Introduction
In the USA and other regions of the world, bladder
cancer is one of the most common forms of urologic cancers
(1). Furthermore, bladder cancer is
the primary cause of death among urinary tumors in China (2). An individual afflicted with
non-muscle-invasive bladder cancer is susceptible to a high
recurrence rate, due to the aggressive nature of this type of
cancer, and may rapidly progress to muscle-invasive disease
(3). The prognosis of individuals
with muscle-invasive bladder cancer is extremely poor, due to the
high rate of metastasis (4).
It is well known that the most common type of
bladder cancer is bladder urothelial carcinoma. There is a great
deal of evidence that supports the theory that
epithelial-mesenchymal transition (EMT) is pivotal in tumor
invasion and metastasis, since it provides cells with a more motile
and invasive phenotype (5–7). The primary characteristics of EMT is the
disappearance of epithelial cell polarity, acquisition of
mesenchymal cell properties (8) and
abnormal E-cadherin and N-cadherin expression (9). The EMT process is extremely important
for progression of the tumor. E-cadherin and N-cadherin are
considered biomarkers of EMT (10).
In tumor development, E-cadherin and N-cadherin functions are
varied. However, the exact process of EMT remains unclear, and the
function of E-cadherin and N-cadherin double-negative expression is
rarely studied. In order to gather additional knowledge regarding
the process of EMT, the present study investigated E-cadherin and
N-cadherin double-negative expression in various pathological
grades of infiltrative bladder urothelial carcinoma tissues, and
examined the biological characteristics of these cells and deduced
the association of E-cadherin and N-cadherin expression with
EMT.
Materials and methods
Tissue samples and cell culture
Human bladder cancer tissues were obtained from
patients at Nanfang Hospital, which is affiliated to the Southern
Medical University (Guangzhou, China). The Bioethics Committee of
Nanfang Hospital approved the present study. All participants were
informed about the purpose of the study and provided their written
consent. Three specimens were collected (male; aged 65, 83 and 88
years), which were pathologically diagnosed biopsy specimens of
low-, mid- and high-level infiltrative bladder urothelial
carcinoma. Human urinary bladder grade II carcinoma 5637,
transitional cell carcinoma UMUC-3 and invasive bladder carcinoma
EJ cells were obtained from Guangzhou Jennio Biological Technology
Co., Ltd., (Guangzhou, China), and the cells were preserved in the
laboratory. The cells were cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific Inc., Waltham, MA, USA) that contained 10% fetal
bovine serum (FBS; HyClone™, GE Healthcare, Logan, UT, USA) at 37°C
in a 5% CO2 humidified incubator.
Immunofluorescence of tissue
samples
Tissue samples were cut into 10 µm thick sections by
freeze-sectioning. The sections of tissue samples were placed onto
12×12 mm glass slides. The 10 µm-thick sections were fixed with
fixing liquid (70% acetone and 30% anhydrous methanol; 4°C
precooling) for 10 min, washed 3 times with phosphate-buffered
saline (PBS; 1 wash/5 min), blocked in PBS with 5% bovine serum
albumin (BSA; Beyotime Institute of Biotechnology, Shanghai, China)
for 30 min, washed again with PBS, then incubated at 4°C in a
refrigerator overnight with rabbit polyclonal anti-E-cadherin (Cell
Signaling Technology, Inc., Danvers, MA, USA; dilution, 1:100;
catalog no. 3195p) and mouse monoclonal anti-N-cadherin antibodies
(Abcam, Cambridge, MA, USA; dilution, 1:100; catalog no. ab98952).
Following an additional wash with PBS, the sections were incubated
with species-specific secondary antibodies (goat anti-mouse Dylight
488, catalog no. ZF-0512; and goat anti-rabbit Dylight 594, catalog
no. ZF-0516; Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China; dilution, 1:100) at 37°C for 90 min and washed with
PBS. Subsequently, the sections were incubated with fluoroscein
isothiocyanate (FITC)-phalloidin (catalog no. P5282; Sigma-Aldrich,
St. Louis, MO, USA) at 37°C for 30 min in the dark and washed with
PBS again, and the slides were then stained with
4′,6-diamidino-2-phenylindole (DAPI; Zhongshan Golden Bridge
Biotechnology Co., Ltd.) for nuclear staining. Images were capyured
and analyzed with a microscope (Olympus Corporation, Tokyo,
Japan).
