Introduction
Solitary fibrous tumors (SFTs) are rare,
non-functional, and generally benign mesenchymal tumors, with an
estimated age-standardized incidence of 1.4 per million population
(1,2).
Despite the fact that SFTs are mainly intrathoracic (1), on rare occasions they can appear in
extrapleural sites, such as the retroperitoneal (3) and pelvic regions (4). In total, 10–20% of SFTs are classified
as malignant, and eventually lead to mortality (1). Previous studies have reported the
association of malignant SFTs with non-islet cell tumor
hypoglycemia (NICTH) due to the secretion of insulin-like growth
factor (IGF)-II by the tumor cells (1,3,5,6).
Furthermore, certain SFTs have been shown to recur >10 years
after the resection of the original tumor, regardless of whether
the original tumors were benign or malignant (7).
The present study reports a case of malignant pelvic
SFT with NICTH due to IGF-II secretion in a 72-year-old male
patient. The tumor recurred ~12 years after the first surgery,
despite the presumed complete excision of the original tumor, which
is a rare occurrence. The tumor was evaluated using several imaging
tests, as well as pathological, immunohistochemical and western
blot analyses. A second surgery was performed for the excision of
the recurrent tumor, which may have been incomplete due to the
tumor's strong adhesiveness and anatomical features. A
postoperative computed tomography (CT) scan showed no evidence of
