Introduction
Prostate cancer is one of the leading causes of
cancer-associated mortality in men. Prostate cancer cell growth is
dependent on the presence of androgens. With an estimated incidence
of 233,000 novel cases and 29,480 mortalities in 2014, it is the
most frequently diagnosed cancer and second most frequent cause of
cancer mortality in American males (1). Although androgen deprivation therapy
(ADT) is the standard treatment for metastatic prostate cancer, the
tumors tend to relapse following a disease-free period and exhibit
androgen-independent proliferation (2), which is termed castration-resistant
prostate cancer (CRPC). However, the mechanism underlying CRPC
development remains unclear. Previous studies into CRPC have
focused on the androgen receptor (AR), including AR amplification
and overexpression (3), AR gene
mutations (4,5) and the functions of AR co-regulators
(6,7).
Specifically, AR activity has been shown to be activated by
co-regulators at low androgen levels (8), suggesting a critical role of AR
co-regulators in prostate cancer progression.
Wang et al (7)
found evidence of a relationship between the Wnt/β-catenin and
androgen signaling pathways (7).
β-Catenin, an AR co-regulator, physically interacts with AR through
the ligand-binding domain of AR and the first 6 armadillo repeats
of β-catenin (9). In prostate cancer
cells, β-catenin nuclear localization results in an increased
number of AR/β-catenin complexes, thus altering target gene
activation. β-catenin activity is regulated by phosphorylation,
which leads to its degradation, resulting in decreased nuclear
accumulation and decreased interaction with AR. Glycogen synthase
kinase-3β (GSK-3β) promotes the phosphorylation and subsequent
degradation of β-catenin via the ubiquitin pathway (10).
CD147, also known as extracellular matrix
metalloproteinase inducer (EMMPRIN), is highly expressed on the
surface of the majority of cancer cells (11). During tumorigenesis, CD147 contributes
to metastasis, drug resistance and angiogenesis (12–14). Our
previous studies have demonstrated that CD147 is important in
prostate cancer cell invasion, metastasis and autophagy (12,15). In
addition, CD147 regulates the canonical Wnt/β-catenin signaling
pathway to accelerate lung tumorigenesis (16). The present study aimed to determine
whether CD147 mediates the interaction between β-catenin and AR
during prostate cancer progression.
Materials and methods
Cell culture and stable transfection
of LNCaP cells
The human prostate LNCaP cell line was obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA).
LNCaP cells were maintained in RPMI 1640 medium (Gibco,
ThermoFisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal calf serum (FCS; ThermoFisher Scientific, Inc.) at 37°C
in an atmosphere of 95% air and 5% CO2. To investigate
the role of the Akt/GSK-3β pathway in the regulation of β-catenin
by CD147, cells were cultured with or without 20 µM LY294002 (Cell
Signaling Technology; Danvers, MA, USA) for 24 h. The pGV248
lentiviral vector was used as the backbone for the CD147 shRNA
construct. Lentiviral constructs and high-titer viruses were
provided by Jikai Genechem Company (Jikai Genechem, Shanghai,
China). Cells that expressed low levels of CD147 were termed
LNCaP/shCD147 cells. LNCaP/Scramble cells (negative control) were
established by transfection of LNCaP cells with the pGV112 vector
containing a control shRNA sequence. The target sequences for the
CD147 and control shRNA duplexes were 5′-GTCGTCAGAACACATCAACT-3′
and CAGTCGCGTTTGCGACTGG, respectively. Lentiviral infection was
performed following the manufacturer's protocol. Briefly,
1×106 cells were seeded per well in 12-well plates 16–18
h prior to the experiment. Recombinant lentiviral vectors (10 µg)
were produced by co-transfecting 293T cells with the lentivirus
expression plasmid (10 µg) and packaging plasmid (10 µg, pHelper
1.0 and pHelper 2.0) using Lipofectamine 2000 (Invitrogen,
Shanghai, China). After 48 h, the supernatants were collected and
concentrated.
Western blotting
Cells were washed twice with PBS and then lysed with
RIPA lysis buffer (Beyotime, Inc., Nanjin, China) containing a
cocktail of protease inhibitors (Roche Diagnostics, Mannheim,
Germany). To extract specific protein compartments, the
Compartmental Protein Extraction Kit (Millipore, Billerica, MA,
USA) was used according to the manufacturer's protocol. Cell
lysates containing 30 µg of protein were separated on 10% sodium
dodecyl sulfate (SDS)-polyacrylamide gels and transferred to
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA). The membranes were incubated with primary antibodies against
β-catenin (cat no. 8480), p-β-catenin (Ser 33/37/Thr 41; cat no.
