–175. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng YG, Han WD, Zhao YL, Huang K, Si YL,
Wu ZQ and Mu YM: Induction of the LRP16 gene by estrogen promotes
the invasive growth of Ishikawa human endometrial cancer cells
through the downregulation of E-cadherin. Cell Res. 17:869–880.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perlino E, Loverro G, Maiorano E, Giannini
T, Cazzolla A, Napoli A, Fiore MG, Ricco R, Marra E and Selvaggi L:
Down-regulated expression of transforming growth factor beta 1 mRNA
in endometrial carcinoma. Br J Cancer. 77:1260–1266. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaul YD and Seger R: The MEK/ERK cascade:
From signaling specificity to diverse functions. Biochim Biophys
Acta. 1773:1213–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|