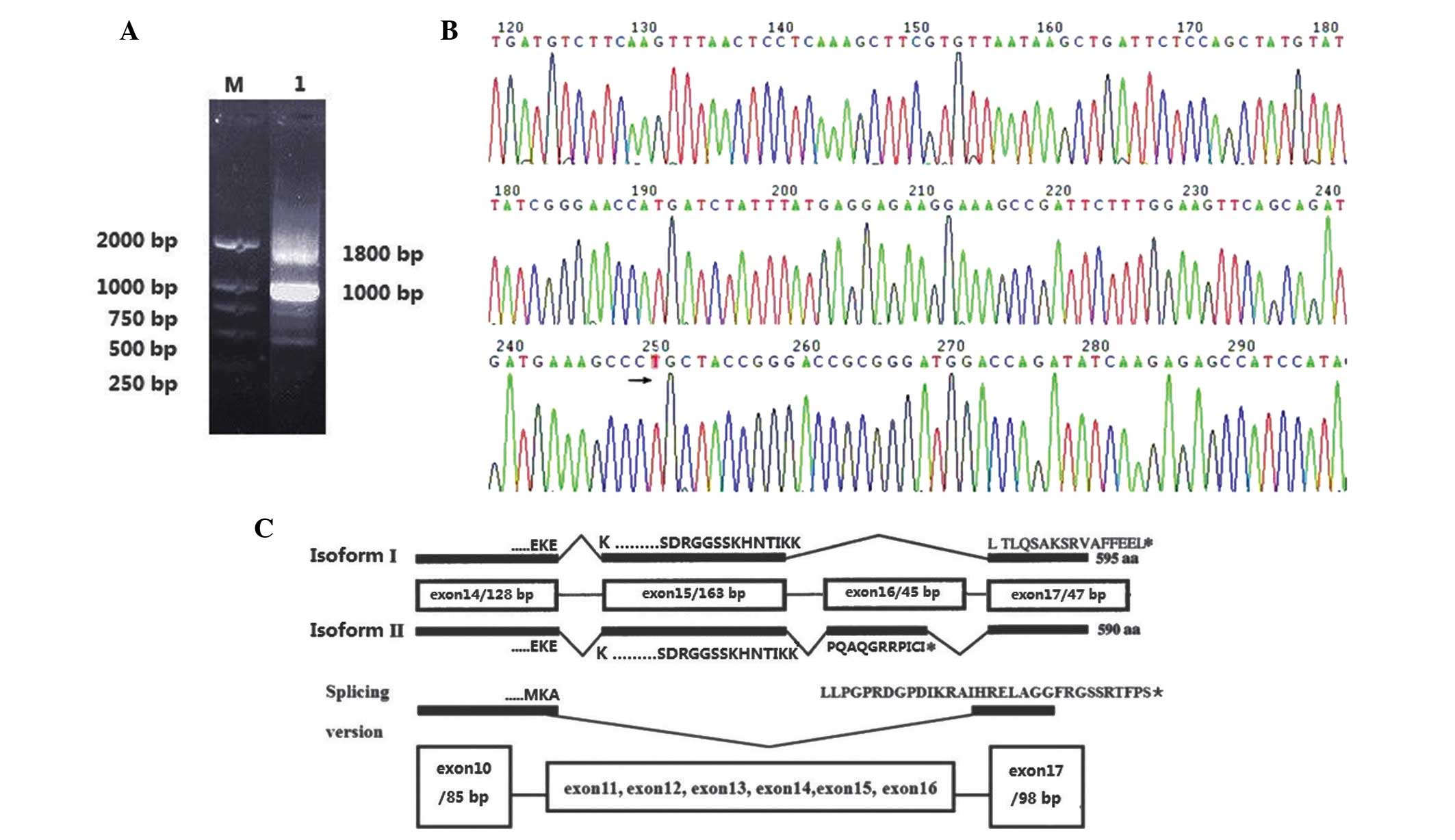
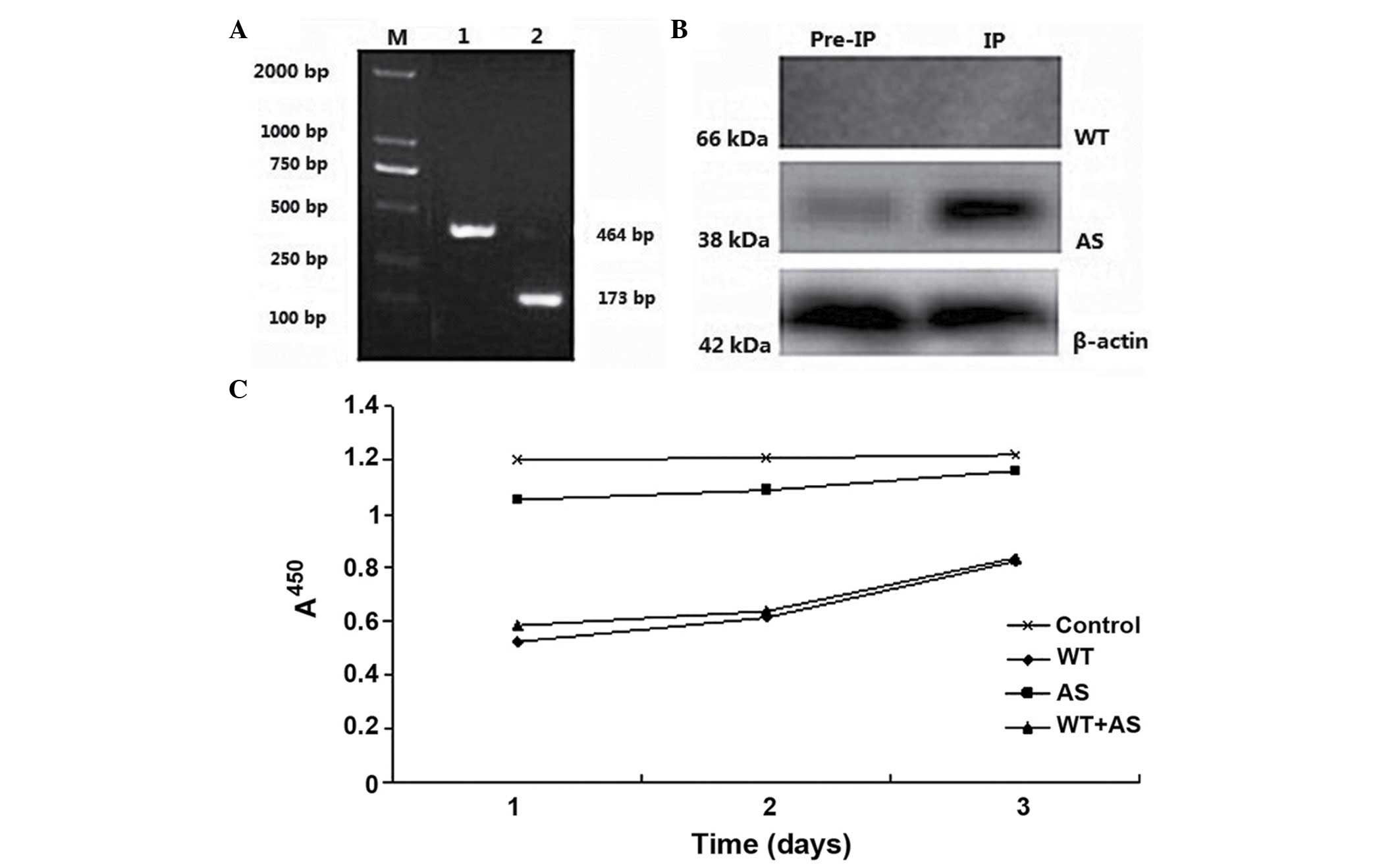
|
1
|
Mahanthappa NK, Anton ES and Matthew WD:
Glial growth factor 2, a soluble neuregulin, directly increases
Schwann cell motility and indirectly promotes neurite outgrowth. J
Neurosci. 16:4673–4683. 1996.PubMed/NCBI
|
|
2
|
Shepard TH, Tucci DL, Grant GA and Kaylie
DM: Management of hearing in pediatric NF2. Otol Neurotol.
33:1066–1070. 2012.PubMed/NCBI
|
|
3
|
Diensthuber M, Lenarz T and Stöver T:
Neurotrophic factor expression in vestibular schwannoma. An
overview. Laryngorhinootologie. 85:731–737. 2006.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hexter A, Jones A, Joe H, Heap L, Smith
MJ, Wallace AJ, Halliday D, Parry A, Taylor A, Raymond L, et al:
Clinical and molecular predictors of mortality in neurofibromatosis
2: A UK national analysis of 1192 patients. J Med Genet.
52:699–705. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Drouet A, Le Moigne F, Salamé D, Quesnel
L, Motolese C, des Portes V, Guilloton L and Pinson S: Type 2
neurofibromatosis: Intergenerational differences in genetic and
clinical expression. Arch Pediatr. 21:1233–1240. 2014.(In French).