Immunofluorescence of bladder cancer
cells
Human bladder cancer cells (1×105) were
cultured in a confocal dish and subjected to immunofluorescence
analysis at a 80–90% confluence of cells. The cells were washed 3
times with PBS (1 wash/5 min). Following fixing with 4%
paraformaldehyde, permeabilization with 0.2% Triton X-100 and
washing with PBS, the cells were blocked using 5% BSA in PBS for 30
min. Subsequently, the cells were washed with PBS, then incubated
at 4°C overnight with anti-E-cadherin and anti-N-cadherin
antibodies (dilution, 1:100). Following washing with PBS, the cells
were incubated with species-specific secondary antibodies (goat
anti-mouse Dylight 488; goat anti-rabbit Dylight 594; dilution
1:100) at 37°C for 90 min. Following another wash with PBS, the
cells were incubated with FITC-phalloidin at 37°C for 2 min in the
dark. Subsequent to a final wash with PBS, the nuclei of the cells
were stained with DAPI, and images were photographed and analyzed
with an Olympus microscope.
Western blotting
Protein samples were extracted from bladder cancer
cells with M-PER™(Mammalian Protein Extraction Reagent, catalog no.
78501; Thermo Fisher Scientific Inc.), Equivalent quantities of
proteins (50 µg) were separated with 6% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and subsequently transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk in PBS with
Tween 20 and incubated at 4°C overnight with the primary
antibodies, rabbit polyclonal anti-E-cadherin and mouse monoclonal
anti-N-cadherin antibody, and a mouse monoclonal anti-Tubulin
antibody (Cell Signaling Technology, Inc.; catalog no. T6199;
dilution, 1:1,000). The cells were then incubated with secondary
antibodies conjugated with horseradish peroxidase (HRP)
(anti-rabbit or anti-mouse; catalog no. 7074 and 7076,
respectively; dilution, 1:1,000; Cell Signaling Technology, Inc.
Danvers, MA, USA) for 1 h at room temperature. Detection of the
protein bands was performed by a FluorChem® FC2 Imaging
System (Alpha Innotech, San Leandro, CA, USA).
Cell proliferation assay
The bladder cancer 5637, UMUC-3, and EJ cells were
seeded in a 96-well plate at a density of 600 cells/well, and
incubated for 7 days. Following the incubation period, a cell
proliferation assay was performed by adding 10 µl cell counting
kit-8 solution (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) at a set time each day over the 7 day period.
Following incubation for 2 h with CCK-8, the absorbance values were
detected by EnSpire® 2300 multilabel reader
(PerkinElmer, Inc., Waltham, MA, USA) at 570 nm.
Migration abilities in vitro
In total, 200 µl serum-free RPMI-1640 media
containing tumor cells (5×104 cells/well) was added to
the upper chamber of a Transwell chamber (Corning Incorporated,
Corning, NY, USA), and 500 µl RPMI-1640 containing 10% FBS was
added to the lower chamber as a chemo-attractant. Following
incubation for 12 h (37°C; 5% CO2), the non-migratory
tumor cells were removed with cotton swabs. Subsequently, the
migratory tumor cells that were located on the lower surface of
membrane were fixed for 25 min using 4% paraformaldehyde. The cells
were then stained with hematoxylin for 20 min. Following rinsing
with PBS, the Transwell chambers were inspected via inverted
microscopy in 5 random visual fields.
Invasion abilities in vitro
In total, 50 µl Matrigel (dilution, 1:5 with
RPMI-1640) was added to a Transwell chamber. A total of 200 µl
serum-free RPMI-1640 containing tumor cells (1×105
cells/well) was added to the upper chamber, and 500 µl RPMI-1640
containing 10% FBS was added to the lower chamber as a chemotactic
factor. Following incubation for 36 h (37°C; 5% CO2),
the non-invading tumor cells were removed with cotton swabs.
Subsequently, 4% paraformaldehyde was used to fix the invading
tumor cells that were located in the lower surface of membrane for
25 min. The cells were then stained with hematoxylin for 20 min.
Following rinsing with PBS, the Transwell chambers were inspected
via inverted microscopy in 5 random visual fields.
Plate colony formation test
In total, ~2×102 cells/well were added to
6-well culture plates. Following incubation for 8 days (37°C; 5%
CO2), 4% paraformaldehyde was used to fix the tumor
cells for 25 min. The cells were washed 3 times with PBS and
stained with hematoxylin for 25 min, and rinsed again with PBS. The
number of colonies (≥50 cells) were counted under a microscope, and
the following equation was used: Colony formation efficiency =
(number of colonies / number of inoculating cells) × 100%.
