Introduction
Thyroid cancer is the most common carcinoma of the
endocrine glands and represents ~1% of all malignancies (1). Papillary thyroid carcinoma (PTC),
follicular thyroid carcinoma (FTC), and anaplastic and medullary
thyroid carcinomas comprise >98% of all thyroid malignancies
(2). While 80–85% of thyroid
carcinomas are well-differentiated (PTC and FTC) and have a
favorable prognosis, anaplastic thyroid cancer has an unfavorable
prognosis and a fatal outcome.
According to reports from Western countries, PTC
comprises 75–80% of all thyroid neoplasms. This carcinoma
frequently demonstrates metastasis to the regional lymph nodes and
exhibits multicentricity in the thyroid gland. FTC accounts for
10–20% of the thyroid carcinoma cases in the United States. In
contrast to PTC, FTC is likely to metastasize to distant organs
rather than the regional lymph nodes (1,2). These
carcinomas generally have an indolent character, but following
dedifferentiation of the lesion to become an undifferentiated
carcinoma, it exhibits rapid growth with an adverse prognosis
(1,2).
Different cytogenetic events and oncogenic mechanisms occur in
thyroid carcinoma (3–6); in particular, the ret proto-oncogene/PTC
rearrangement is a specific genetic alteration observed in
papillary carcinoma, but never in undifferentiated thyroid cancer
(7). This oncogenic fusion protein
fails to induce the carcinogenesis of mature thyrocytes (8), but introduction into the germ line is
sufficient to induce PTC (9),
indicating that the initiation of thyroid carcinoma may occur using
transformed stem cells prior to or during terminal commitment.
According to the cancer stem cell hypothesis, only a
rare subset of cells is able to initiate and sustain tumor growth.
The existence of these cells, called cancer stem cells (CSCs) or
cancer-initiating cells, was first demonstrated in acute myeloid
leukemia (10), and was successively
described in other hematological and solid tumors (11–18),
including thyroid tumors (19). This
small subpopulation of CSCs with unlimited proliferative potential
possesses tumorigenic capacity and is consequently responsible for
the development and maintenance of tumors (19). Thus, CSCs are a primary therapeutic
target for complete tumor eradication.
Therefore, the present study investigated the
cytotoxic effects of different chemotherapeutic agents on PTC
spheres isolated and characterized at the Research Laboratories,
Mediterranean Institute of Oncology (Viagrande, Italy). It was
found that the PTC spheres were resistant to the chemotherapeutic
drugs applied, which is consistent with the poor therapeutic effect
observed when using conventional chemotherapy on relapsed or
resistant PTC patients. Conversely, the drugs were effective on
differentiated PTC (DPTC) cells, suggesting that undifferentiated
cells become sensitive after differentiation.
Since the majority of chemotherapeutic agents act
through the cell cycle (20) by
inducing cell death, the present study also investigated cell cycle
features, including sub-G0, G0/G1, S and G2/M.
Materials and methods
Isolation and culture of PTC
spheres
Papillary thyroid CSCs were obtained from 10
surgically-resected samples at the Mediterranean Institute of
Oncology (Catania, Italy) between January 2008 and June 2012, as
previously reported (19). The
patient sample included 4 males and 6 females (age range, 36–78
years). All patients were informed of the study purpose and
provided written informed consent. The study was approved by the
Mediterranean Institute of Oncology Ethical Committee. Briefly,
following mechanical and enzymatic dissociation of the tissue, the
cells were cultured in serum-free Dulbecco' modified Eagle's
medium/F12 medium containing 20 ng/ml epidermal growth factor (EGF)
and 10 ng/ml basic fibroblast growth factor. These experimental
conditions allowed the selection and growth of the tumor spheres.
Factor deprivation and addition of 10% fetal bovine serum to the
medium induced undifferentiated PTC (UPTC) cells to adhere to the
flask and acquire the typical morphological features of
differentiated cells.