either recurrence or metastasis, and, at the time of writing, the
patient remained asymptomatic at 9 months post-surgery.
Case report
A 72-year-old male patient with intermittent loss of
consciousness was admitted to another hospital. Laboratory data
revealed a reduced blood glucose level of 28 mg/dl (normal level,
75–109 mg/dl), and the patient was deemed to be in a hypoglycemic
coma. Subsequent hypoglycemic attacks occurred frequently,
necessitating a more extensive examination. Hormonal tests revealed
elevated levels of serum IGF-II by western blotting (molecular
weight, 20.9 kDa). Other markers were found to be suppressed,
including serum C-peptide (0.2 ng/ml; normal level, 1.1–3.3 ng/ml),
IGF-I (52.0 ng/ml; normal level, 63.0–206.0 ng/ml), growth hormone
(GH) (<0.05 ng/ml; normal level, <0.42 ng/ml) and
immunoreactive insulin (<0.1 µU/ml; normal level, <0.4
µU/ml). Abdominal CT and magnetic resonance imaging (MRI) scans
revealed a pelvic tumor measuring ~7 cm in diameter. On the basis
of these findings, an IGF-producing tumor was suspected. Primary
surgery was performed on May 2002, and the tumor was completely
excised. Microscopically, the tumor was diagnosed as an
IGF-II-producing SFT, based on its positive immunoreactivity for
cluster of differentiation 34 (CD34) and IGF-II. Furthermore,
partial hemorrhagic and necrotic findings, as well as high mitotic
activity, suggested a malignant phenotype. The tumor was ultimately