Tumorigenicity in vivo assay
The Ethics Committee of the Southern Medical
University approved experimental procedures with animals in the
present study (contract no., 2011016). Female, 5-week-old, immune
deficient mice (n=9) were maintained at the Center of Experimental
Animals, Southern Medical University. All mice were maintained at
room temperature under specific pathogen-free conditions and
exposed to 12 h light/dark cycles. A total of 3 animals (per group)
were kept in each cage with ad libitum access to water and
food.
The left and right flanks of the immune deficient
mice received subcutaneous injections with tumor cells (100 µl;
2×106 cells; n=3 per group). The tumor size was measured
with a ruler every three days between day 6 and day 24 following
the injection. Four weeks later, the subcutaneous tumors were
resected and the mice were sacrificed. The subcutaneous tumor was
cut into 10-µm-thick sections using freeze-sectioning and observed
by hematoxylin and eosin (H&E) staining. The tumor volume was
calculated with the following formula: Tumor volume = d2
× D / 2, where d is the shortest diameter and D is the longest
diameter (11).
Statistical analysis
SPSS version 13.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Data was expressed as the
mean ± standard deviation. Statistical analysis was performed with
Student's t-test between two groups, or one-way analysis of
variance for more than three groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
E-cadherin and N-cadherin
double-negative expression in bladder urothelial carcinoma
tissues
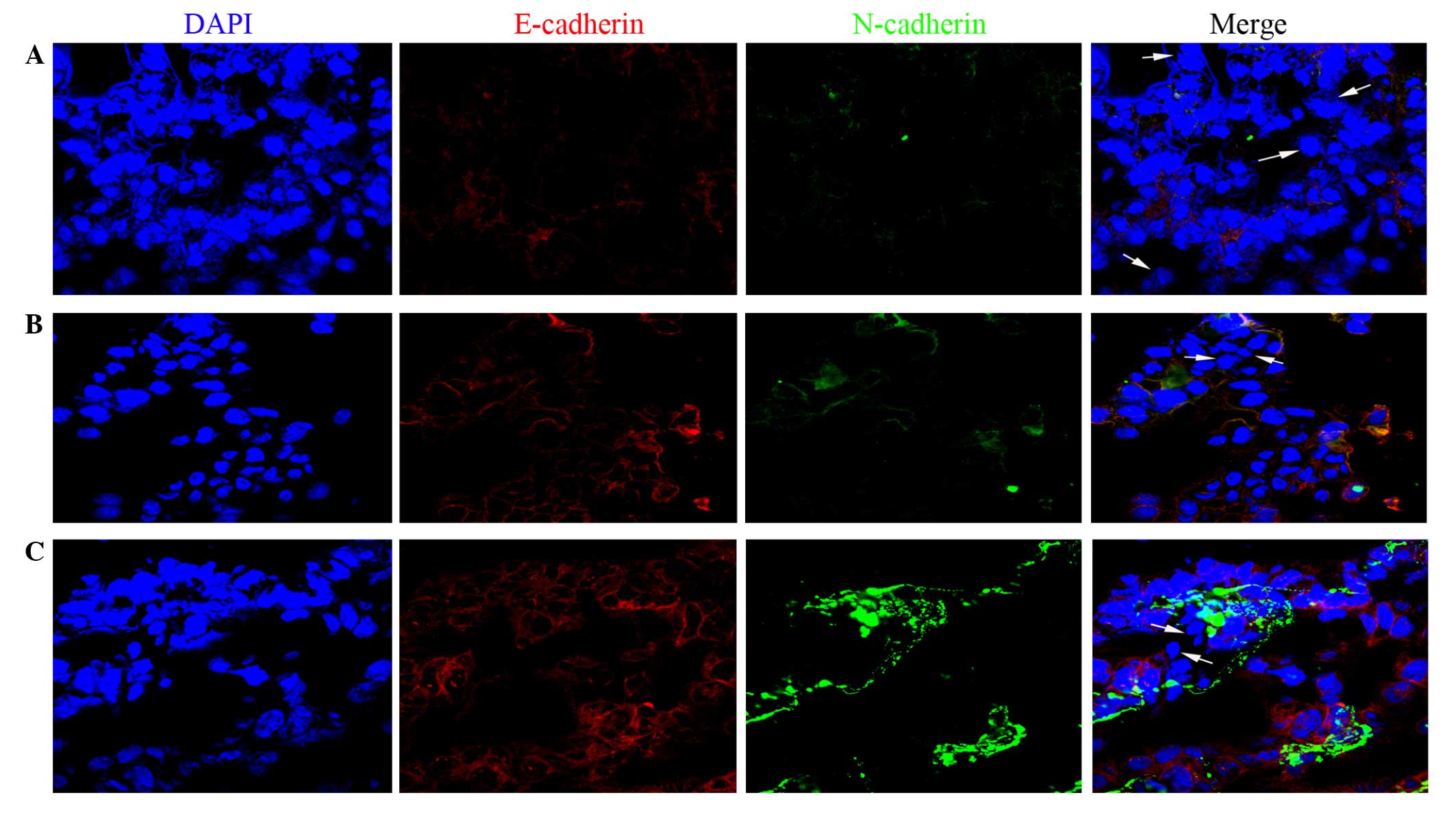
Immunofluorescence analysis of tissue samples
revealed that E-cadherin and N-cadherin double-negative expression
was detected in low-, mid- and high-level infiltrative bladder
urothelial carcinoma (Fig. 1).
