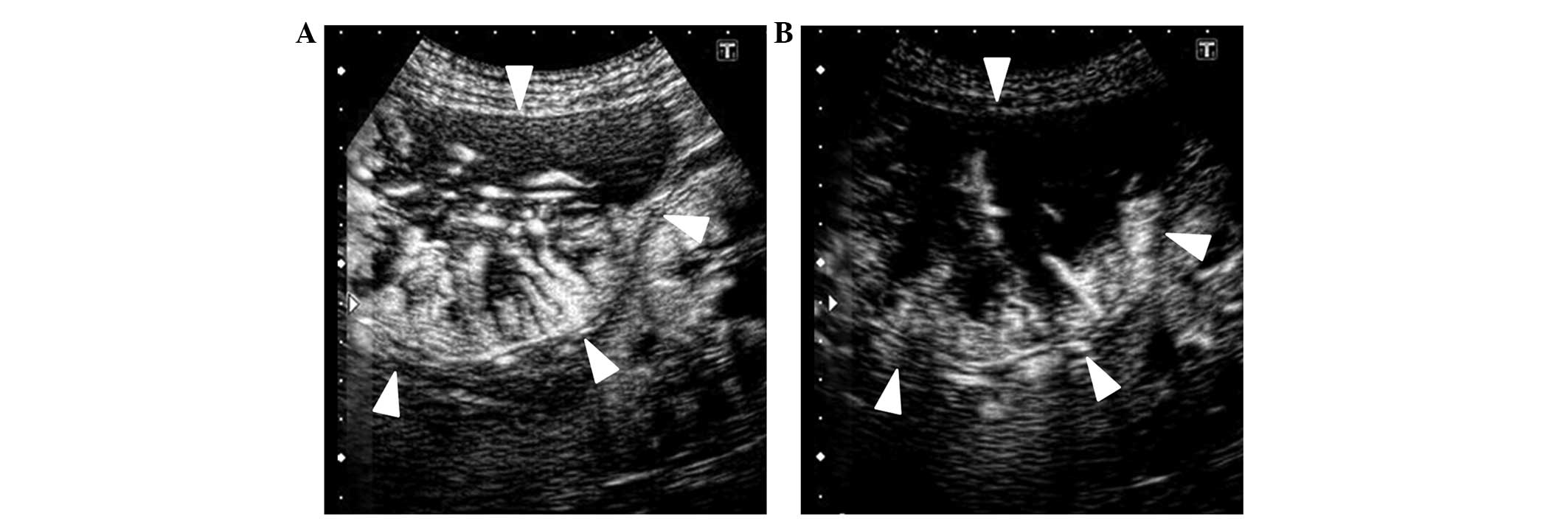
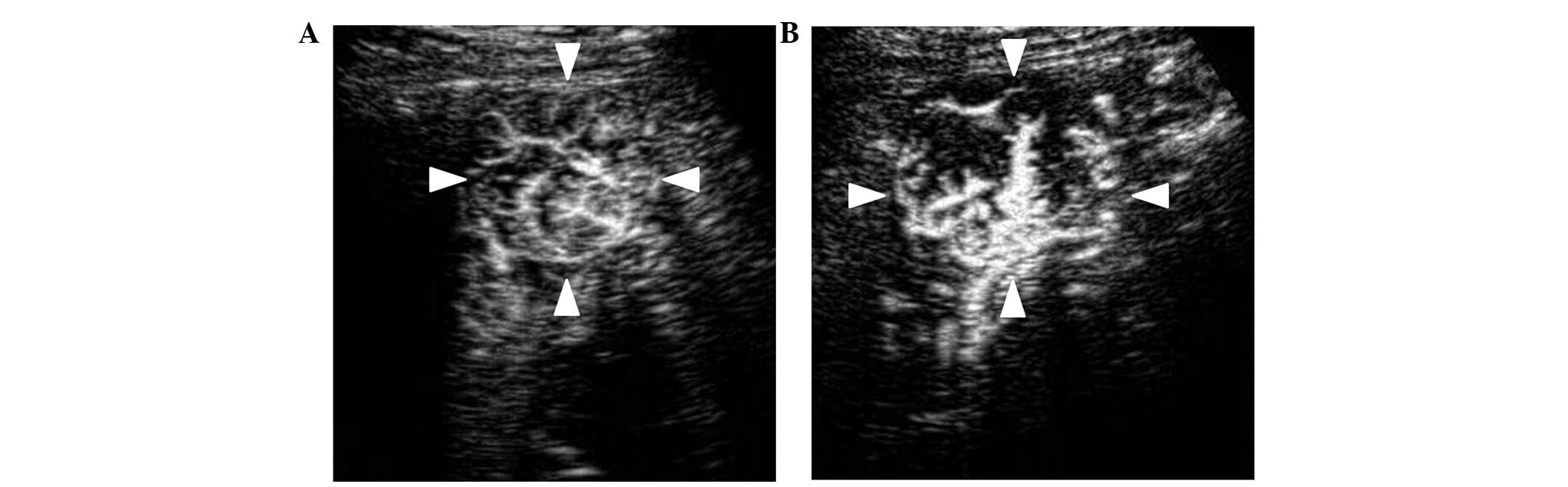
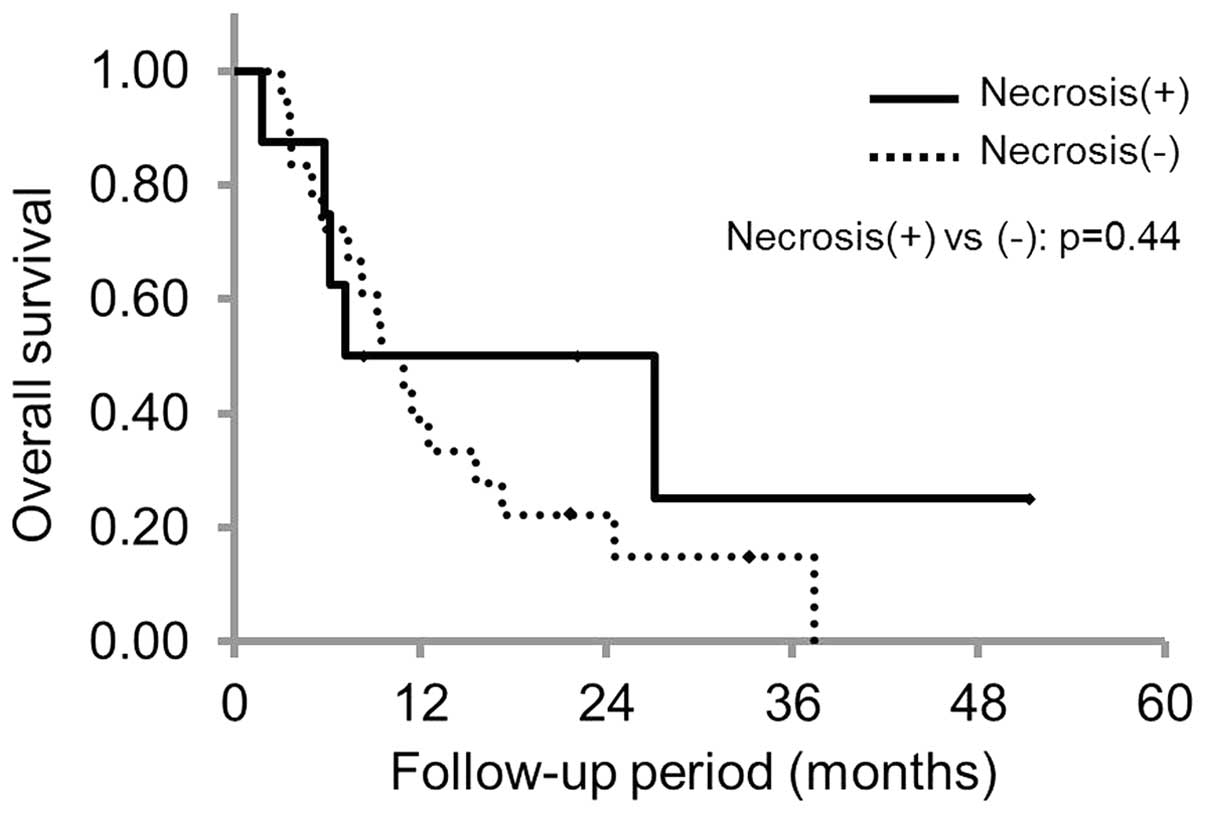
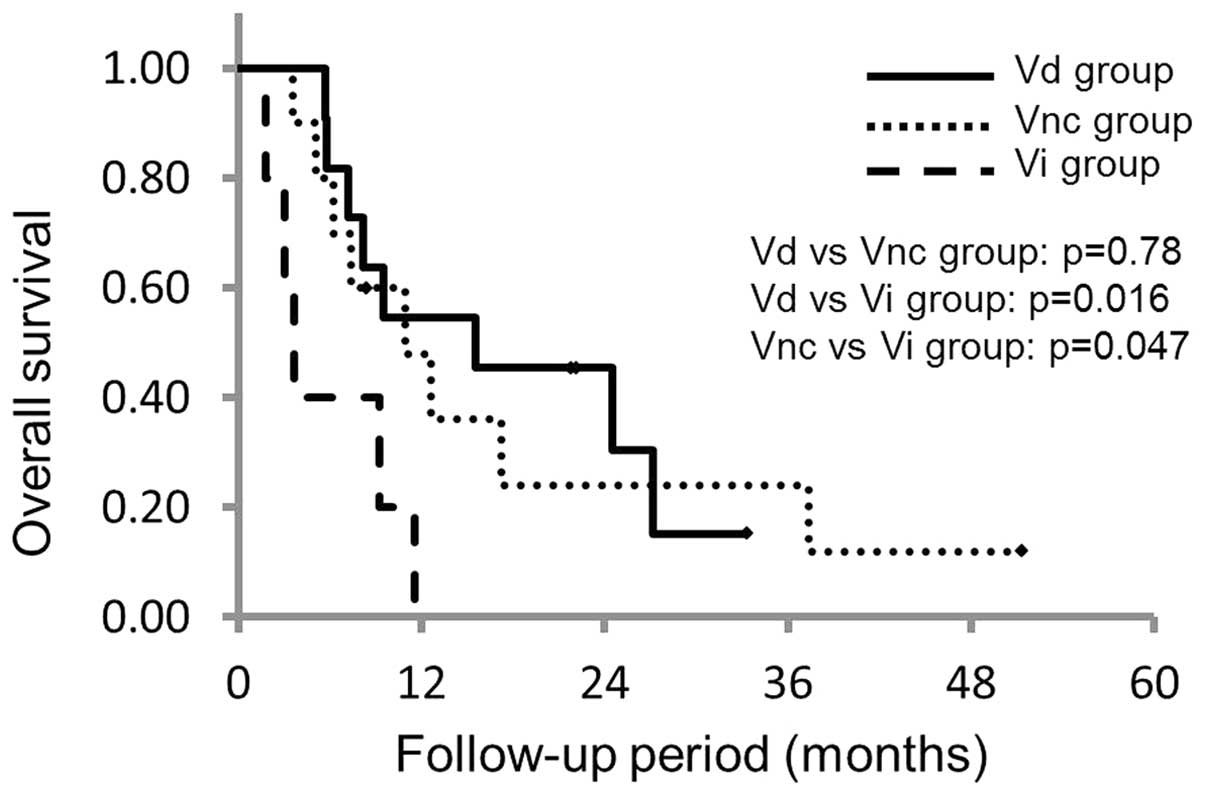
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH,
Zhang YQ, Lin XJ and Lau WY: A prospective randomized trial
comparing percutaneous local ablative therapy and partial
hepatectomy for small hepatocellular carcinoma. Ann Surg.
243:321–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yi HM, Zhang W, Ai X, Li KY and Deng YB:
Radiofrequency ablation versus surgical resection for the treatment
of hepatocellular carcinoma conforming to the Milan criteria:
Systemic review and meta-analysis. Int J Clin Exp Med. 7:3150–3163.