diagnosed as a malignant pelvic SFT with NICTH due to IGF-II, and
regular follow-up CT scans and laboratory examinations were
performed for 5 years, with no evidence of tumor recurrence or
hypoglycemia, which were then discontinued at the patient's own
prerogative.
Approximately 12 years later, the patient began to
re-experience episodes of loss of consciousness. Laboratory
examinations revealed low blood glucose level (40 mg/dl), and an
enhanced CT scan revealed a poorly-enhanced homogeneous tumor
measuring ~90 mm in diameter, spreading within the pelvis. The
tumor appeared to consist of 3 major components that were located
ventrally to the sacral bone, immediately above the bladder and
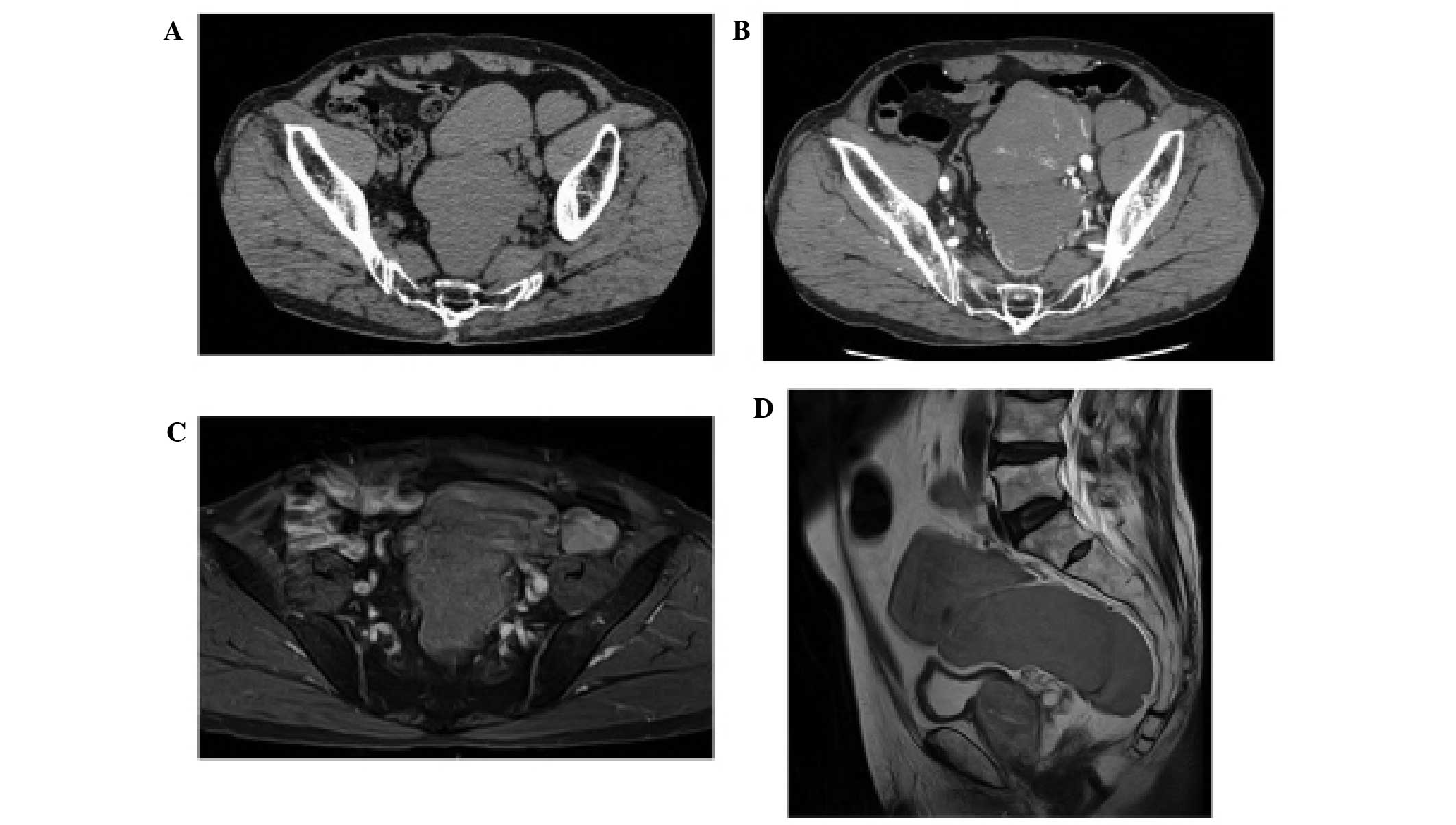
ventrally to the left common iliac artery (Fig. 1A and B). Pelvic SFT recurrence was
suspected, and the patient was referred to the Department of
Urology, Tokyo Women's Medical University (Tokyo, Japan) on June
2014 for further evaluation and treatment. Endocrinological tests
revealed that the levels of GH and IGF-I were suppressed (GH,
<0.03 ng/ml; IGF-I, 27 ng/ml). An abdominal MRI scan showed a
homogenous tumor with a low contrast-enhancement occupying the
pelvis. T2-weighted images revealed a tumor that was iso- to
hyper-intense with low diffusion (Fig. 1C
and D), while the T1-weighted images demonstrated that the
tumor had clear margin. In addition, 18F-fluorodeoxyglucose
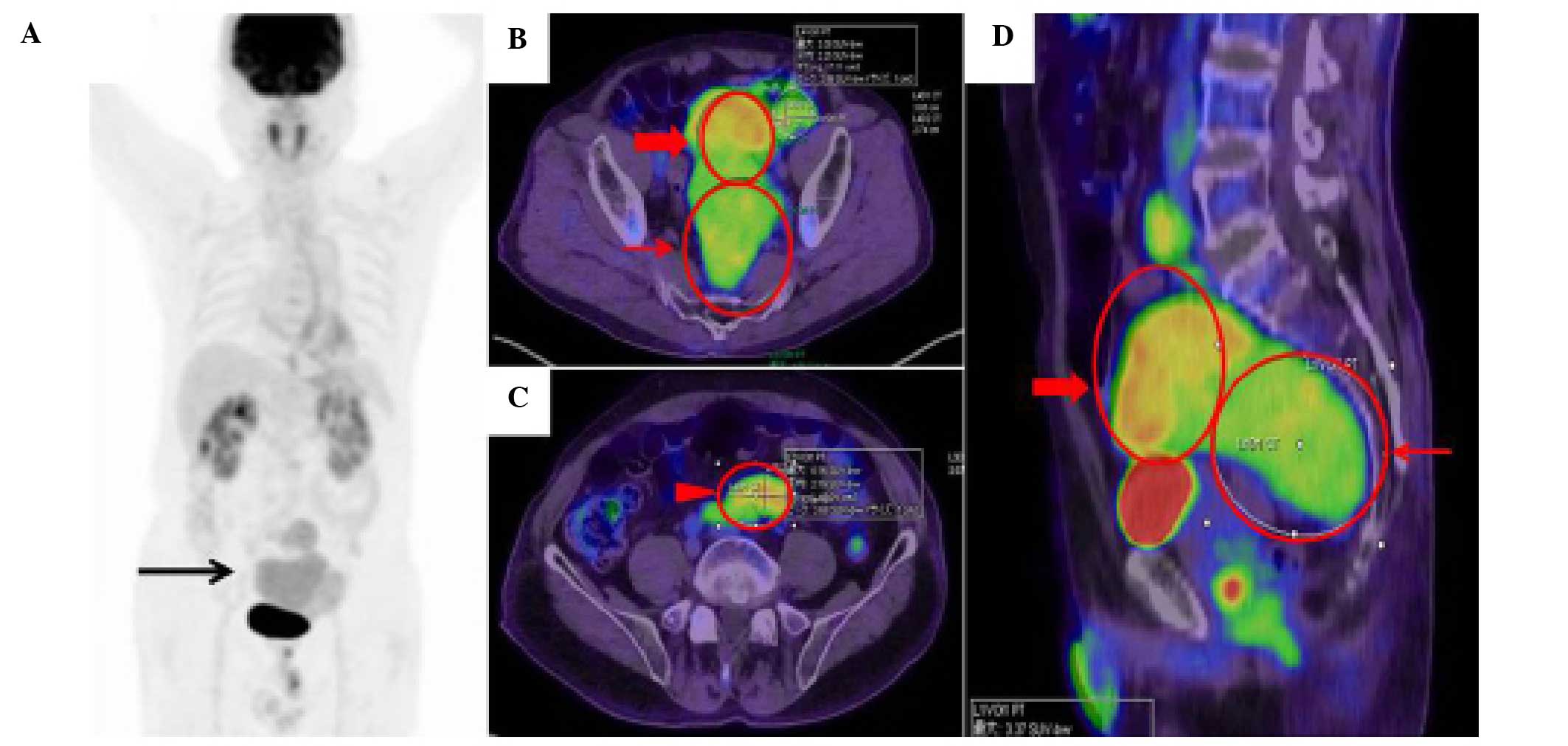