9561), p-Akt (Ser 473; cat no. 4060), p-GSK-3β (Ser 9; cat no.
9322) and prostate-specific antigen (PSA; cat no. 5365) (1:1,000;
Cell Signaling Technology; Danvers, MA, USA) overnight at 4°C.
β-Actin was used as a loading control (1:2000; Epitomics,
Burlingame, CA, USA). Primary antibody binding was detected using a
horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody
(1:3,000; cat no. A0208; Beyotime Institute of Biotechnology) and
was visualized using an enhanced chemiluminescence (ECL) detection
system (Pierce, ThermoFisher Scientific, Inc., Rockford, IL,
USA).
Immunoprecipitation
Cells were lysed using lysis buffer containing 20 mM
Tris-HCl (pH 7.8), 0.5% Nonidet P-40, 137 mM NaCl, 50 µM EDTA, and
protease inhibitors (Roche Diagnostics, Mannheim, Germany), and
protein extracts were incubated with 2 µg anti-AR mouse IgG
antibody (cat no. sc-31358; Santa Cruz Biotechnology, Dallas, TX,
USA) overnight at 4°C, followed by the addition of protein A/G
agarose beads (Santa Cruz Biotechnology) for 2 h. After 3 washes
with 0.5 ml of lysis buffer, the pellets were suspended in SDS
sample buffer, boiled for 5 min, and analyzed on 10%
SDS-polyacrylamide gels. Proteins were transferred to PVDF
membranes (Millipore, Billerica, MA, USA), and western blot
analysis was performed with a rabbit anti-β-catenin antibody
(1:1,000; Cell Signaling Technology, Danvers, MA, USA). Primary
antibody binding was detected using a horseradish peroxidase
(HRP)-conjugated anti-rabbit IgG antibody (1:3,000; cat no. A0208;
Beyotime Institute of Biotechnology) and was visualized using an
enhanced chemiluminescence (ECL) detection system (Pierce,
ThermoFisher Scientific, Inc., Rockford, IL, USA). Control
immunoprecipitation experiments were performed with normal immune
serum (Beyotime, Inc., Nanjin, Jiangsu, China).
Cell growth assays
Cell growth was determined using the Cell Counting
Kit-8 (CCK-8, Dojindo, Japan). Briefly, cells were seeded in
96-well plates at a density of 3×103 cells/well and
cultured for 4, 8 and 12 days. CCK-8 solution was added to each
well, and cells were incubated for an additional 3 h. Each
experiment was performed in triplicate and repeated 3 times. The
absorbance at 450 nm was measured using a microplate reader
(Bio-Rad Laboratories, Hercules, CA, USA). Inhibition rate
(%)=(A450, control group-A450,
experimental group)/A450, control
groupx100.
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance (ANOVA) was used to compare
significant differences in the means among all treatment groups,
and Bonferroni's correction was used to identify significant
statistical differences between the means of individual treatments.