Flow cytometry
The cells were checked for cluster of
differentiation (CD)133 stem cell marker by cytofluorimetric
analysis Freshly isolated cells were washed with cold
phosphate-buffered saline (PBS) containing 1% bovine serum albumin
and exposed to mouse monoclonal anti-CD133/1 primary antibody
(clone AC133; dilution, 1:10; catalog no., 130-090-422; Miltenyi
Biotec Inc., Cambridge, MA, USA) primary antibody or isotype
control (mouse IgG1). Subsequent to being washed, the cells were
labeled with phycoerthyrin-conjugated donkey anti-mouse secondary
antibody (dilution, 1:100; catalog no., 715-116-150; Jackson
ImmunoResearch Labs, West Grove, PA, USA) and fluorescence
intensity was evaluated using a FACScan EPICS® XL™ flow
cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Musashi cDNA was amplified after total RNA was
isolated from the UPTC and DPTC cells using the RNeasy Mini Spin
Column kit (Qiagen GmbH, Hilden, Germany) according to the
manufacturer's protocols. Total RNA was reverse transcribed with
random examers. Briefly, 1.0 µg RNA was incubated for 5 min at
70°C, then the sample was placed on ice and reverse transcribed
with M-MLV H- DNA polymerase (Promega, Madison, WI, USA) for 10 min
at 25°C followed by 1 h incubation at 42°C. For conventional PCR,
cDNA was diluted 1:5 with H2O and a 299 bp fragment was
amplified using the GoTaq® Green Master Mix (Promega)
and the following PCR primers (MWG-Biotech AG, Ebersberg, Germany)
for Musashi DNA: GIOL393, sense 5′-CAAGATGTTCATCGGGGGACTCAGTT-3′
and GIOL394, antisense 5′-TATTGCTTCACGTCCTCCACCGTC-3′. The cycling
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 35 cycles of 62°C for 30 sec and extension at 72°C
for 60 sec. Brain tumor stem cell (BTSC) cDNA was used as a
positive control. The experiment was performed in triplicate using
the Biometra TProfessional Gradient thermocycler (Biometra GmbH,
Göttingen, Germany).
Chemotherapy resistance studies
Following dissociation, 3,000 cells obtained from
PTC spheres and DPTC cells were seeded in 96-well plates. For the
chemotherapy resistance studies, chemotherapeutic drugs were added
to the cells at the following concentrations: 100 nM bortezomib
(PS-341), 5 mM Taxol, 500 ng/ml cisplatin, 0.5 µM etoposide
(VP-16), 5 mM doxorubicin and 1 µM vincristine. After 24, 48 and 72
h, cell viability was evaluated using the CellTiter 96®
AQueous One Solution Cell Proliferation Assay (Promega) according
to the manufacturer's protocol. This assay is based on the
reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-car-
boxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt to a
colored formazan product. The formazan is measured by absorbance at
490 nm, which is directly proportional to the number of living
cells. The plates were read using a microplate reader (Synergy HT;
BioTek, Winooski, VT, USA). Survival was expressed as the
percentage of viable cells in the treated sample relative to the
untreated control cells. Cell count was evaluated by Trypan blue
exclusion test (catalog no., ECM0990D; EuroClone SpA, Pero MI,
Italy). Data are presented as the mean of three independent
experiments performed with the two experimental procedures.
Cell cycle analysis
Following dissociation, 5,000 cells obtained from
PTC spheres and DPTC cells were washed and resuspended in cold 80%
ethanol to a final concentration of 0.5×106 cells/ml for
1 h at 4°C. The ethanol-fixed cells were centrifuged at 300 × g for
10 min to remove ethanol and the pellet was resuspended in
propidium iodide staining reagent (0.1% triton X-100, 0.1 mM
ethylenediaminetetraacetic acid, 0.05 mg/ml RNase A and 50 µg/ml
propidium iodide). The cells were stored in the dark at room
temperature for ~3 h. The cells were then analyzed with a flow
cytometer (FC500; Beckman Coulter Inc.) for cell cycle
analysis.
