Introduction
Pancreatic cancer is the fourth leading cause of
cancer-associated mortality in western countries (1). Pancreatic cancer has the highest
mortality rate of all major cancers, with a 5-year overall survival
of 5–6% across all disease stages. Survival rate has not improved
substantially in the past 30 years. The number of novel cases is
increasing, in the next decade an increase of 50% is expected
(2). The prognosis of patients with
metastatic pancreatic cancer (PC) is extremely poor (1). The modest results of current treatments
(first/second-line) reveal the requirement for novel therapeutic
strategies (2). Surgery remains the
only curative treatment for pancreatic cancer patients with a
5-year survival rate of 10–20% and patients diagnosed with
metastatic disease median overall survival range from 6–11 months
with systemic chemotherapy (3–6). The MPACT
trial results revealed that nab-paclitaxel plus gemcitabine
improved the overall survival (OS), progression-free survival (PFS)
and response rate of patients compared with gemcitabine treatment
alone (7). However, the associated
toxicity limited its use in patients with a poor performance status
(PS). Consequently these patients usually received single-agent
chemotherapy.
The present study reports a case of a patient with
metastatic PC with high comorbidity and poor PS [Eastern
Cooperative Oncology Group (ECOG) 2] (8), who developed bone and lung metastases
with elevated levels of gastrointestinal cancer-associated
carbohydrate antigen 19-9 (CA19–9). Adjusted doses of
nab-paclitaxel plus gemcitabine were administered as first-line
palliative chemotherapy, through which a partial response was
obtained, since the levels of CA19-9 were decreased (9) and bone pain was alleviated. Therefore,
improving the quality of life of the patient.
Case report
A 61-year-old Caucasian female patient was diagnosed
with stage I invasive lobular right breast carcinoma (pT1c-pN0-M0)
(10) at Hospital General
Universitario Gregorio Marañón (Madrid, Spain) in April 2006, where
all subsequent treatment was provided. Estrogen receptor (ER) was
30%, progesterone receptor (PR) status and c-erbB were negative and
Ki-67 was 60%. Following a right radical mastectomy and homolateral
axillary lymphadenectomy (0/14 tested nodes were positive), the
patient received adjuvant chemotherapy (4 cycles, intravenously,
cycles every 21 days; 60 mg/m2 adriamycin and 600
mg/m2 cyclophosphamide). Subsequently, anastrozole
administration was initiated; however, due to intolerance,
anastrozole was changed to letrozole 3 months later (oral
administration for 5 years).
In June 2010, a nodule was detected in the
pancreatic head/neck. A thoracoabdominal computed tomograph (CT)
scan and echo-endoscopy revealed a focal hypodense lesion measuring
2.5 cm in diameter (1.7×1.5 cm) at the junction of the body and
neck of the pancreas, which was closely attached to the portal,
superior-mesenteric and splenic vein confluence, and small lymph
nodes (<1 cm in diameter) in the celiac, gastrohepatic and
interaortocaval regions, without distant involvement. The CA19-9
levels were observed to be elevated (62 IU/ml; normal range, 2–37
IU/ml).
Pancreatic adenocarcinoma was diagnosed and
confirmed by fine-needle aspiration puncture and positron emission
tomography (PET)-CT (Biograph, Siemens, Germany), which
demonstrated that there was increased metabolic activity in the
tumor, without other macroscopic evidence of tumor activity.
Neoadjuvant chemoradiation was administered to the
patient: Gemcitabine, (1,000 mg/m2; intravenous; days 1,
8 and 15), followed by external beam radiotherapy (total dose, 50.4
Gy; 5 fractions/week of 1.8 Gy/fraction) with tegafur (1,200 mg/day
orally, throughout radiotherapy treatment) as the sensitizing
agent. Non-Response Evaluation Criteria in Solid Tumors
(non-RECIST) (11) partial response
was confirmed by CT, and a complete pancreaticoduodenectomy with
splenectomy and vascular portal resection, including the splenic
and superior-mesenteric veins with D2-lymphadenectomy, was
performed. Intraoperative radiotherapy (1,000 Gy) was successfully
delivered over the vascular risk area. The postoperative course of
the patient was favourable with no significant findings.