P<0.05 was considered statistically significant. All statistical
analyses were performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
CD147 regulates β-catenin nuclear
localization
β-Catenin is a multifunctional protein that has
structural importance in the adhesive junction complex and the
ability to bind to AR to stimulate AR-mediated gene transcription
(17). β-catenin expression was
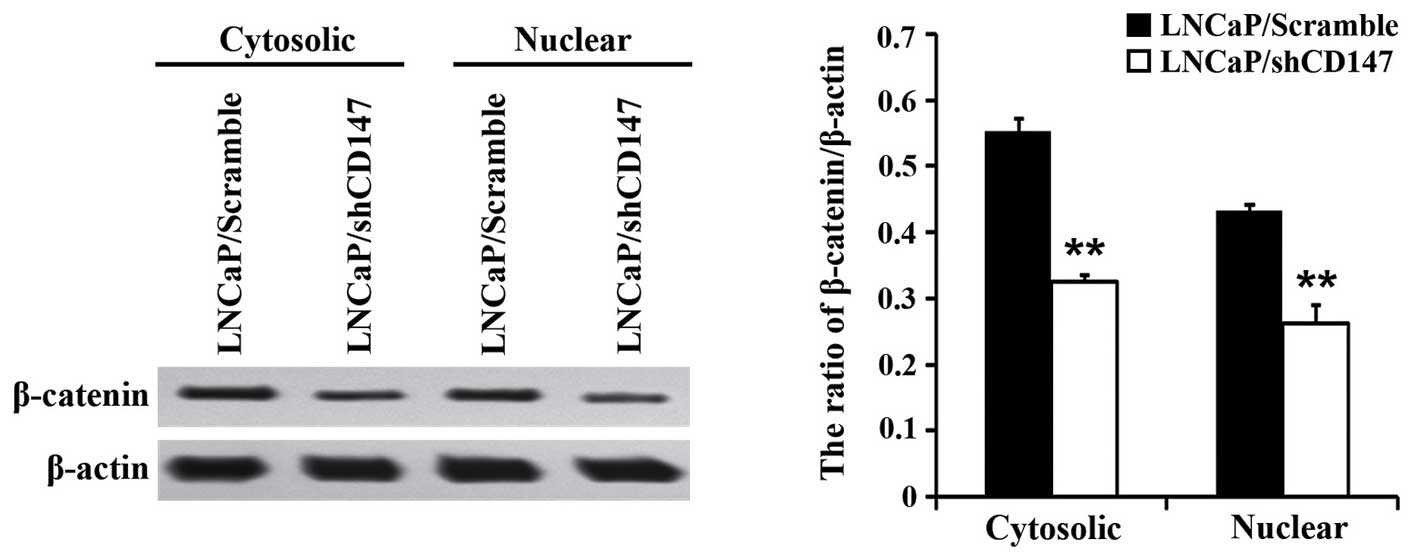
measured in LNCaP/shCD147 and LNCaP/Scramble cells by western
blotting. LNCaP/shCD147 cells exhibited significantly lower
β-catenin protein levels in the cytoplasm (P<0.01) and nucleus
(P<0.01) compared with LNCaP/Scramble cells (Fig. 1). These results indicate that CD147 is
a key molecule involved in β-catenin localization.
CD147 regulates β-catenin stability
via the Akt/GSK-3β signaling pathway in LNCaP cells and affects
AR/β-catenin protein-protein interactions
β-Catenin stability is triggered by GSK-3β, a
well-known target of Akt; therefore, the present study focused on
Akt/GSK-3β signaling. GSK-3β activity is suppressed by
phosphorylation on Ser 9 by Akt. To investigate the role of the
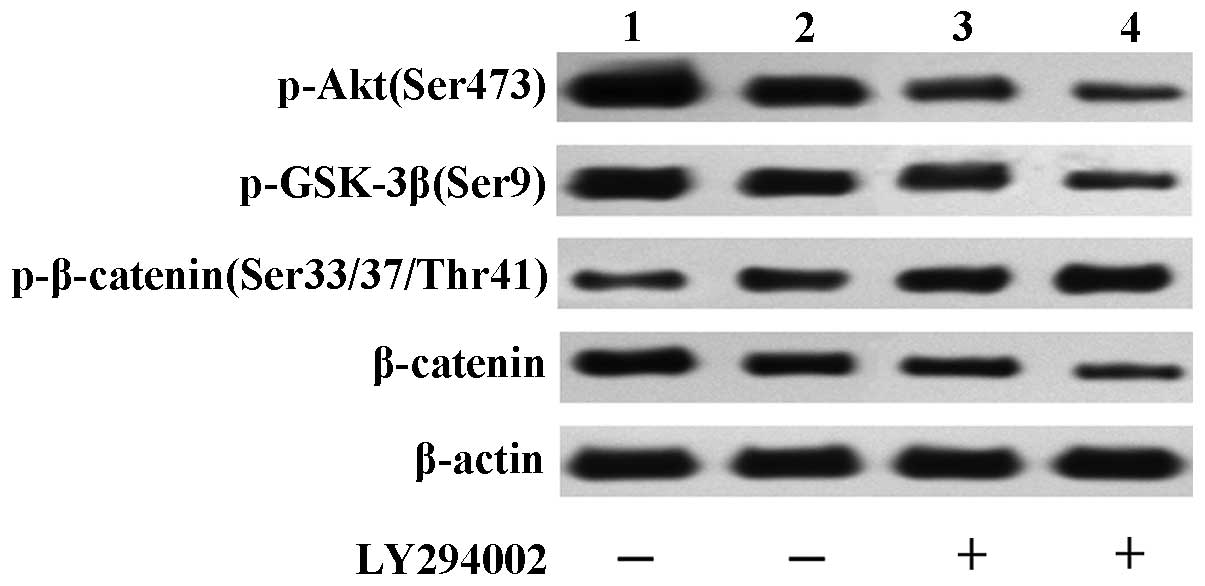
Akt/GSK-3β pathway in the regulation of β-catenin by CD147, the
expression of p-Akt (Ser473), p-GSK-3β (Ser9), p-β-catenin
(Ser33/37/Thr41) and β-catenin was examined in LNCaP/shCD147 and