Therefore, the assay demonstrated that E-cadherin and N-cadherin
double-negative expression was present in infiltrative bladder
urothelial carcinoma.
E-cadherin and N-cadherin expression
in human bladder cancer 5637, UMUC-3 and EJ cells
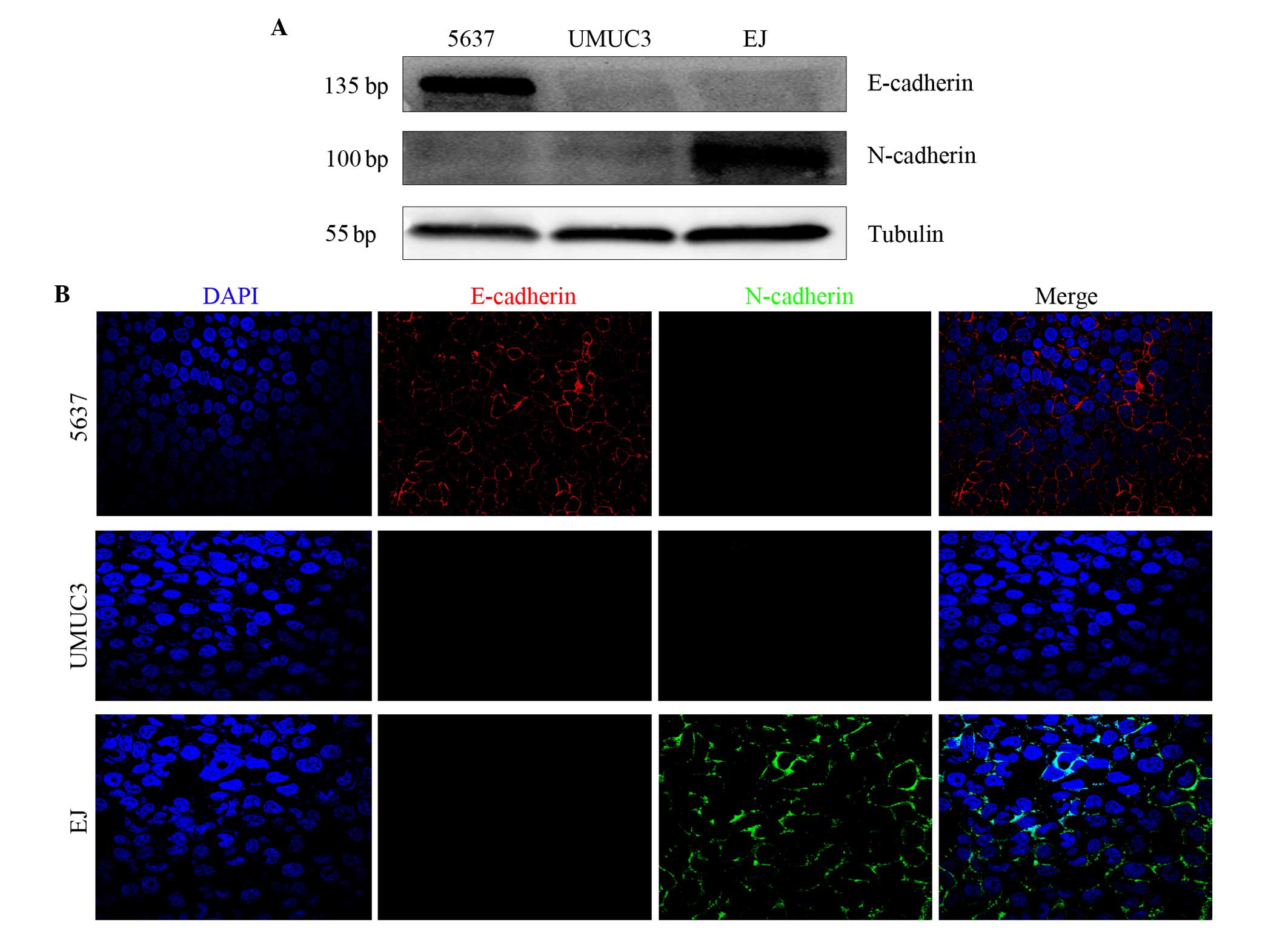
Western blotting revealed that E-cadherin and
N-cadherin double-negative expression was present in UMUC-3 cells.