Histopathology on the resected tumor confirmed an
invasive ductal adenocarcinoma of the pancreas (ypT2-pN0-M0) with
extensive stromal changes and hyalinization, and focal invasion of
the peripancreatic tissue; peripancreatic lymph nodes and
large-diameter vessels were not involved.
Subsequently, 3 cycles of gemcitabine adjuvant
chemotherapy (1,000 mg/m2; intravenous; days 1, 8 and
15) was administered, following which the CA19-9 levels were
observed to be normal (15 IU/ml). During treatment, dose delays
occurred due to toxicity in the patient (grade 3
asthenia/neutropenia; grade 2 anemia), and subsequently the patient
presented chemotherapy-induced post-pancreatectomy diabetes
mellitus, exocrine pancreatic insufficiency, chronic post-radiation
enteritis and recurrent acute cholangitis associated with chronic
bile duct dilatation (thrombotic cholangiopathy).
In February 2011, post-chemotherapy PET-CT did not
reveal any evidence of disease, but vascular thrombosis of portal
and superior-mesenteric veins was observed. Therefore, the patient
was administered with enoxaparin (1 mg/kg/12 h, subcutaneously, for
6 months).
In October 2012, a simple left mastectomy was
performed for grade 2 microinvasive breast carcinoma (2 mm in size)
without axillar involvement [ER/PR, negative; Ki-67, 60%; human
epidermal growth factor receptor 2 (HER2) gene amplification,
positive; HER2/centromere enumerator probe 17 ratio, >2.2
(fluorescence in situ hybridization)]. No adjuvant therapy
was administered.
In January 2013, the patient presented with intense
lumbar pain, and chest/abdomen/pelvis CT revealed the presence of a
sclerotic lesion involving the L5 vertebral body, without spinal