2014.PubMed/NCBI
|
|
4
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43–9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang YS, Adnane J, Trail PA, Levy J,
Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A,
et al: Sorafenib (BAY 43–9006) inhibits tumor growth and
vascularization and induces tumor apoptosis and hypoxia in RCC
xenograft models. Cancer Chemother Pharmacol. 59:561–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hotte SJ and Hirte HW: BAY 43–9006: Early
clinical data in patients with advanced solid malignancies. Curr
Pharm Des. 8:2249–2253. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Kubo S, Takayasu K, Sakamoto M,
Tanaka M, Ikai I, Furuse J, Nakamura K and Makuuchi M: Liver Cancer
Study Group of Japan (Committee for Response Evaluation Criteria in
Cancer of the Liver, Liver Cancer Study Group of Japan): Response
evaluation criteria in cancer of the liver (RECICL) proposed by the
liver cancer study group of Japan (2009 revised version). Hepatol
Res. 40:686–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi H, Charnsangavej C, Faria SC,
Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA and
Benjamin RS: Correlation of computed tomography and positron
emission tomography in patients with metastatic gastrointestinal
stromal tumor treated at a single institution with imatinib
mesylate: Proposal of new computed tomography response criteria. J
Clin Oncol. 25:1753–1759. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Watanabe M, Shiozawa K, Takahashi M, Wakui
N, Otsuka Y, Kaneko H, Tanikawa K, Shibuya K, Kamiyama N and Sumino
Y: Parametric imaging using contrast-enhanced ultrasound with
Sonazoid for hepatocellular carcinoma. J Med Ultrasonics. 37:81–86.
2010. View Article : Google Scholar
|
|
14
|
Shiozawa K, Watanabe M, Kikuchi Y, Kudo T,
Maruyama K and Sumino Y: Evaluation of sorafenib for hepatocellular
carcinoma by contrast-enhanced ultrasonography: A pilot study.
World J Gastroenterol. 18:5753–5758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugimoto K, Moriyasu F, Kamiyama N, Metoki
R, Yamada M, Imai Y and Iijima H: Analysis of morphological
vascular changes of hepatocellular carcinoma by microflow imaging
using contrast-enhanced sonography. Hepatol Res. 38:790–799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Liu GJ, Lu MD, Xu HX and Xie XY:
Evaluation of the vascular archirecture of hepatocellular carcinoma
by micro flow imaging: Pathologic correlation. J Ultrasound Med.
26:461–467. 2007.PubMed/NCBI
|
|
17
|
Linden RA, Trabulsi EJ, Forsberg F,
Gittens PR, Gomella LG and Halpern EJ: Contrast enhanced ultrasound
flash replenishment method for directed prostate biopsies. J Urol.
178:2354–2358. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo M: Hepatocellular carcinoma 2009 and
beyond: From the surveillance to molecular targeted therapy.
Oncology. 75(Suppl 1): 1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao H, Yao JL, Wang Y and Zhou KR:
Detection of small hepatocellular carcinoma: Comparison of dynamic
enhancement magnetic resonance imaging and multiphase
multirow-detector helical CT scanning. World J Gastroenterol.
13:1252–1256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruix J, Sala M and Llovet JM:
Chemoembolization for hepatocellular carcinoma. Gastroenterology.
127(Suppl 1): S179–S188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moschouris H, Malagari K, Gkoutzios P,
Kalokairinou M, Stamatiou K, Chatzimichail K, Kornezos I,
Karagiannis E, Kiltenis M and Papadaki MG: Intermediate and
advanced hepatocellular carcinoma treated with the antiangiogenic
agent sorafenib. Evaluation with unenhanced and contrast-enhanced
ultrasonography. Med Ultrason. 14:87–94. 2012.PubMed/NCBI
|
|
23
|
Tanaka H, Iijima H, Higashiura A, Yoh K,
Ishii A, Takashima T, Sakai Y, Aizawa N, Iwata K, Ikeda N, et al:
New malignant grading system for hepatocellular carcinoma using the
Sonazoid contrast agent for ultrasonography. J Gastroenterol.
49:755–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato K, Tanaka S, Mitsunori Y, Mogushi K,
Yasen M, Aihara A, Ban D, Ochiai T, Irie T, Kudo A, et al:
Contrast-enhanced intraoperative ultrasonography for vascular
imaging of hepatocellular carcinoma: Clinical and biological
significance. Hepatology. 57:1436–1447. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matsui O, Kadoya M, Kameyama T, Yoshikawa
J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R and Ida
M: Benign and malignant nodules in cirrhotic livers: Distinction
based on blood supply. Radiology. 178:493–497. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakamoto M, Hirohashi S and Shimosato Y:
Early stages of multistep hepatocarcinogenesis: Adenomatous
hyperplasia and early hepatocellular carcinoma. Hum Pathol.
22:172–178. 1991. View Article : Google Scholar : PubMed/NCBI
|