LNCaP/Scramble cells cultured with or without 20 µM LY294002, a
specific inhibitor of class I phosphatidylinositol-3-kinases
(PI3Ks). Expression of p-Akt (Ser473) and p-GSK-3β (Ser9) were
down-regulated in LNCaP/shCD147 cells, as expected, which was
consistent with the down-regulation of β-catenin and the
up-regulation of p-β-catenin (Ser33/37/Thr41) (Fig. 2). In support of these findings,
exposure to LY294002 significantly decreased p-Akt (Ser473),
p-GSK-3β (Ser9) and β-catenin levels and increased p-β-catenin
(Ser33/37/Thr41) levels in LNCaP/shCD147 cells (Fig. 2). β-Catenin binds to AR and stimulates
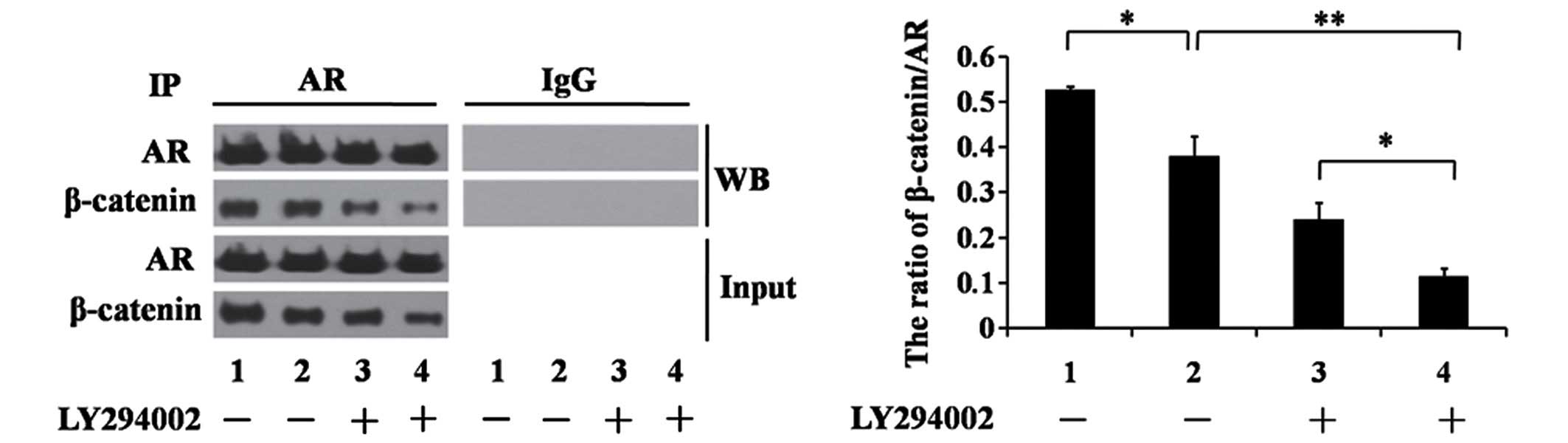
AR-mediated gene transcription. Protein extracts from
LNCaP/Scramble and LNCaP/shCD147 cells were immunoprecipitated with
an anti-AR antibody, followed by SDS-PAGE and western blotting
analysis using an anti-β-catenin antibody with normal IgG as a
negative control. Fig. 3 demonstrates
that introducing CD147-shRNA into LNCaP cells reduced the formation
of the AR/β-catenin complex. As expected, AR/β-catenin complex
formation was lowest when LNCaP/shCD147 cells were treated with
LY294002 (Fig. 3; P<0..01). These
results suggest that CD147 enhances the stability of β-catenin via
the Akt/GSK-3β pathway, which leads to increased AR/β-catenin
protein-protein interactions.