canal invasion. The results of bone biopsy and PET-CT confirmed a
diagnosis of sclerotic metastasis from moderately differentiated
adenocarcinoma of pancreatic origin (cytokeratin (CK) 7, CA19-9,
CK17 and CK19, positive; CK20, ER/PR and c-erb2, negative) without
macroscopic evidence of active malignant disease in other
locations. Chemotherapy, zoledronic acid (4 mg every 28 days,
continuously) and radiotherapy at the L5 vertebra were proposed,
but the patient refused chemotherapy. Therefore, zoledronic acid
and external beam radiotherapy treatment was initiated to the
lumbar spine in March-April 2013 (10×300 cGy, 10 fractions; total
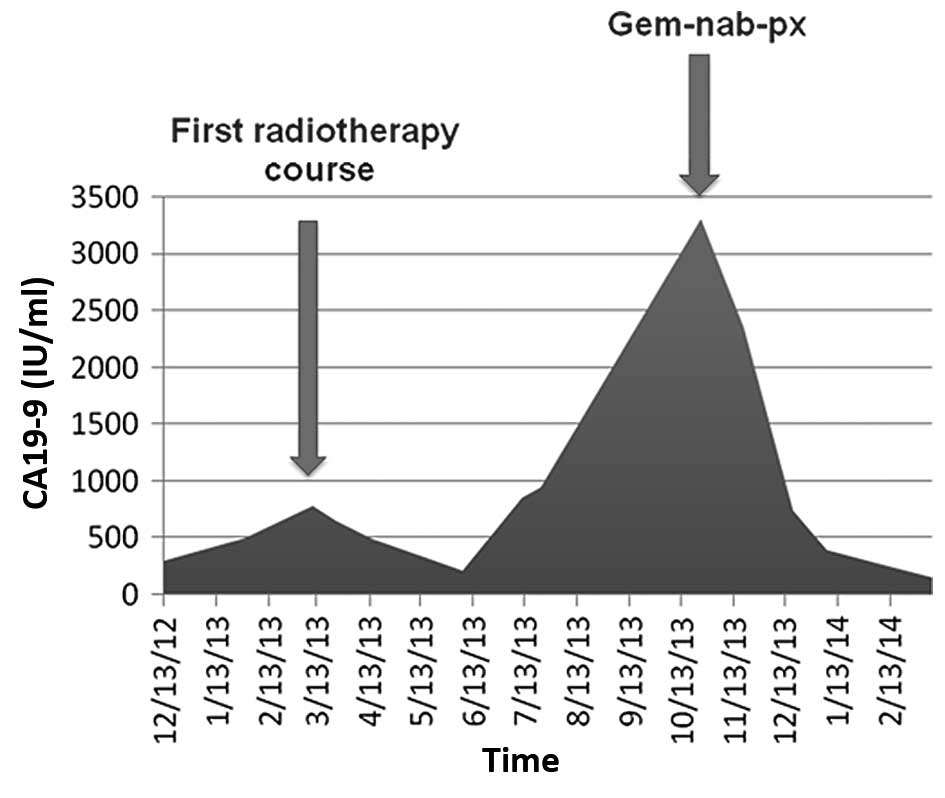
dose, 30 Gy). Bone pain improved and CA19-9 levels decreased in
June 2013 (650 and 215 IU/ml, pre- and post-radiotherapy). However,
1 month later, intense lumbar pain in the L5 reappeared and the
CA19-9 levels increased to 858 IU/ml; management with first-stage
analgesia failed [paracetamol and non-steroidal anti-inflammatory
drug (NSAID)]. The restaging evaluation did not reveal any evidence
of malignancy in other locations. Chemotherapy and surgery were
rejected by the patient; therefore, the patient received
reirradiation over the same region (5×400 cGy, 5 fractions; total
dose, 20 Gy), which had a poor analgesic response.
In October 2103, during the follow-up period, the
lumbar/sacral bone pain increased and novel abdominal pain
appeared. PET-CT demonstrated malignant activity in the
abdominopelvic lymph nodes, several bone lesions at T11, T12 and L5
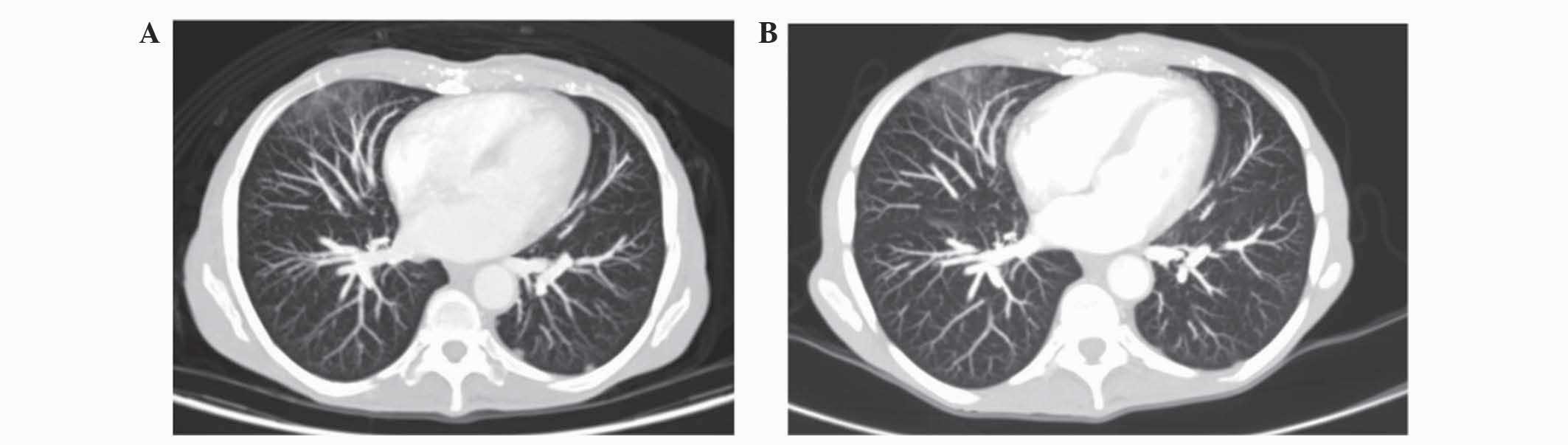
vertebrae and bilateral lung nodules (Fig. 1A); a questionable secondary
involvement of the left adrenal gland was also detected.