However, E-cadherin positive and N-cadherin negative expression was
identified in 5637 cells, while E-cadherin negative and N-cadherin
positive expression was identified in EJ cells (Fig. 2A). Immunofluorescence analysis of the
bladder cancer cells demonstrated the same result as the western
blot analysis (Fig. 2B). Therefore,
the two assays revealed that E-cadherin and N-cadherin
double-negative expression was detected only in UMUC-3 cells.
Functional comparison of bladder
cancer 5637, UMUC-3 and EJ cells
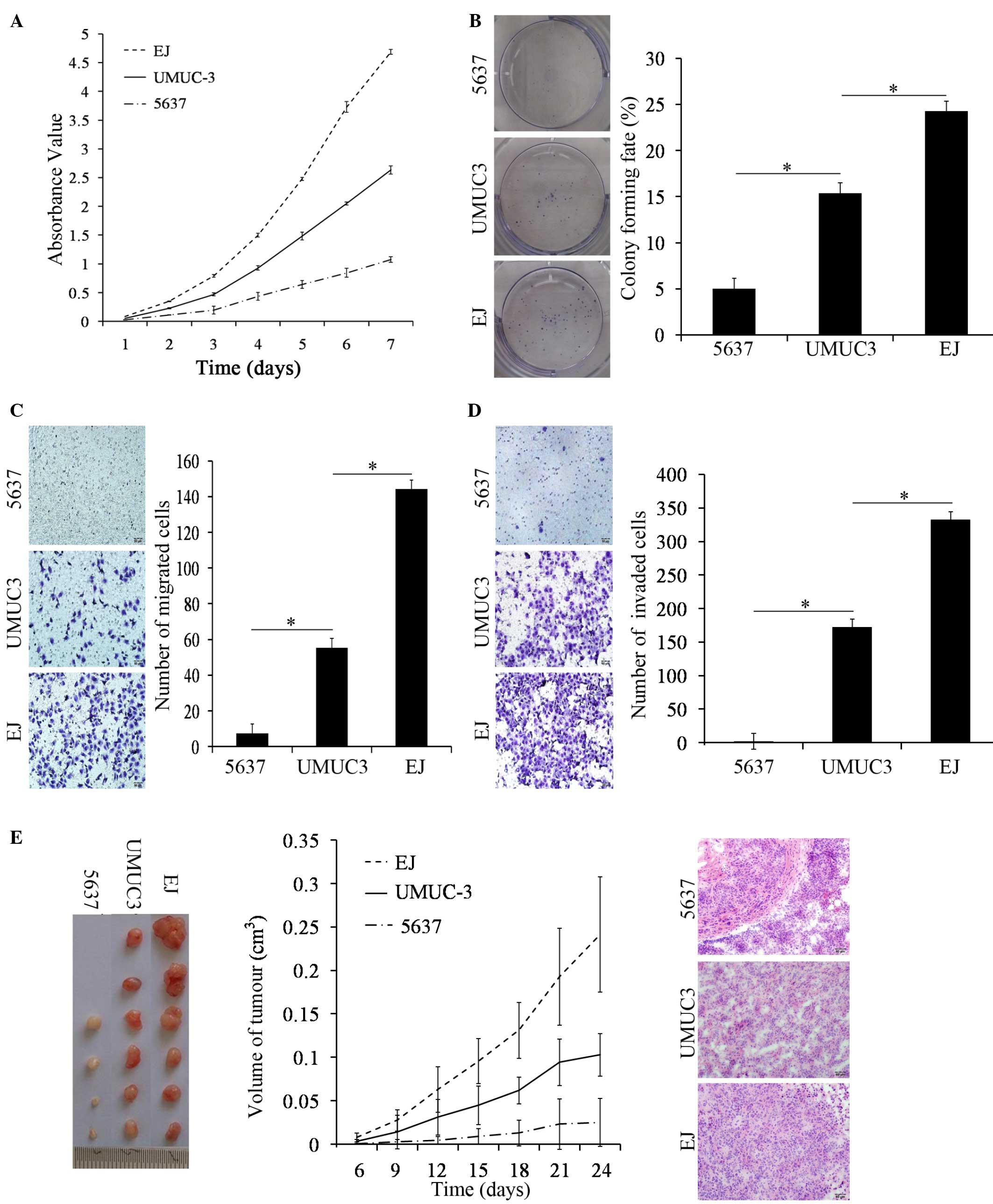
A cell proliferation assay revealed that the ability
of UMUC-3 cells to proliferate was significantly increased compared
with 5637 cells on day 3, 4, 5, 6 and 7 using a CCK-8 assay
(P<0.001); however, the cell proliferative abilities of the
UMUC-3 cells was significantly weaker compared with the EJ cells
(P=0.004; Fig. 3A). The plate colony
formation assay revealed that UMUC-3 cells formed larger and more
numerous colonies compared with the 5637 cells (P<0.001).