CD147 promotes AR-mediated gene
expression
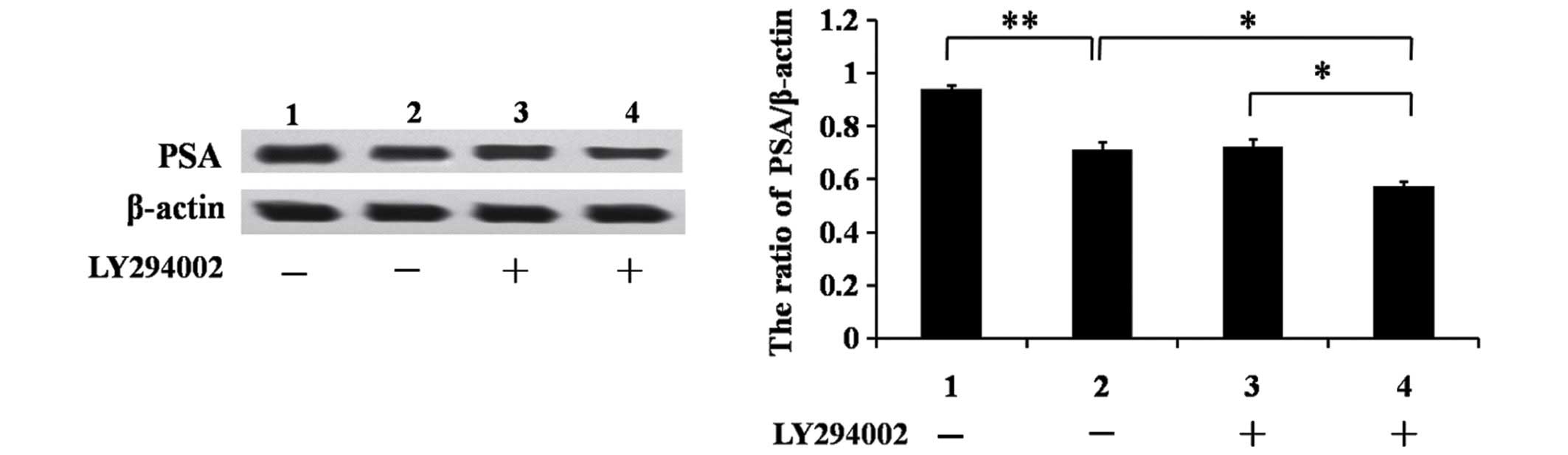
PSA is a well-established AR target gene in human
prostate cancer. Therefore, the potential effect of CD147 on
AR-mediated PSA protein expression was investigated using western
blot analysis. As shown in Fig. 4,
shRNA targeting CD147 resulted in reduced PSA protein levels
(P<0.05). In addition, LNCaP cells were treated with LY294002
and PSA protein levels were further reduced in LNCaP/shCD147 cells
(Fig. 4; P<0.05). Consistent with
the above results, CD147 appears to enhance the expression of
downstream AR target proteins via the Akt/GSK-3β/β-catenin
pathway.