Reirradiation was dismissed and the patient underwent chemotherapy.
The patient was not eligible for FOLFIRINOX and capecitabine due to
her ECOG PS of 2, history of chronic diarrhea and
post-pancreatectomy malabsorption. As gemcitabine had been
previously administered, weight-adjusted gemcitabine (400
mg/m2; intravenously; days 1, 8 and 15) plus
nab-paclitaxel (50 mg/m2; intravenously; days 1, 8 and
15) was administered in November 2013 as first-line palliative
chemotherapy every 28 days.
Patient characteristics prior to treatment were as
follows: Weight, 46 kg; height, 164 cm; body mass index, 17.1; ECOG
PS, 2; CA19-9, 3306 IU/ml; and analgesia (oral oxycodone 40 mg/12
h). Following the second chemotherapy cycle in January 2014,
chest/abdomen/pelvis CT revealed stabilization of bone lesions and
abdominal lymph nodes and no pulmonary metastasis (Fig. 1B). In addition, treatment lead to a
progressive CA19-9 reduction (Fig. 2)
and bone and abdominal pain improvement (analgesic response in
lumbosacral spine). The CA19-9 levels of the patient decreased
markedly (285 IU/ml), analgesia was gradually reduced to 5 mg/12 h,
and an improvement in nocturnal rest and slight body weight
increase were noted. Overall, the tolerance was acceptable.
Toxicities included grade 2 anemia/lymphocytopenia/asthenia,
non-neutropenic fever associated with cholangitis and grade 1
diarrhea, vomiting, hepatotoxicity and lower-limb edema.
Chemotherapy was continued at the same dose, due to
the good maintained response, including the excellent analgesic
response and a decreased in ECOG PS to 1. Eventually, the patient
succumbed to acute cholangitis in April 2014.
Discussion
The present case report demonstrates the efficacy
and safety of combination therapy of nab-paclitaxel plus
gemcitabine in a patient with stage IV PC with an ECOG PS of 2 and
several comorbidities, including breast carcinoma and bone (L5
vertebral body) and lung metastases.
Isolated bone metastasis in PC is uncommon (12); therefore, in the present study, the
malignancy and origin of the bone lesion was confirmed by biopsy
prior to treatment (pancreatic adenocarcinoma instead of breast
cancer). For this advanced PC, radical surgery at the L5 was
dismissed, due to a lack of clear benefit. With regard to
chemotherapy treatment options, 4 different regimens of first-line
chemotherapy that have shown an OS benefit in advanced PC have been
reported: Gemcitabine (13),
gemcitabine plus erlotinib (14),
FOLFIRINOX (15) and nab-paclitaxel
plus gemcitabine (7).
For the present patient, who had previously received
gemcitabine monotherapy (adjuvant and neo-adjuvant), oral erlotinib
and FOLFIRINOX were not considered due to malabsorption, since the
patient had diarrhea due to exocrine pancreatic insufficiency and
chronic post-radiation enteritis, and the unfavourable toxic
profile of these two combinations (14,15).
Nab-paclitaxel plus gemcitabine combination treatment has
demonstrated efficacy in patients with PC. In the MPACT trial
(7) [Karnofsky PS ≥70, equivalent to
ECOG PS ≤2 (16)] nab-paclitaxel plus
gemcitabine combination improved the OS and PFS of patients
compared with patients treated with gemcitabine alone. Another
clinical case reported that in a patient with ECOG PS of 3
nab-paclitaxel plus gemcitabine combination treatment was also
efficient (17).
In the MPACT trial (7), a ≥90% reduction in the CA19-9 levels was
observed primarily in patients treated with nab-paclitaxel plus
gemcitabine versus patients treated with gemcitabine alone (31 vs.
14%; P<0.001). In addition, the nab-paclitaxel plus gemcitabine
combination treatment was associated with a longer median survival
time (13.5 vs. 8.2 months; ≥90 vs. <90% CA19-9 decrease). In the
present patient a ≥CA19-9 reduction was also observed.