(FDG)-positron emission tomography/CT was performed (Fig. 2). The maximal intensity projection
image showed a moderate FDG uptake in the pelvic tumor (Fig. 2A). The maximum standardized uptake
value was 3.37 (Fig. 2B and D), 4.56
(Fig. 2B and D) and 4.16 (Fig. 2C) in each compartment of the tumor
located ventral to the sacral bone, right above the bladder, and
ventral to the left common iliac artery, respectively.
The tumor was diagnosed as a recurrent pelvic SFT
and a second surgery was performed. Intraoperatively, the tumor was
found to be fairly fragile and adhesive, and to have directly
invaded the left ureter and perirectal fat tissue. The maximum
amount of tumor possible was removed. The resected tumor tissue was
immediately fixed with 20% formalin, embedded in paraffin, and
subjected to histopathological diagnosis and immunohistochemistry.
Paraffin sections (4-µm-thick) were stained with hematoxylin and
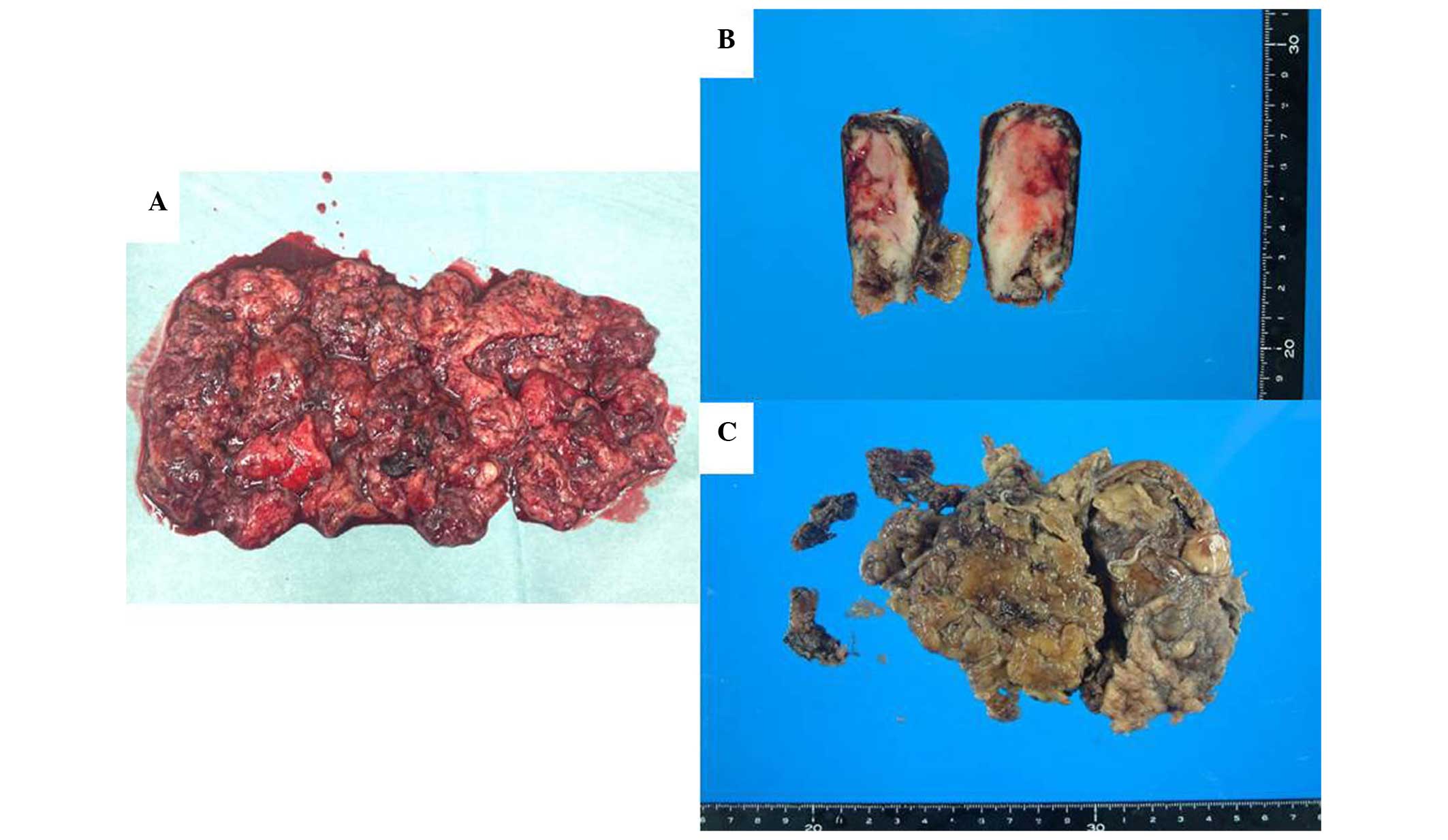
eosin for routine histopathological diagnosis. Macroscopically, the
tumor was grayish-white with prominent hemorrhage and necrosis
(Fig. 3). Pathologically, the tumor
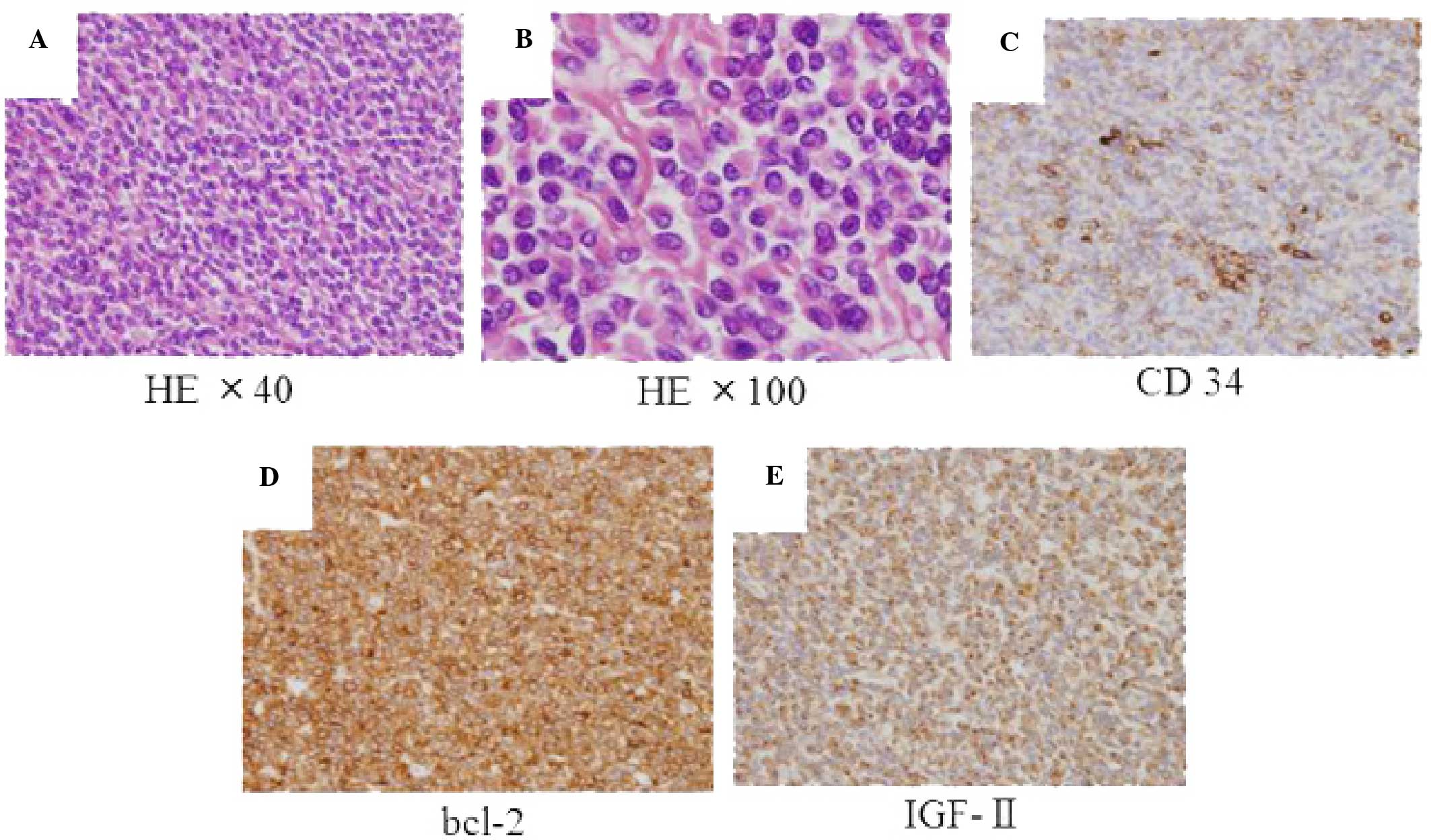
revealed mild-sized spindle cells, densely arranged along a
collagen background, with round and oval nuclei and a fine granular
eosinophilic cytoplasm (Fig. 4A and
B).
For immunohistochemistry, the paraffin sections were
stained using an autostainer (Ventana Medical Systems, Inc.,
Tucson, AZ, USA). Briefly, sections were deparaffinized in xylene,