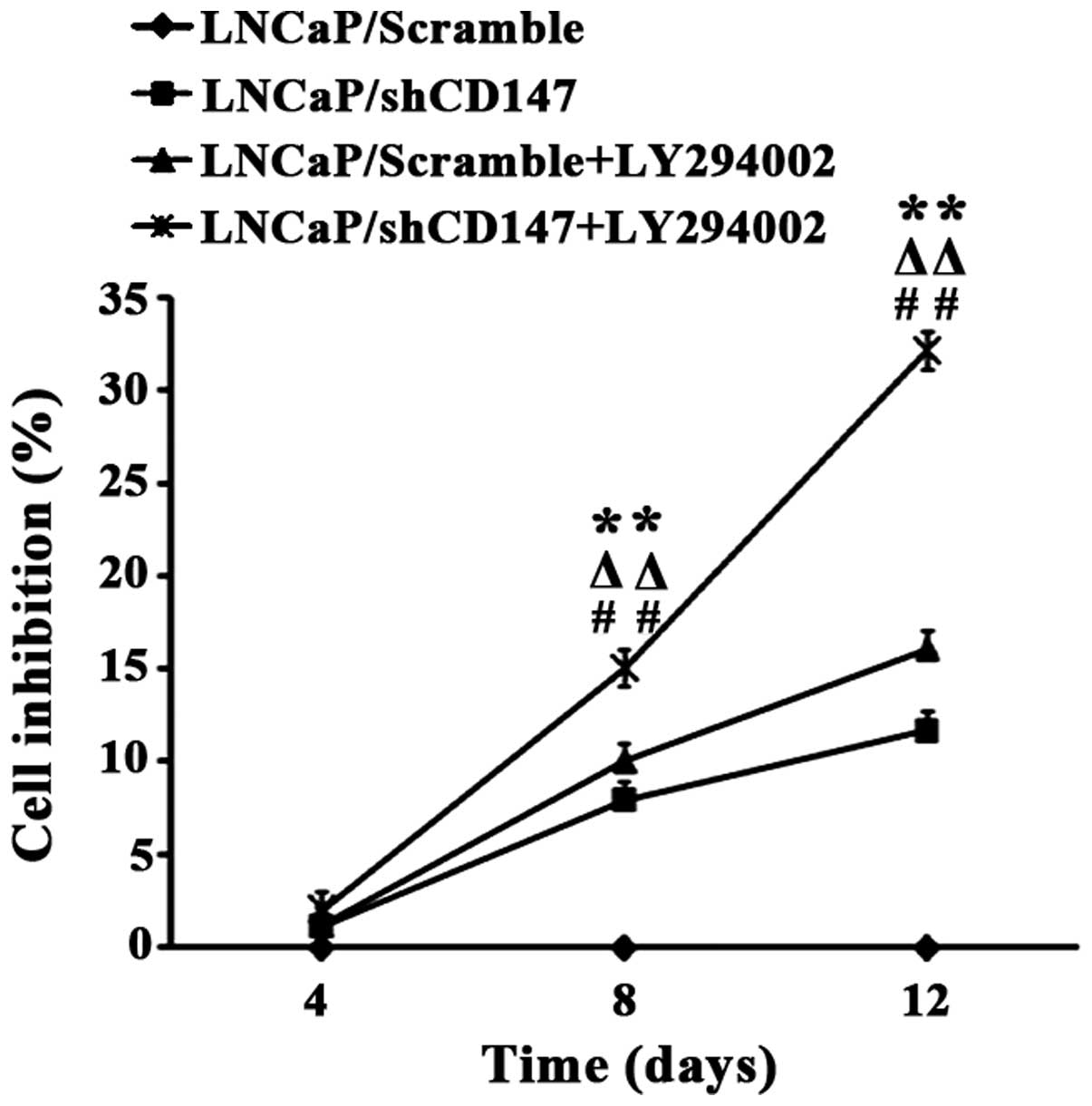
CD147 enhances the growth of prostate
cancer cells
The role of CD147 in the regulation of prostate
cancer cell growth was also investigated because LNCaP cells are
androgen-sensitive and AR-positive. Cell growth was measured using
the CCK8 assay. As shown in Fig. 5,
knocking down CD147 expression in LNCaP cells inhibited cell growth
compared with control Scramble expression (P<0.01). Moreover,
the growth inhibition rate in the LNCaP/shCD147 group was markedly
increased by LY294002 treatment on days 4, 8 and 12 compared with
the control LNCaP/shCD147 group (P<0.01).
Discussion
The AR signaling pathway has been demonstrated to be
involved in CRPC. Emerging evidence indicates that β-catenin is
more highly expressed in prostate cancer than in normal prostate
tissue and that it is involved in advanced prostate cancer
(18). In prostate cells, β-catenin
directly interacts with AR and acts as a co-activator to enhance
androgen-induced AR-mediated transcription. A previous study
demonstrated that the dysregulation of β-catenin and the
androgen-signaling pathway induces cell growth and contributes to
the initiation and progression of prostate cancer (19).