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Zhang X, Zhang Z, Yang T, Wang Z
and Wu H: The role of NF2 gene mutations and pathogenesis-related
proteins in sporadic vestibular schwannomas in young individuals.
Mol Cell Biochem. 392:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuga Y, Ohnishi H, Kodama Y, Takakura S,
Hayashi M, Yagi R, Fukutome K, Matsushima K, Okamoto K, Taomoto K
and Takahashi H: Cerebral and spinal cord tanycytic ependymomas in
a young adult with a mutation in the NF2 gene. Neuropathology.
34:406–413. 2014.PubMed/NCBI
|
|
8
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrilli A, Copik A, Posadas M, Chang LS,
Welling DB, Giovannini M and Fernández-Valle C: LIM domain kinases
as potential therapeutic targets for neurofibromatosis type 2.
Oncogene. 33:3571–3582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Cooper J, Karajannis MA and
Giancotti FG: Merlin: A tumour suppressor with functions at the
cell cortex and in the nucleus. EMBO Rep. 13:204–215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmucker B, Tang Y and Kressel M: Novel
alternatively spliced isoforms of the neurofibromatosis type 2
tumor suppressor are targeted to the nucleus and cytoplasmic
granules. Hum Mol Genet. 8:1561–1570. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rustgi AK, Xu L, Pinney D, Sterner C,
Beauchamp R, Schmidt S, Gusella JF and Ramesh V: Neurofibromatosis
2 gene in human colorectal cancer. Cancer Genet Cytogenet.
84:24–26. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lepont P, Stickney JT, Foster LA, Meng JJ,
Hennigan RF and Ip W: Point mutation in the NF2 gene of HEI-193
human schwannoma cells results in the expression of a merlin
isoform with attenuated growth suppressive activity. Mutat Res.
637:142–1451. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang LS, Akhmametyeva EM, Wu Y, Zhu L and
Welling DB: Multiple transcription initiation sites, alternative
splicing and differential polyadenylation contribute to the
complexity of human neurofibromatosis 2 transcripts. Genomics.
79:63–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Barker CL, Baillie BK, Hammond-Kosack KE,
Jones JD and Jones DA: Dominant-negative interference with defence
signalling by truncation mutations of the tomato Cf-9 disease
resistance gene. Plant J. 46:385–399. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doherty JK, Ongkeko W, Crawley B, Andalibi
A and Ryan AF: ErbB and Nrg: Potential molecular targets for
vestibular schwannoma pharmacotherapy. Otol Neurotol. 29:50–57.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angelo LS, Maxwell DS, Wu JY, Sun D, Hawke
DH, McCutcheon IE, Slopis JM, Peng Z, Bornmann WG and Kurzrock R:
Binding partners for curcumin in human schwannoma cells: Biologic
implications. Bioorg Med Chem. 21:932–939. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prabhakar S, Taherian M, Gianni D, Conlon
TJ, Fulci G, Brockmann J, Stemmer-Rachamimov A, Sena-Esteves M,
Breakefield XO and Brenner GJ: Regression of schwannomas induced by
adeno-associated virus-mediated delivery of caspase-1. Hum Gene
Ther. 24:152–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cockburn K, Biechele S, Garner J and
Rossant J: The Hippo pathway member Nf2 is required for inner cell
mass specification. Curr Biol. 23:1195–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cačev T, Aralica G, Lončar B and
Kapitanović S: Loss of NF2/Merlin expression in advanced sporadic
colorectal cancer. Cell Oncol (Dordr). 37:69–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thurneysen C, Opitz I, Kurtz S, Weder W,
Stahel RA and Felley-Bosco E: Functional inactivation of NF2/merlin
in human mesothelioma. Lung Cancer. 64:140–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kros J, de Greve K, van Tilborg A, Hop W,
Pieterman H, Avezaat C, Lekanne Dit Deprez R and Zwarthoff E: NF2
status of meningiomas is associated with tumour localization and
histology. J Pathol. 194:367–372. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dilwali S, Patel PB, Roberts DS, Basinsky
GM, Harris GJ, Emerick KS and Stankovic KM: Primary culture of
human Schwann and schwannoma cells: Improved and simplified
protocol. Hear Res. 315:25–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sainio M, Jääskeläinen J, Pihlaja H and
Carpén O: Mild familial neurofibromatosis 2 associates with
expression of merlin with altered COOH-terminus. Neurology.
54:1132–1138. 2000. View Article : Google Scholar : PubMed/NCBI
|