Western blot analysis
Proteins (30 µg) extracted from the UPTC and DPTC
cells were processed for western blot analysis. Nitrocellulose
membranes were incubated with the following primary anti-human
antibodies: Rabbit polyclonal anti-p27 (clone, C-19; dilution,
1:200; catalog no., sc-528), rabbit polyclonal anti-cyclin E
(clone, M-20; dilution, 1:500; catalog no., sc-481), mouse
monoclonal anti-BCL2-like 1 isoform 1 (Bcl-xL; clone, H-5;
dilution, 1:500; catalog no., sc-8392), and goat polyclonal
anti-actin (clone, I-19; dilution, 1:500; catalog no., sc-1616)
(all obtained from Santa Cruz Biotechnology Inc., Dallas, TX, USA).
After washing with PBS, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG
(dilution, 1:2,000; catalog no., sc-2005), goat anti-rabbit
(dilution, 1:5,000; catalog no., sc-2313) and donkey anti-goat IgG
(dilution, 1:5,000; catalog no., sc-2020) secondary antibodies (all
obtained from Santa Cruz Biotechnology Inc.). For detection, the
enhanced chemiluminescence kit (ECL plus; GE Healthcare Life
Sciences, Chalfont, UK) was used.
Statistical analysis
All statistical analysis was performed using
Microsoft Excel 14.5.3 software (Microsoft Corporation, Redmond,
WA, USA). Data are presented as the mean ± standard deviation. A
paired t-test was used to analyze the statistical significance of
the results. P<0.05 was considered to indicate a statistically
significant difference.
Results
RNA-binding protein Musashi is
expressed by UPTC spheres but not by DPTCs
As previously reported, experimental conditions
based on culturing freshly dissociated tumor cells in serum-free
medium containing EGF and bFGF, allowed the selective growth of
cellular clusters resembling tumor spheres (12,17,18,19,21).
In the present study, such spheres maintained an undifferentiated
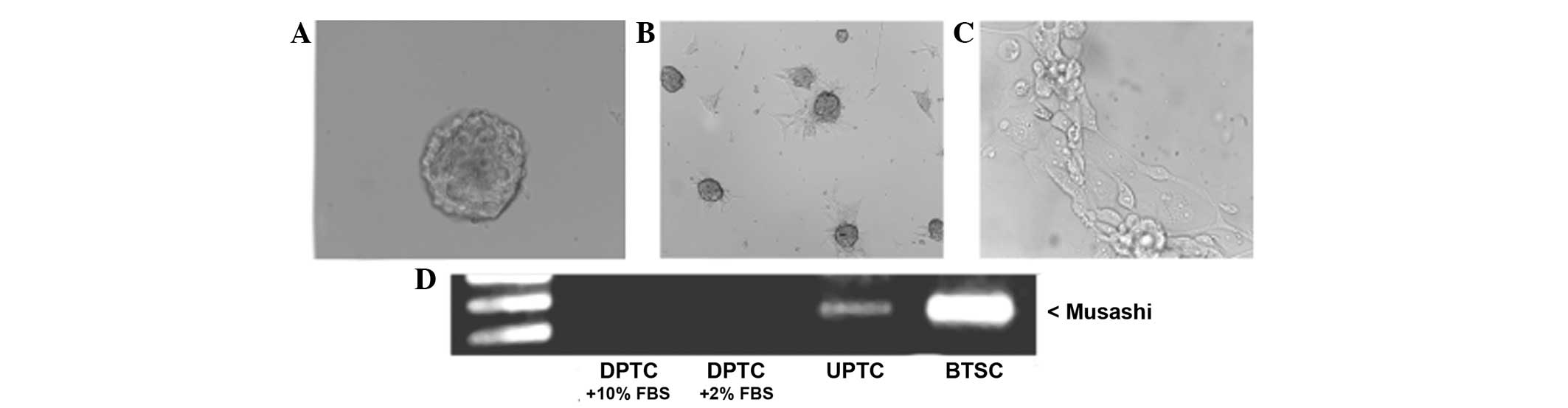
state (UPTC), as confirmed by morphological analysis (Fig. 1A) and the expression of stem cell
markers such as CD133 (data not shown), as found in previous
studies (17,22). Morphological analysis revealed that
these cell cultures were exclusively formed by cellular clusters
resembling so called ‘tumor spheres’. In the presence of 10% serum,
the PTC spheres became adherent and acquired the typical
morphological features of differentiated cells (DPTC), which is a
fibroblast-like phenotype (Fig. 1B and
C); furthermore, CD133 antigen was lost, confirming its
specific expression in the undifferentiated cells (data not
shown).