CD147 serves an important role in tumor biology; it
inhibits cancer cell anoikis and promotes cancer cell invasion and
metastasis. In the present study, a previously unknown effect of
CD147 on the β-catenin signaling pathway in prostate cancer was
identified. It was demonstrated that knockdown of CD147 expression
decreased β-catenin levels and disrupted the nuclear accumulation
of active β-catenin in LNCaP cells. Decreased β-catenin level may
result from the GSK-3β-mediated phosphorylation of β-catenin on Ser
33/37 and Thr 41. The phosphorylated form of β-catenin is
ubiquitinated by the β-TrCP ubiquitin E3 ligase degraded by the
proteasome (20). The data in the
present study demonstrated that CD147 knockdown inhibited the
phosphorylation of GSK-3β on Ser 9 and increased the
phosphorylation of β-catenin on Ser 33/37 and Thr 41, which can
induce β-catenin ubiquitination. The results demonstrated that
knockdown of CD147 expression could potentially lead to an increase
GSK-3β-mediated phosphorylation of β-catenin. Phosphorylation of
β-catenin leads to its instability and decreases its accumulation
in the nucleus. AR-mediated target gene transcription is essential
for prostate cancer growth and progression (21). β-catenin acts as an AR co-activator
and enhances AR-mediated gene transcription (9). PSA, a critical downstream AR target
gene, is an important biomarker of disease onset and progression in
prostate cancer. In the present study, it was observed that the
interaction between β-catenin and AR was decreased, ultimately
leading to the inhibition of PSA expression in LNCaP/shCD147 cells
compared with LNCaP/Scramble cells. A previous study showed that
dysregulation of the Wnt and androgen signaling pathways induces
cell growth and directly contributes to the progression of prostate
cancer (19). In the present study,
the data indicated that decreased CD147 expression inhibited
AR-positive LNCaP cell growth, which is consistent with the role of
CD147 in enhancing AR-mediated transcription. These results suggest
that CD147 contributes to novel molecular cross-talk between
Wnt/β-catenin signaling and AR activity.
The class I PI3K/Akt pathway regulates a wide
spectrum of cellular processes, including cell cycle progression,
cell survival and migration (18).
Dysregulation of the PI3K/Akt pathway promotes tumorigenesis and
angiogenesis in various cancer types (18). Our prior study demonstrated that CD147
activates the class I PI3K/Akt pathway in prostate cells (15). GSK-3β also serves an important role in
suppressing tumor progression through the cytoplasmic
phosphorylation and degradation of β-catenin via the Akt signaling
pathway (22). Therefore, the present
authors hypothesized that the PI3K/Akt pathway may be involved in
the regulation of β-catenin by CD147. It was demonstrated that
decreasing CD147 levels inhibited the Akt phosphorylation and
significantly decreased GSK-3β activity through N-terminal
phosphorylation at Ser 9 and that LY294002, a specific inhibitor of
class I PI3Ks, further destabilized β-catenin. Therefore, PI3K/Akt
signaling is potentially involved in CD147-mediated β-catenin
stabilization, resulting in significant inhibition of the
interaction between β-catenin and AR and decreased PSA levels.
The current study prevents evidence that CD147 acts
as an upstream signal that modulates Akt/GSK-3β/β-catenin/AR
signaling network and promote prostate cell proliferation. These
results provide novel insights into the function of CD147 during
tumor progression.
Acknowledgements
The present study was supported by grants from the
China National Natural Science Foundation (grant no. 81202031) and
the Science and Technology Development Program of Jilin Province
(grant no. 20130101154JC).
References
|
1
|
Scher HI, Solo K, Valant J, Todd MB and
Mehra M: Prevalence of prostate cancer clinical states and
mortality in United States: Estimates using a dynamic progression
model. Plos One. 10:e01394402015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lorente D and De Bono JS: Molecular
alterations and emerging targets in castration resistant prostate
cancer. Eur J Cancer. 50:753–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koivisto P, Kononen J, Palmberg C, Tammela
T, Hyytinen E, Isola J, Trapman J, Cleutjens K, Noordzij A,
Visakorpi T and Kallioniemi OP: Androgen receptor gene
amplification: A possible molecular mechanism for androgen
deprivation therapy failure in prostate cancer. Cancer Res.
57:314–319. 1997.PubMed/NCBI
|
|
4
|
Tilley WD, Buchanan G, Hickey TE and
Bentel JM: Mutations in the androgen receptor gene are associated
with progression of human prostate cancer to androgen independence.