rehydrated in graded ethanol and immersed in 0.3% hydrogen peroxide
to quench the intrinsic peroxidase. Following incubation with
normal sera of the animals in which the secondary antibodies were
raised, antigen retrieval was performed by autoclaving the samples
in Tris-ethylenediaminetetraacetic acid buffer (pH 9.0) in a
pressure cooker. Subsequently, the samples were incubated with the
following primary antibodies diluted 200-fold: Anti-B-cell lymphoma
2 (bcl-2) (clone 124; Dako, Glostrup, Denmark), anti-CD34 (clone
QBEnd 10; Dako) and anti-IGF-II (clone S1F2; Upstate Biotechnology,
Inc., Lake Placid, NY, USA). Subsequently, the sections were rinsed
with phosphate-buffered saline, and treated with biotinylated
secondary antibodies and streptavidin-conjugated horseradish
peroxidase. The labeled antigens were visualized using Ventana
Universal DAB kit (catalog no., 518-100431; Ventana Medical
Systems, Inc.), and the sections were counterstained with
hematoxylin. The tumor cells were immunohistologically positive for
IGF-II, CD34, bcl-2 and vimentin, but negative for c-kit and S-100.
The Ki-67 proliferative index was <5% (Fig. 4C–E). Abnormal mitosis was mild and the
Ki-67 proliferative index was low; however, according to two
pathologists of the Department of Pathology, Tokyo Women's Medical
University, the tumor appeared to be malignant due to its
hypercellularity, infiltrative growth, gross-necrosis and cellular
atypia.