Notably, it was found that the RNA-binding protein
Musashi, associated with stem cell identity and recently considered
as a master regulator in a number of stem cell populations
(23,24), was expressed by the UPTC spheres,
while its expression was lost in the DPTCs (Fig. 1D).
UPTC cells are highly resistant to
different chemotherapeutic agents
The establishment of PTC stem-like cell cultures may
allow the direct evaluation of the cytotoxic activity of
antineoplastic agents on the putative cells responsible for tumor
growth and spreading, which represent the optimal cellular targets
for successful therapies. Therefore, the present study investigated
the cytotoxic effect of different chemotherapeutic agents on PTC
spheres. Taxol, cisplatin, VP-16, doxorubicin, vincristine and
PS-341 were used at doses comparable with the higher plasma levels
reached in patients. Since in preliminary experiments these cells
proved to be rather resistant to chemotherapeutic drugs after 24
and 48 h of treatment, the viability of the UPTC cells was
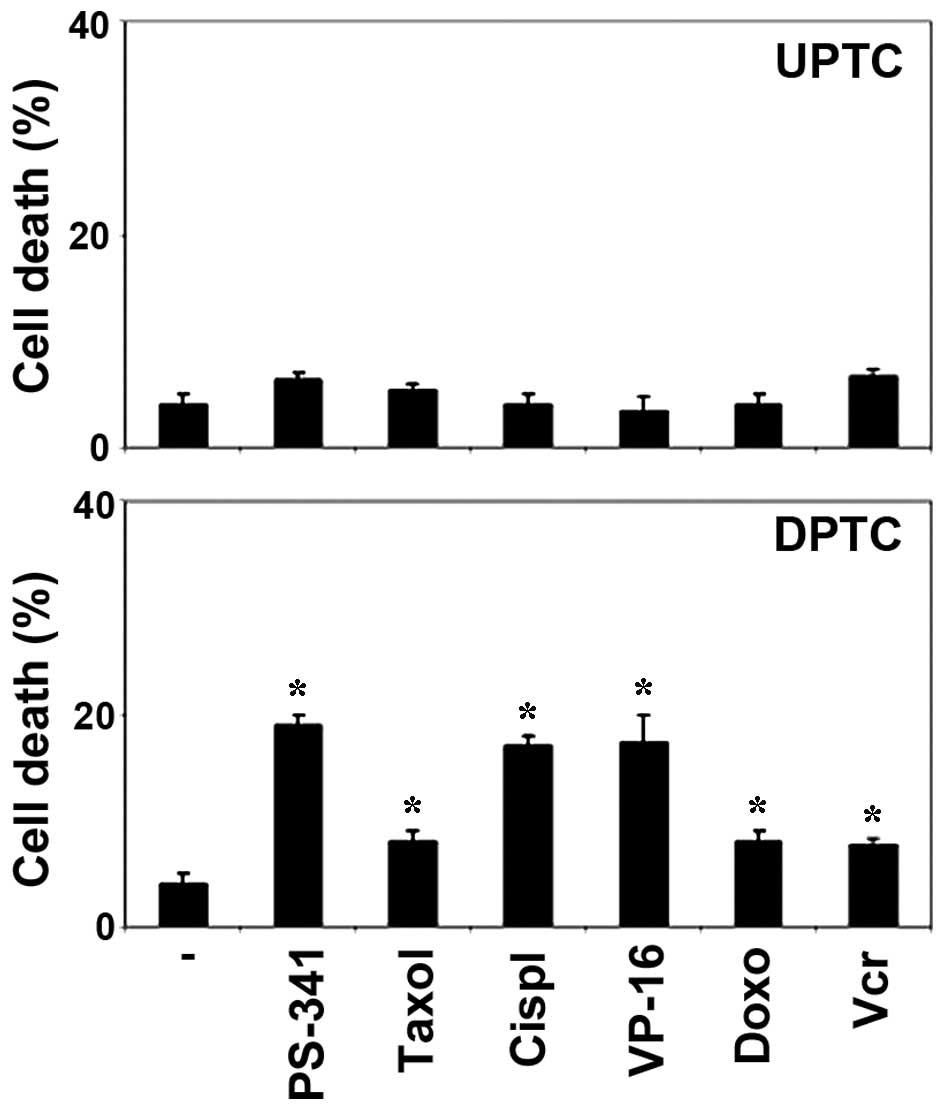
evaluated after 3 days of treatment. These drugs displayed modest
cytotoxic activity in all UPTC cells examined at any time point
(P>0.05). Conversely, the drugs were effective against the DPTC
cells after 24 h, suggesting that undifferentiated cells become
sensitive after differentiation (P<0.05; Fig. 2). Thus, similar to glioblastoma and
lung cancer stem cells (18,25), PTC spheres are resistant to
chemotherapeutic drugs, which is in agreement with the poor
therapeutic effect observed when using conventional chemotherapy on