However, in comparison to EJ cells, there was a significant
decrease in the quantity of colonies of UMUC-3 cells (P<0.001;
Fig. 3B). The results of the two
assays revealed that the proliferative abilities of UMUC-3 cells
was decreased compared with EJ cells and increased compared with
5637 cells.
Using the same number of cells and the same
incubation conditions, UMUC-3 cells exhibited a significant
increase in motility and invasion abilities compared with 5637
cells (both P<0.001). However, in comparison to EJ cells, the
motility and invasion abilities of the UMUC-3 cells exhibited a
significant decrease (both P<0.001; Fig. 3C and D). Therefore, the migration and
Matrigel invasion assays revealed that the transmembrane activity
of UMUC-3 cells was decreased compared with EJ cells and increased
compared with 5637 cells.
Under the same conditions, an injection of UMUC-3
cells led to the development of increased tumor volumes compared
with 5637 cells in immune deficient mice (P<0.001). Compared
with an injection of EJ cells in immune deficient mice, the
subcutaneous tumor volume of UMUC-3 cells was significantly smaller
(P=0.017; Fig. 3E). The change in
tumor volume of the 5637 cells group change was not significant
with time elapsed (P=0.138), however, the tumor volumes of the
UMUC-3 and EJ cell groups significantly increased with time elapsed
(both P<0.001; Fig. 3E). The
morphology of subcutaneous tumor sections were stained with H&E
and observed under a microscope; the tumor cells showed diffuse
patchy distribution, with a clearly abnormal shape, deeply stained
nuclei and common mitotic figures (Fig.
3E). Consequently, the animal assays revealed that the in
vivo tumorigenic abilities of the UMUC-3 cells were decreased
compared with the EJ cells and increased compared with the 5637
cells.
Discussion
Greenberg and Hay (12) proposed the theory of EMT in 1982; it
was revealed that cultured lens epithelial cells morphologically
converted into mesenchyme-like cells with pseudopodia in the gel.
EMT is a multistep process. The primary characteristics of EMT are
the disappearance of epithelial cell polarity and acquisition of
mesenchymal cell properties (8). The
important barrier functions of epithelial cells are promoted by
their tight cell-cell junctions (13), and the disappearance of cell-cell
junctions may cause morphological alterations and increase the
invasion and metastatic abilities of epithelial cells. The primary
mediator of cell-cell junction is cadherin, a transmembrane
glycoprotein, that is observed in epithelial tissue. Cadherin is a
calcium-dependent adhesion protein, which promotes tight cell-cell
junction molecules that promote the formation and growth of
malignant tumors (14). The primary
cadherins identified are E-cadherin, P-cadherin and N-cadherin
(15). E-cadherin not only adjusts
the connections of nearby epithelial cells, but also maintains cell
phenotype and cell polarity. Therefore, E-cadherin is an extremely
key role in epithelial cell-cell junctions (16). E-cadherin is also crucial for tumor
suppression as it prevents tumor cell invasion (17). Several studies have revealed that the
loss of E-cadherin function may induce EMT and increase tumor
invasion and metastasis (18–21).
N-cadherin was first identified in muscle and neural
cells; however, a recent study revealed that N-cadherin was also
identified in mesenchymal cells (22). N-cadherin is located at the adherens
junction, where it promotes dynamic contact between the matrix and
cells, and between the cells themselves (23). The cytoplasmic expression of
N-cadherin participates in multiple intracellular signaling
pathways (24). Much study has
indicated that N-cadherin may increase tumor cell motility and
promote tumor cell metastasis and invasion in numerous experimental
models (25). In addition, N-cadherin
has been used as a biomarker of mesenchymal differentiation in the
study of EMT (26).