Clin Cancer Res. 2:277–285. 1996.PubMed/NCBI
|
|
5
|
Gottlieb B, Beitel LK, Wu JH and Trifiro
M: The androgen receptor gene mutations database (ARDB): 2004
update. Hum Mutat. 23:527–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chmelar R, Buchanan G, Need EF, Tilley W
and Greenberg NM: Androgen receptor coregulators and their
involvement in the development and progression of prostate cancer.
Int J Cancer. 120:719–733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang G, Wang J and Sadar MD: Crosstalk
between the androgen receptor and beta-catenin in
castrate-resistant prostate cancer. Cancer Res. 68:9918–9927. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinlein CA and Chang C: Androgen receptor
(AR) coregulators: An overview. Endocr Rev. 23:175–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang F, Li X, Sharma M, Sasaki CY, Longo
DL, Lim B and Sun Z: Linking beta-catenin to androgen-signaling
pathway. J Biol Chem. 277:11336–11344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Li Y, Semenov M, Han C, Baeg GH,
Tan Y, Zhang Z, Lin X and He X: Control of beta-catenin
phosphorylation/degradation by a dual-kinase mechanism. Cell.
108:837–847. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishibashi Y, Matsumoto T, Niwa M, Suzuki
Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K, et al:
CD147 and matrix metalloproteinase-2 protein expression as
significant prognostic factors in esophageal squamous cell
carcinoma. Cancer. 101:1994–2000. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang L, Wu G, Yu L, Yuan J, Fang F, Zhai
Z, Wang F and Wang H: Inhibition of CD147 expression reduces tumor
cell invasion in human prostate cancer cell line via RNA
interference. Cancer Biol Ther. 5:608–614. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grass GD, Dai L, Qin Z, Parsons C and
Toole BP: CD147: Regulator of hyaluronan signaling in invasiveness
and chemoresistance. Adv Cancer Res. 123:351–373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szubert S, Szpurek D, Moszynski R, Nowicki
M, Frankowski A, Sajdak S and Michalak S: Extracellular matrix
metalloproteinase inducer (EMMPRIN) expression correlates
positively with active angiogenesis and negatively with basic
fibroblast growth factor expression in epithelial ovarian cancer. J
Cancer Res Clin Oncol. 140:361–369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang F and Wang L, Zhang S, Fang Q, Hao F,
Sun Y, Zhao L, Chen S, Liao H and Wang L: CD147 modulates autophagy
through the PI3K/Akt/mTOR pathway in human prostate cancer PC-3
cells. Oncol Lett. 9:1439–1443. 2015.PubMed/NCBI
|
|
16
|
Sidhu SS, Nawroth R, Retz M,
Lemjabbar-Alaoui H, Dasari V and Basbaum C: EMMPRIN regulates the
canonical Wnt/beta-catenin signaling pathway, a potential role in
accelerating lung tumorigenesis. Oncoqene. 29:4145–4156. 2010.
|
|
17
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Abrams SL, Montalto G, D'Assore AB, Libra M, Nicoletti F,
Maestro R, et al: Multifaceted roles of GSK-3 and Wnt/β-catenin in
hematopoiesis and leukemogenesis: Opportunities for therapeutic
intervention. Leukemia. 28:15–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scher HI and Sawyers CL: Biology of
progressive, castration-resistant prostate cancer: Directed
therapies targeting the androgen-receptor signaling axis. J Clin
Oncol. 23:8253–8261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verras M and Sun Z: Roles and regulation
of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett.
237:22–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu G, Xu G, Schulman BA, Jeffrey PD,
Harper JW and Pavletich NP: Mol Cell. 11:1445–1456. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Q, Youn H, Tang J, Tawfik O, Dennis K,
Terranova PF, Du J, Raynal P, Thrasher JB and Li B:
Phosphoinositide 3-OH kinase p85 alpha and p110 beta are essential
for androgen receptor transactivation and tumor progression in
prostate cancers. Oncogene. 27:4569–4579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takahashi-Yanaga F and Sasaguri T: Drug
development targeting the glycogen synthase kinase-3beta
(GSK-3beta)-mediated signal transduction pathway: Inhibitors of the
Wnt/beta-catenin signaling pathway as novel anticancer drugs. J
Pharmacol Sci. 109:179–183. 2009. View Article : Google Scholar : PubMed/NCBI
|