relapsed or resistant PTC patients.
UPTC and DPTC cells show different
cell cycle features and anti-apoptotic protein expression
Since the majority of chemotherapeutic agents acts
through the cell cycle and/or by activation of apoptotic mechanism
to induce cell death (20,26,27), it is
likely that defects or dysregulation of different steps of these
pathways may be important determinants of resistance to anticancer
drugs. To this aim, the present study investigated cell cycle
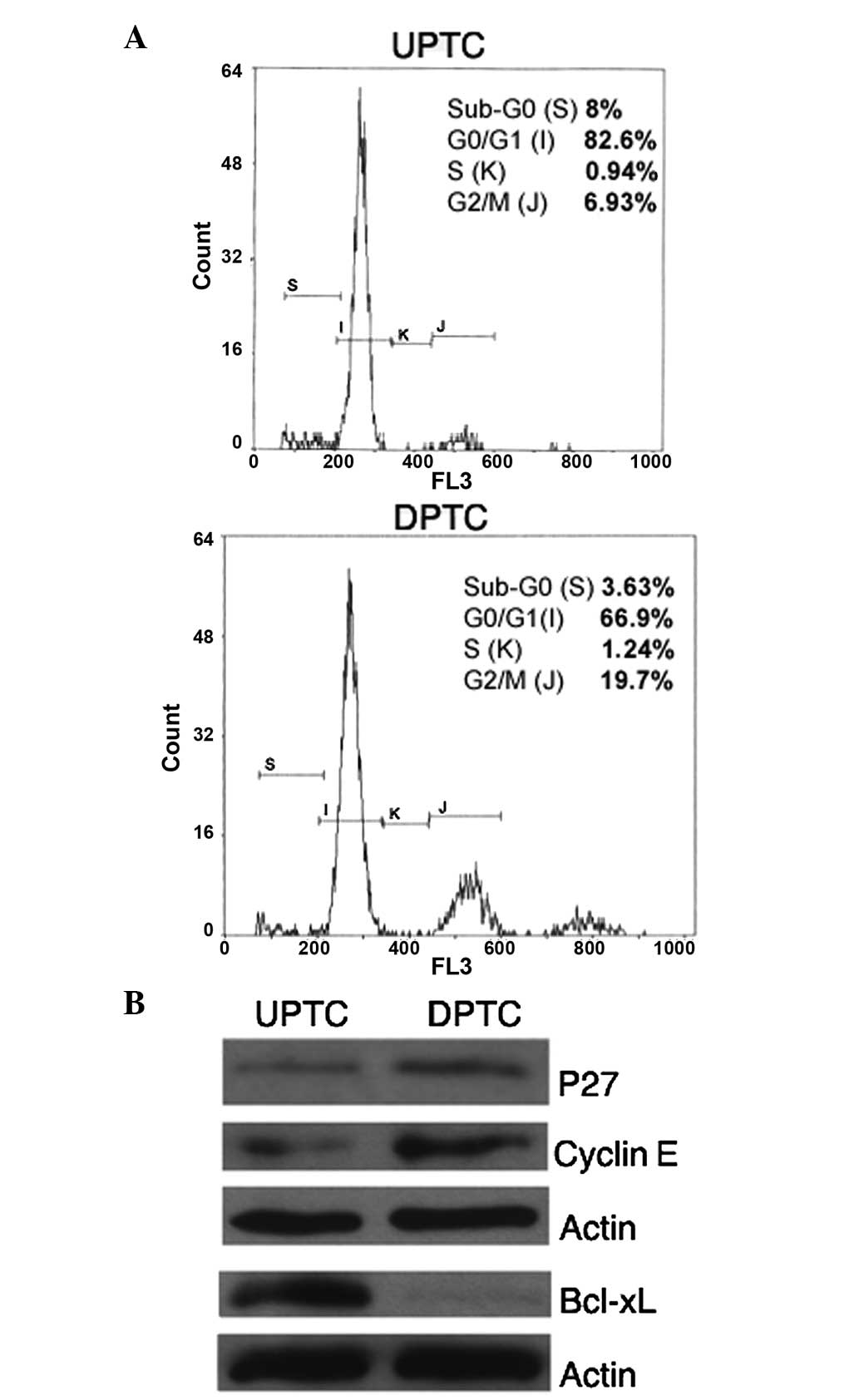
features and found that the UPTC cells had an higher percentage of
cells (82.6%) in a quiescent status (G0/G1)
than the DPTC cells (66.9%) (Fig.
3A). In addition, the UPTC and DPTC cells exhibited differing
cell cycle protein expression; in particular, the p27 and cyclin E
proteins, involved in G0-G1 progression, were
expressed to a lesser degree in the UPTC cells compared with the
DPTC cells (Fig. 3B). Furthermore,
the expression level of the anti-apoptotic protein Bcl-xL was also
evaluated and Bcl-xL was found to be strongly expressed by the UPTC
cells, but not expressed by the DPTC cells (Fig. 3B).
Discussion
Growing evidence suggests that CSCs are responsible
for tumor initiation, growth, metastasis, therapy resistance,
relapse and poor prognosis. It has recently been demonstrated that
CSCs isolated from different types of cancer tissues as tumor
spheres are able to reproduce the original human tumor in
immunocompromised mice (10–19). These cells should be primary
therapeutic target in order to achieve complete eradication of the