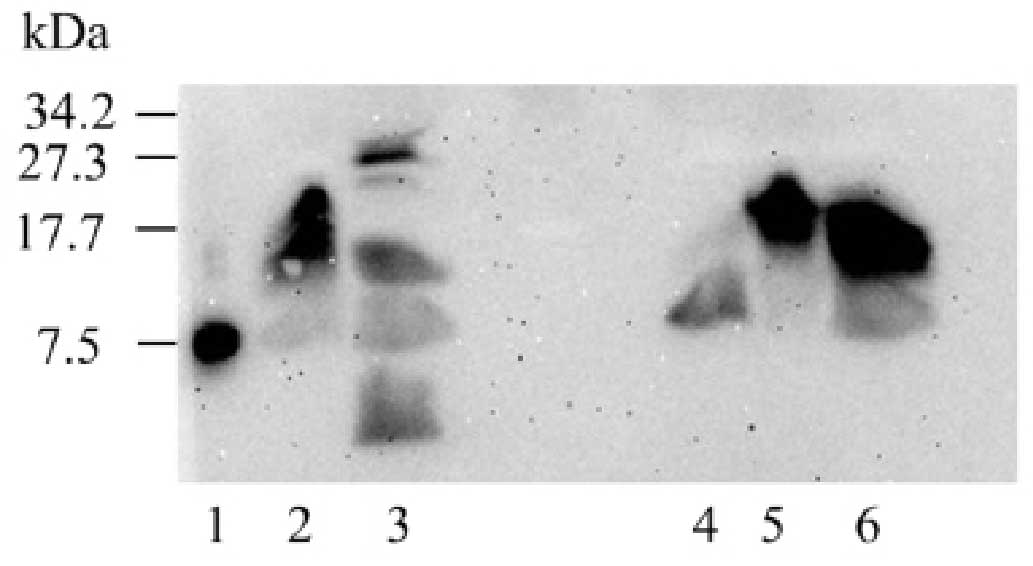
Western blot analysis was performed by the
Department of Hypertension and Endocrinology of Tokyo Women's
Medical University to investigate the preoperative heterogeneity of
serum IGF-II forms and the retention of IGF-II postoperatively
(Fig. 5). As shown in Fig. 5 (lane 2), the patient's serum
contained the high molecular weight (HMW)-form of IGF-II. This
result was similar to the results of other tumors secreting IGF-II,
obtained from previously studied patients (Fig. 5; lanes 5 and 6). HMW-IGF-II
disappeared 4 days following the procedure, although several other
bands appeared (Fig. 5; lane 3). At
13 days post-surgery, the patient presented with rectal
perforation, and a rectal resection and colostomy were immediately
performed. Since the tumor was found to be adhesive and to have
directly invaded the left ureter and perirectal fat tissue,
long-time and careful observations were required to ensure that no
tumor traces remained after surgery. Thus, the patient was followed
up by blood examination every 1 or 2 months, and by imaging
examination such as CT every 3 months. No tumor recurrence or
hypoglycemic symptoms were observed on the follow-up by blood
examination every 1 or 2 months and imaging examination, such as CT
scan, every 3 months for 9 months after the surgery, and no further
treatments were performed.
Discussion
Certain patients with SFTs experience frequent
hypoglycemic episodes, which are associated with IGF-II production.
It has been reported that, while ~4% of pleural SFTs are associated
with hypoglycemia, the incidence rate of hypoglycemia in
retroperitoneal cases is ~11.5% (1,3,5). While high levels of IGF-II are generally
detected in the serum or tumor cells, Daughaday et al
(6) reported an increase in the HMW
form of IGF-II in patients with NICTH. The screening and detection
of suppressed IGF-I levels and increased serum IGF-II/IGF-I ratios
may serve as useful diagnostic markers (8). A deficiency in functional GH could serve
as a secondary marker for NICTH. Low IGF-I levels in patients with
NICTH are attributed to chronic attenuation of GH secretion, due to
the negative feedback of IGF-II (9).
Several studies have shown that NICTH due to HMW-IGF-II can be
diagnosed using western blot analysis (10–12). Hata
et al (10) examined
HMW-IGF-II by using preoperative and postoperative blots and
demonstrated that HMW-IGF-II disappeared postoperatively, which is
considered to be a useful therapeutic index. In the present case,
perioperative western blot analysis was also performed, which
revealed the disappearance of HMW-IGF-II, but also the appearance
of several unidentified protein bands. We speculated that this
result may have been due to either hemolysis in the blood samples,
artifacts caused by obtaining the postoperative sample too soon, or
incomplete removal of the tumor.
Surgical removal is considered the gold standard for
the treatment of SFTs (13,14). Resectability is the most important
factor influencing the outcome, and complete excision should allow
for a favorable prognosis without recurrence or metastasis, even if
the tumor is malignant (15).
However, Baldi et al (7)
reported a number of SFT cases that relapsed >10 years after
initial diagnosis, despite a history of surgical excision of the
primary tumor. Additionally, it is possible that certain inoperable
cases are encountered, which are due to a poor general condition of
the patient, metastasis or severe infiltration to other tissues and
organs. Several studies have indicated that the length of time
between the first and second recurrence is shorter compared with
the time between the initial diagnosis and the first recurrence in
frequently-recurring SFTs, regardless of whether surgery was
performed (7,10). Baldi et al (7) suggested that the inherent limitations of
surgery may result in unavoidable recurrence or metastasis, since
the anatomy of the retroperitoneum and pelvis make complete
resection challenging. Thus, high rates of local failure could
occur even for benign SFTs, due to incomplete removal. Frequent
surgical resection for recurrent tumors causes aggressive adhesion,
making complete surgical resection a considerable challenge.