N-cadherin and E-cadherin are typical cadherins, and
are biomarkers of EMT (10). The
prominent characteristic of EMT is E-cadherin decrease and
N-cadherin increase, also referred to as cadherin switching. In the
majority of tumor cells, cadherin switching plays an extremely
crucial role (27). Cadherin
switching has been observed to be an extremely crucial process in
bladder cancer development (28), and
cadherin switching promotes tumor invasion and metastasis in
bladder cancer development (29,30). Much
study has demonstrated the potential effect of EMT in bladder
cancer development (5,6,31).
Although there are numerous studies concerning EMT, the study of
E-cadherin and N-cadherin double-negative expression has rarely
been examined, and the exact process of EMT remains unclear.
The present study demonstrated that E-cadherin and
N-cadherin double-negative expression is observed in non-muscle
invasive bladder cancers using immunohistochemical staining
(32). In addition, the present study
detected E-cadherin and N-cadherin double-negative expression in
low-, mid- and high-level infiltrative bladder urothelial carcinoma
using immunofluorescence assays. These results suggest that
E-cadherin and N-cadherin double-negative expression exists in
bladder urothelial carcinoma. It is known that the prominent
characteristic of EMT is E-cadherin decrease and N-cadherin
increase. Therefore, in order to study the characteristics of
bladder cancer cells with E-cadherin and N-cadherin double-negative
expression and deduce its association with EMT, bladder cancer
cells of E-cadherin positive and N-cadherin negative expression,
and E-cadherin negative and N-cadherin positive expression were
selected as controls. Western blotting and immunofluorescence
assays revealed that E-cadherin and N-cadherin double-negative
expression were identified in the bladder cancer UMUC-3 cell line.
E-cadherin positive and N-cadherin negative expression were
identified in the bladder cancer 5637 cell line, and E-cadherin
negative and N-cadherin positive expression were identified in the
bladder cancer EJ cell line. By comparing the functions among these
three cell lines, the present study demonstrated that the
biological characteristics of UMUC-3 cells were significantly
stronger compared with 5637 cells, and significantly weaker
compared with EJ cells.
In conclusion, the present study revealed that
E-cadherin and N-cadherin double-negative expression exists in
bladder urothelial carcinoma. Analysis of the biological
characteristics of bladder cancer cells with E-cadherin and
N-cadherin double-negative expression were performed by comparing
these type of cells with bladder cancer cells of E-cadherin
positive and N-cadherin negative expression, and E-cadherin
negative and N-cadherin positive expression. The present results
suggest that the biological characteristics of bladder cancer cells
with E-cadherin and N-cadherin double-negative expression were
significantly stronger compared with bladder cancer cells of
E-cadherin positive and N-cadherin negative expression. However,
the biological characteristics of double-negative cells were
significantly weaker compared with bladder cancer cells that
exhibited E-cadherin negative and N-cadherin positive expression.
Therefore, the biological characteristics of bladder cancer cells
with E-cadherin and N-cadherin double-negative expression existed
between bladder cancer cells of E-cadherin positive and N-cadherin
negative expression and bladder cancer cells of E-cadherin negative
and N-cadherin positive expression. Overall, the present study
deduces that the status of E-cadherin and N-cadherin
double-negative expression may participate in the process of EMT in
the pathogenesis of bladder urothelial carcinoma. The present study
may aid in the understanding of the process and effect of EMT in
the pathogenesis of bladder cancer.
Acknowledgements
The present study was supported by the Chinese
National Natural Science Foundation (grant no. 81272844) and
Educational Scientific Research Project of Guangdong Province
(grant no. 2013KJCX0039).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pasin E, Josephson DY, Mitra AP, Cote RJ
and Stein JP: Superficial bladder cancer: An update on etiology,
molecular development, classification, and natural history. Rev
Urol. 10:31–43. 2008.PubMed/NCBI
|
|
4
|
Yun SJ and Kim WJ: Role of the
epithelial-mesenchymal transition in bladder cancer: From prognosis
to therapeutic target. Korean J Urol. 54:645–650. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baumgart E, Cohen MS, Neto Silva B, Jacobs
MA, Wotkowicz C, Rieger-Christ KM, Biolo A, Zeheb R, Loda M,
Libertino JA and Summerhayes IC: Identification and prognostic
significance of an epithelial-mesenchymal transition expression
profile in human bladder tumors. Clin Cancer Res. 13:1685–1694.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995,
Discussion-5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu GL, Yang HJ, Liu T and Lin YZ:
Expression and significance of E-cadherin, N-cadherin, transforming
growth factor-β1 and Twist in prostate cancer. Asian Pac J Trop
Med. 7:76–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development and disease. J Cell Biol. 172:973–981. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB,
Liu W, Qi Q, Lu N, Tao L, Wang XT, et al: Isolation and
characterization of cancer stem like cells in human glioblastoma
cell lines. Cancer Lett. 279:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenburg G and Hay ED: Epithelia
suspended in collagen gels can lose polarity and express
characteristics of migrating mesenchymal cells. J Cell Biol.