malignancy. Therefore, the establishment of PTC stem-like cell
cultures may enable the direct evaluation of the cytotoxic activity
of antineoplastic agents on the putative cells responsible for the
growth and spread of thyroid tumors, which represent optimal
cellular targets for successful therapies. For this purpose, the
present study isolated PTC spheres from surgical pathological
tissues and PTC stem-like cell cultures were set up and
characterized. Notably, the present study found that the
undifferentiated cells expressed Musashi, an RNA-binding protein
associated with stem cell identity, and that once differentiated,
the expression of the protein was lost. Subsequently, the present
study investigated the cytotoxic effect of several chemotherapeutic
agents on the PTC spheres. It was found that the PTC spheres were
resistant to all the chemotherapeutic drugs tested, which is in
line with the poor therapeutic effect observed for the use of
conventional chemotherapy on relapsed or resistant PTC patients.
This is also in agreement with various studies that revealed that
CSCs are crucial in chemoresistance against different anti-tumoral
drugs, including cisplatin, paclitaxel, VP-16 and doxorubicin
(28–31), in a variety of tumors, including
glioblastoma (28), breast (29), ovarian (30) and prostate (31) cancers. However, in the present study,
the drugs became effective on the cells once they were induced to
differentiate into DPTC cells, suggesting that the undifferentiated
cells become sensitive to the treatment only after
differentiation.