Furthermore, incomplete resection may actually cause tumor
dissemination. These factors may explain why the time between the
first and second recurrence is shorter. Thus, intraoperative
findings and tumor location should be considered an associated risk
for recurrence or metastasis in conjunction with pathological
findings. In the present case, intraoperative findings and
postoperative western blot analysis results indicated that a second
recurrence will most likely occur more quickly than the first
recurrence.
Although there are currently no consensus guidelines
for curative treatments other than surgery, several previous
studies have reported other effective treatments. Hosaka et
al (16)reported that a malignant
pelvic IGF-II-secreting SFT was successfully treated via
intra-arterial chemotherapy with cisplatin and carboplatin and
concurrent radiotherapy for 5 years. Although the patient had NICTH
prior to treatment (as in the present case), hypoglycemic attacks
were resolved in tandem with tumor shrinkage (16). Other studies reported that
glucocorticoid therapy may be effective against hypoglycemia caused
by IGF-II-producing tumors or tumor growth itself (11,17).
Furthermore, several studies have suggested that imatinib mesylate
is an effective therapy for unresectable SFTs (12,18,19).
Yamada et al (20) reported
that the Akt-mammalian target of rapamycin pathway is activated in
~50% of SFTs and is associated with the upregulation of receptor
tyrosine kinases. Furthermore, sunitinib malate may be efficacious
due to its anti-platelet-derived growth factor receptor-β activity
(21). Recent genetic research
revealed that the recurrent fusion of two genes, NGFI-A binding
protein 2 and signal transducer and activator of transcription 6,
both located at chromosomal region 12q31, was identified as a
SFT-specific chimeric fusion gene by next-generation sequencing and
reverse transcription polymerase chain reaction (22–24).
Finally, the development of new-generation drugs that target
tumor-promoting genes or proteins is also essential. In the present
case, intraoperative findings and western blot analysis results
suggested that the resection of the tumor was incomplete, since the
tumor was found to be adhesive and to have directly invaded the
left ureter and perirectal fat tissue. Thus, adjuvant therapies
such as the ones discussed, should be investigated in an effort to
prevent recurrence, as current options do not appear to be
effective.
In conclusion, the present study reports a rare case
of malignant pelvic NICTH-inducing SFT with IGF-II secretion, which
recurred ~12 years after the presumed complete resection of the
primary tumor. The present case report suggested that the safe and
complete resection of tumors may sometimes be challenging to
perform, due to their adhesiveness or physical presentation;
therefore, the indications for surgery should be considered with
caution.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for the English language editing.
Glossary
Abbreviations
Abbreviations:
|
SFT
|
solitary fibrous tumor
|
|
NICTH
|
non-islet cell tumor hypoglycemia
|
|
IGF
|
insulin-like growth factor
|
|
CT
|
computed tomography
|
|
GH
|
growth hormone
|
|
HMW
|
high-molecular-weight
|
References
|
1
|
Briselli M, Mark EJ and Dickersin GR:
Solitary fibrous tumors of the pleura: Eight new cases and review
of 360 cases in the literature. Cancer. 47:2678–2689. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thorgeirsson T, Isaksson HJ, Hardardottir
H, Alfredsson H and Gudbjartsson T: Solitary fibrous tumors of the
pleura: An estimation of population incidence. Chest.
137:1005–1006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takizawa I, Saito T, Kitamura Y, Arai K,
Kawaguchi M, Takahashi K and Hara N: Primary solitary fibrous tumor
(SFT) in the retroperitoneum. Urol Oncol. 26:254–259. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsushimi T, Yagi T, Tomozawa N and Ohnishi
H: Retroperitoneal solitary fibrous tumor of the pelvis with
pollakiuria: A case report. BMC Res Notes. 5:5932012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mentzel T, Bainbridge TC and Katenkamp D:
Solitary fibrous tumour: Clinicopathological, immunohistochemical,
and ultrastructural analysis of 12 cases arising in soft tissues,
nasal cavity and nasopharynx, urinary bladder and prostate.
Virchows Arch. 430:445–453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Daughaday WH, Emanuele MA, Brooks MH,
Barbato AL, Kapadia M and Rotwein P: Synthesis and secretion of
insulin-like growth factor II by a leiomyosarcoma with associated
hypoglycemia. N Engl J Med. 319:1434–1440. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baldi GG, Stacchiotti S, Mauro V, Dei Tos
AP, Gronchi A, Pastorino U, Duranti L, Provenzano S, Marrari A,
Libertini M, et al: Solitary fibrous tumor of all sites: Outcome of
late recurrences in 14 patients. Clin Sarcoma Res. 3:42013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuda I, Hizuka N, Ishikawa Y, Yasumoto
K, Murakami Y, Sata A, Morita J, Kurimoto M, Okubo Y and Takano K:
Clinical features of insulin-like growth factor-II producing
non-islet-cell tumor hypoglycemia. Growth Horm IGF Res. 16:211–216.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ron D, Powers AC, Pandian MR, Godine JE
and Axelrod L: Increased insulin-like growth factor II production
and consequent suppression of growth hormone secretion: A dual
mechanism for tumor-induced hypoglycemia. J Clin Endocrinol Metab.