An ongoing Spanish randomized phase I study
(FRAGANCE) is evaluating the efficacy of nab-paclitaxel plus
gemcitabine treatment in patients with PC (ECOG PS 2) (10). The promising results of phase I
demonstrated the feasibility of this combination
(standard-dose/low-dose regimen) in these patients, which is
supported by the present results.
The present patient received nab-paclitaxel plus
gemcitabine every 28 days. The excellent clinical response and
early CA19-9 reduction rendered a dose increase unnecessary. Pain
levels of the patient could be markedly controlled by reducing the
oxycodone dose. The toxicity observed was consistent with that
observed in the MPACT (7) and
FRAGANCE (phase-I) (18) trials.
In conclusion, the findings of the present study
demonstrated that nab-paclitaxel plus gemcitabine combination
treatment is a favorable treatment option with acceptable tolerance
for patients with metastatic PC and other comorbidities.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures. American Cancer Society. Atlanta, GA: 2014.
|
|
2
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neoptolemos JP, Dunn JA, Stocken D, Almond
J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C, et
al: Adjuvant chemoradiotherapy and chemotherapy in resectable
pancreatic cancer: A randomised controlled trial. Lancet.
358:1576–1585. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adsay NV, Basturk O, Cheng JD and Andea
AA: Ductal neoplasia of the pancreas: nosologic, clinicopathologic,
and biologic aspects. Semin Radiat Oncol. 15:254–264. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bilimoria KY, Bentrem DJ, Ko CY, Stewart
AK, Winchester DP and Talamonti MS: National failure to operate on
early stage pancreatic cancer. Ann Surg. 246:173–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hoff DD, Ervin T, Arena FP, et al:
Increased survival in pancreatic cancer with nab-paclitaxel plus
gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET, et al: Toxicity and response criteria of the
Eastern Cooperative Oncology Group. Am J Clin Oncol. 5:649–655.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrone CR, Finkelstein DM, Thayer SP,
Muzikansky A, Fernandez-delCastillo C and Warshaw AL: Perioperative
CA19-9 levels can predict stage and survival in patients with
resectable pancreatic adenocarcinoma. J Clin Oncol. 24:2897–2902.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours (7th). Wiley-Blackwell.
Hoboken, NJ, USA: 3241–3244. 2009.
|
|
11
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, et al: New guidelines to
evaluate the response to treatment in solid tumors. European
Organization for Research and Treatment of Cancer, National Cancer
Institute of the United States, National Cancer Institute of
Canada. J Natl Cancer Inst. 92:205–216. 2009. View Article : Google Scholar
|
|
12
|
Borad MJ, Saadati H, Lakshmipathy A,
Campbell E, Hopper P, Jameson G, Von Hoff DD and Saif MW: Skeletal
metastases in pancreatic cancer: A retrospective study and review
of the literature. Yale J Biol Med. 82:1–6. 2009.PubMed/NCBI
|
|
13
|
Burris HA 3rd, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, et al: Improvements in survival and
clinical benefit with gencitabine as first-line therapy for
patients with advanced pancreas cancer: A randomized trial. J Clin
Oncol. 15:2403–2413. 2007.
|
|
14
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buccheri G, Ferrigno D and Tamburini M:
Karnofsky and ECOG performance status scoring in lung cancer: A
prospective, longitudinal study of 536 patients from a single
institution. Eur J Cancer. 32A:1135–1141. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shakir AR: A Near-Complete response to
treatment with gemcitabine plus nab (®)-Paclitaxel in a
patient with metastatic pancreatic cancer and poor performance
status: A case report. Case Rep Oncol. 7:711–717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guillen-Ponce C, López R, Macarulla T,
Rivera F, Cubillo A, Carrato A, et al: A phase I/II trial to
evaluate the efficacy and safety of nab-paclitaxel in combination
with gemcitabine for the treatment of frail patients with advanced
or metastatic pancreatic cancer: Safety results of the Phase I
trial. Ann Oncol. 25(suppl 4): iv2382014.
|