Since the majority of chemotherapeutic agents act
through the cell cycle and the activation of apoptosis to induce
cell death in susceptible cells, the altered expression of proteins
involved in such mechanisms could explain the chemoresistance
phenomenon found in UPTC cells. Indeed, chemotherapy preferentially
targets fast proliferating cells. In addition, it is now generally
theorized that normal and cancer stem cells remain in a quiescent
status for the majority of time (32,33), thus
preventing the attack by most chemotherapeutic drugs. The present
study investigated cell cycle features and found that UPTC cells
exhibited a higher percentage of cells (82.6%) in a quiescent
status (G0/G1) compared to DPTC cells (66.9%). Similarly, the
present study demonstrated, by western blot analysis, a reduced
expression of p27 and cyclin E, cell cycle proteins involved in
G0-G1 progression, in UPTC cells compared with DPTC cells. In
addition, several studies report that BCL-2 family proteins are
critical role for cancer cell survival and chemoresistance
(34,35). For this reason, the present study
evaluated the expression of the anti-apoptotic protein, Bcl-xL by
western blot analysis, and revealed that it was strongly expressed
by UPTC cells, whereas it was almost totally absent in DPTC
cells.
The data obtained by the present study may explain
the resistance of UPTC cells to chemotherapeutic drugs. However,
further studies are required to better understand the
chemoresistance mechanisms in papillary thyroid CSCs, allowing the
development of novel successful therapies for thyroid cancer
treatment.
Acknowledgements
The authors would like to thank Mr. G. Anastasi
(Mediterranean Institute of Oncology-Research) for providing
skillful technical assistance and Dr. Giovanna Calabrese
(Mediterranean Institute of Oncology-Research) for providing
helpful advice and for support with organizing the figures of this
study.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Groot JW, Links TP, Plukker JT, Lips CJ
and Hofstra RM: RET as a diagnostic and therapeutic target in
sporadic and hereditary endocrine tumors. Endocr Rev. 27:535–560.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo T, Ezzat S and Asa SL: Pathogenetic
mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer.
6:292–306. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smallridge RC, Marlow LA and Copland JA:
Anaplastic thyroid cancer: Molecular pathogenesis and emerging
therapies. Endocr Relat Cancer. 16:17–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ivan M, Bond JA, Prat M, Comoglio PM and
Wynford-Thomas D: Activated ras and ret oncogenes induce
over-expression of c-met (hepatocyte growth factor receptor) in
human thyroid epithelial cells. Oncogene. 14:2417–2423. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Salvatore D, Barone MV, Salvatore G,
Melillo RM, Chiappetta G, Mineo A, Fenzi G, Vecchio G, Fusco A and
Santoro M: Tyrosines 1015 and 1062 are in vivo autophosphorylation
sites in ret and ret-derived oncoproteins. J Clin Endocrinol Metab.
85:3898–3907. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Portella G, Vitagliano D, Borselli C,
Melillo RM, Salvatore D, Rothstein JL, Vecchio G, Fusco A and
Santoro M: Human N-ras, TRK-T1, and RET/PTC3 oncogenes, driven by a
thyroglobulin promoter, differently affect the expression of
differentiation markers and the proliferation of thyroid epithelial
cells. Oncol Res. 11:421–427. 1999.PubMed/NCBI
|
|
9
|
Jhiang SM, Sagartz JE, Tong Q,
Parker-Thornburg J, Capen CC, Cho JY, Xing S and Ledent C: Targeted
expression of the ret/PTC1 oncogene induces papillary thyroid
carcinomas. Endocrinology. 137:375–378. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsui W, Huff CA, Wang Q, Malehorn MT,
Barber J, Tanhehco Y, Smith BD, Civin CI and Jones RJ:
Characterization of clonogenic multiple myeloma cells. Blood.