68:701–706. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata T, Tsuruta Y, Takamori S and
Shishikura Y: Non-islet cell tumor hypoglycemia at the second
recurrence of malignant solitary fibrous tumor in the
retroperitoneum and pelvis: A case report. Case Rep Oncol.
5:420–427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsuro K, Kojima H, Okamoto S, Yoshiji H,
Fujimoto M, Uemura M, Yoshikawa M, Nakamura T, Kou S, Nakajima Y
and Fukui H: Glucocorticoid therapy ameliorated hypoglycemia in
insulin-like growth factor-II-producing solitary fibrous tumor.
Intern Med. 45:525–529. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tominaga N, Kawarasaki C, Kanemoto K,
Yokochi A, Sugino K, Hatanaka K, Uekusa T, Fukuda I, Aiba M, Hizuka
N and Uda S: Recurrent solitary fibrous tumor of the pleura with
malignant transformation and non-islet cell tumor-induced
hypoglycemia due to paraneoplastic overexpression and secretion of
high-molecular-weight insulin-like growth factor II. Intern Med.
51:3267–3272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morimitsu Y, Nakajima M, Hisaoka M and
Hashimoto H: Extrapleural solitary fibrous tumor: Clinicopathologic
study of 17 cases and molecular analysis of the p53 pathway. APMIS.
108:617–625. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brunnemann RB, Ro JY, Ordonez NG, Mooney
J, El-Naggar AK and Ayala AG: Extrapleural solitary fibrous tumor:
A clinicopathologic study of 24 cases. Mod Pathol. 12:1034–1042.
1999.PubMed/NCBI
|
|
15
|
England DM, Hochholzer L and McCarthy MJ:
Localized benign and malignant fibrous tumors of the pleura. A
clinicopathologic review of 223 cases. Am J Surg Pathol.
13:640–658. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hosaka S, Katagiri H, Wasa J, Murata H and
Takahashi M: Solitary fibrous tumor in the pelvis: Induced
hypoglycemia associated with insulin-like growth factor II. J
Orthop Sci. 20:439–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pavelić K, Vrbanec D, Marusić S, Levanat S
and Cabrijan T: Autocrine tumour growth regulation by somatomedin
C: An in-vitro model. J Endocrinol. 109:233–238. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prunotto M, Bosco M, Daniele L, Macri' L,
Bonello L, Schirosi L, Rossi G, Filosso P, Mussa B and Sapino A:
Imatinib inhibits in vitro proliferation of cells derived from a
pleural solitary fibrous tumor expressing platelet-derived growth
factor receptor-beta. Lung Cancer. 64:244–246. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Pas T, Toffalorio F, Colombo P, Trifirò
G, Pelosi G, Vigna PD, Manzotti M, Agostini M and de Braud F: Brief
report: Activity of imatinib in a patient with
platelet-derived-growth-factor receptor positive malignant solitary
fibrous tumor of the pleura. J Thorac Oncol. 3:938–941. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamada Y, Kohashi K, Fushimi F, Takahashi
Y, Setsu N, Endo M, Yamamoto H, Tokunaga S, Iwamoto Y and Oda Y:
Activation of the Akt-mTOR pathway and receptor tyrosine kinase in
patients with solitary fibrous tumors. Cancer. 120:864–876. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stacchiotti S, Negri T, Palassini E, Conca
E, Gronchi A, Morosi C, Messina A, Pastorino U, Pierotti MA, Casali
PG and Pilotti S: Sunitinib malate and figitumumab in solitary
fibrous tumor: Patterns and molecular bases of tumor response. Mol
Cancer Ther. 9:1286–1297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barthelmeß S, Geddert H, Boltze C,
Moskalev EA, Bieg M, Sirbu H, Brors B, Wiemann S, Hartmann A,
Agaimy A and Haller F: Solitary fibrous tumors/hemangiopericytomas
with different variants of the NAB2-STAT6 gene fusion are
characterized by specific histomorphology and distinct
clinicopathological features. Am J Pathol. 184:1209–1218. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson DR, Wu YM, Kalyana-Sundaram S,
Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, et al:
Identification of recurrent NAB2-STAT6 gene fusions in solitary
fibrous tumor by integrative sequencing. Nat Genet. 45:180–185.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chmielecki J, Crago AM, Rosenberg M,
O'Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich
MC, Frank DA and Meyerson M: Whole-exome sequencing identifies a
recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet.
45:131–132. 2013. View
Article : Google Scholar : PubMed/NCBI
|