95:333–339. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wheelock MJ and Johnson KR: Cadherins as
modulators of cellular phenotype. Annu Rev Cell Dev Biol.
19:207–235. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nollet F, Kools P and van Roy F:
Phylogenetic analysis of the cadherin superfamily allows
identification of six major subfamilies besides several solitary
members. J Mol Biol. 299:551–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rangel MC, Karasawa H, Castro NP, Nagaoka
T, Salomon DS and Bianco C: Role of Cripto-1 during
epithelial-to-mesenchymal transition in development and cancer. Am
J Pathol. 180:2188–2200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: How does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Derksen PW, Liu X, Saridin F, van der
Gulden H, Zevenhoven J, Evers B, van Beijnum JR, Griffioen AW, Vink
J, Krimpenfort P, et al: Somatic inactivation of E-cadherin and p53
in mice leads to metastatic lobular mammary carcinoma through
induction of anoikis resistance and angiogenesis. Cancer Cell.
10:437–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lehembre F, Yilmaz M, Wicki A, Schomber T,
Strittmatter K, Ziegler D, Kren A, Went P, Derksen PW, Berns A, et
al: NCAM-induced focal adhesion assembly: A functional switch upon
loss of E-cadherin. Embo J. 27:2603–2615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Packer AI, Elwell VA, Parnass JD, Knudsen
KA and Wolgemuth DJ: N-cadherin protein distribution in normal
embryos and in embryos carrying mutations in the homeobox gene
Hoxa-4. Int J Dev Biol. 41:459–468. 1997.PubMed/NCBI
|
|
23
|
Hazan RB, Kang L, Whooley BP and Borgen
PI: N-cadherin promotes adhesion between invasive breast cancer
cells and the stroma. Cell Adhes Commun. 4:399–411. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derycke LD and Bracke ME: N-cadherin in
the spotlight of cell-cell adhesion, differentiation,
embryogenesis, invasion and signalling. Int J Dev Biol. 48:463–476.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jennbacken K, Tesan T, Wang W, Gustavsson
H, Damber JE and Welen K: N-cadherin increases after androgen
deprivation and is associated with metastasis in prostate cancer.
Endocr Relat Cancer. 17:469–479. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Wever O, Pauwels P, De Craene B, Sabbah
M, Emami S, Redeuilh G, Gespach C, Bracke M and Berx G: Molecular
and pathological signatures of epithelial-mesenchymal transitions
at the cancer invasion front. Histochem Cell Biol. 130:481–494.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maeda M, Johnson KR and Wheelock MJ:
Cadherin switching: Essential for behavioral but not morphological
changes during an epithelium-to-mesenchyme transition. J Cell Sci.
118:873–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bryan RT and Tselepis C: Cadherin
switching and bladder cancer. J Urol. 184:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bryan RT, Atherfold PA, Yeo Y, Jones LJ,
Harrison RF, Wallace DM and Jankowski JA: Cadherin switching
dictates the biology of transitional cell carcinoma of the bladder:
Ex vivo and in vitro studies. J Pathol. 215:184–194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lascombe I, Clairotte A, Fauconnet S,
Bernardini S, Wallerand H, Kantelip B and Bittard H: N-cadherin as
a novel prognostic marker of progression in superficial urothelial
tumors. Clin Cancer Res. 12:2780–2787. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Adam L, Zhong M, Choi W, Qi W, Nicoloso M,
Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al:
miR-200 expression regulates epithelial-to-mesenchymal transition
in bladder cancer cells and reverses resistance to epidermal growth
factor receptor therapy. Clin Cancer Res. 15:5060–5072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muramaki M, Miyake H, Terakawa T, Kumano
M, Sakai I and Fujisawa M: Expression profile of E-cadherin and
N-cadherin in non-muscle-invasive bladder cancer as a novel
predictor of intravesical recurrence following transurethral
resection. Urol Oncol. 30:161–166. 2012. View Article : Google Scholar : PubMed/NCBI
|