103:2332–2336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dalerba P and Clarke MF: Cancer stem cells
and tumor metastasis: First steps into uncharted territory. Cell
Stem Cell. 1:241–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schatton T, Murphy GF, Frank NY, Yamaura
K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM,
Weishaupt C, et al: Identification of cells initiating human
melanomas. Nature. 451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C and De
Maria R: Identification and expansion of the tumorigenic lung
cancer stem cell population. Cell Death Differ. 15:504–514. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Todaro M, Iovino F, Eterno V, Cammareri P,
Gambara G, Espina V, Gulotta G, Dieli F, Giordano S, De Maria R and
Stassi G: Tumorigenic and metastatic activity of human thyroid
cancer stem cells. Cancer Res. 70:8874–8885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gascoigne KE and Taylor SS: How do
anti-mitotic drugs kill cancer cells? J Cell Sci. 122:2579–2585.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Forte S, Pagliuca A, Maniscalchi ET,
Gulino R, Calabrese G, Ricci-Vitiani L, Pallini R, Signore M,
Parenti R, De Maria R and Gulisano M: Gene expression analysis of
PTEN positive glioblastoma stem cells identifies DUB3 and Wee1
modulation in a cell differentiation model. PLoS One. 8:e814322013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ke CC, Liu RS, Yang AH, Liu CS, Chi CW,
Tseng LM, Tsai YF, Ho JH, Lee CH and Lee OK: CD-133-expressing
thyroid cancer cells are undifferentiated, radioresistant and
survive radioiodide therapy. Eur J Nucl Mol Imaging. 40:61–71.
2013. View Article : Google Scholar
|
|
23
|
Okano H, Kawahara H, Toriya M, Nakao K,
Shibata S and Imai T: Function of RNA-binding protein Musashi-1 in
stem cells. Exp Cell Res. 306:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sutherland JM, McLaughlin EA, Hime GR and
Siddall NA: The Musashi family of RNA binding proteins: Master
regulators of multiple stem cell populations. Adv Exp Med Biol.
786:233–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eramo A, Ricci-Vitiani L, Zeuner A,
Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C and
De Maria R: Chemotherapy resistance of glioblastoma stem cells.
Cell Death Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Portugal J, Bataller M and Mansilla S:
Cell death pathways in response to antitumor therapy. Tumori.
95:409–421. 2009.PubMed/NCBI
|
|
28
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shafee N, Smith CR, Wei S, Kim Y, Mills
GB, Hortobagyi GN, Stanbridge EJ and Lee EY: Cancer stem cells
contribute to cisplatin resistance in Brca1/p53-mediated mouse
mammary tumors. Cancer Res. 68:3243–3250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu L, McArthur C and Jaffe RB: Ovarian
cancer stem-like side-population cells are tumourigenic and
chemoresistant. Br J Cancer. 102:1276–1283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu T, Xu F, Du X, Lai D, Liu T, Zhao Y,
Huang Q, Jiang L, Huang W, Cheng W and Liu Z: Establishment and
characterization of multi-drug resistant, prostate
carcinoma-initiating stem-like cells from human prostate cancer
cell lines 22RV1. Mol Cell Biochem. 340:265–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilson A, Laurenti E, Oser G, van der Wath
RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L,
Bockamp E, et al: Hematopoietic stem cells reversibly switch from
dormancy to self-renewal during homeostasis and repair. Cell.
135:1118–1129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pece S, Tosoni D, Confalonieri S, Mazzarol
G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG and Di Fiore
PP: Biological and molecular heterogeneity of breast cancers
correlates with their cancer stem cell content. Cell. 140:62–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chonghaile Ni T, Sarosiek KA, Vo TT, Ryan
JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P,
Tai YT, et al: Pretreatment mitochondrial priming correlates with
clinical response to cytotoxic chemotherapy. Science.
334:1129–1133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bettaieb A, Dubrez-Daloz L, Launay S,
Plenchette S, Rébé C, Cathelin S and Solary E: Bcl-2 proteins:
Targets and tools for chemosensitisation of tumor cells. Curr Med
Chem Anticancer Agents. 3:307–318. 2003. View Article : Google Scholar : PubMed/NCBI
|