Introduction
IMPCs are located in distinct, empty spaces
resembling small, extended lymphatic vessels and contain clusters
of cancer cells that adhere tightly to one another (1–4). The
clusters do not have a fibrovascular core (3,5). The
spaces are separated from one another by thin bands of fibrous
tissue and form a structure comparable to a sponge. Nests may also
form with fusiform-like cancer cells filling up single spaces
(6,7),
and are often observed in invasive tumor margins or less frequently
in the tumor center (1,7–9). The
micropapillary structure may constitute one of the morphological
tumor components and coexist with other histological types of
cancer, or it can be the only morphological feature (3,5,8). However, cases of cancer that are formed
of only micropapillary components are extremely rare (8). IMPC cells exhibit reverse polarity,
which results in a characteristic ‘inside-out’ structure, for
example, the basal surface of cells demonstrate properties
typically observed in the apical region. Electron microscopy has
previously confirmed that numerous microvilli line the outer
surface of IMPC cells, which target their secretory activity
towards the surrounding stroma. Furthermore, a limited amount of
mucous secretion has been observed in spaces surrounding tumor cell
nests (10,11). These findings have been supported by
immunohistochemistry using anti-mucin 1, cell surface associated
(MUC1) antibodies, with results demonstrating that MUC1 is
primarily located in the outer surface of epithelial cells in
patients with IMPC compared with the color reaction located in the
apical part of normal glandular cells (12). The reverse polarity of IMPC cells
disturbs adhesion, conditions their malignancy and determines
secretory properties. All the aforementioned morphological features
are responsible for vascular and stromal invasion of tumor cells,
promoting lymph node involvement and metastasis of cancer
cells.
Micropapillary structures were first identified in
invasive ductal breast cancer (13),
and were also later identified in malignant tumors of the ovaries,
urinary bladder, salivary glands and lungs (14–17).
Previous studies have confirmed the presence of these structures in
malignancies of the digestive system (9). In addition, it has been demonstrated
that the presence of a micropapillary component in tumors indicates
aggressiveness and poor clinical outcome (14–18).
Therefore, the present study aimed to investigate the histological
significance of micropapillary components in colorectal carcinoma
compared with conventional colorectal adenocarcinoma.
Materials and methods
Materials
The study group consisted of 115 colorectal cancer
patients, 53 women and 62 men, who had all been treated surgically
in the Second Department of General and Gastroenterological
Surgery, Medical University of Białystok (Białystok, Poland)
between 2009 and 2013. The age of the patients ranged from 34–86
years old. All samples were pathologically diagnosed as colorectal
cancer and diagnoses included the following information:
Macroscopic tumor localization, histopathological type, anal
invasion, primary tumor (pT) status, malignancy stage, lymph node
involvement and metastases to distant organs according to
Tumor-Node-Metastasis (TNM) classification guidelines (19). In addition, peritumoral inflammatory
infiltrate and venous invasion were noted. Peritumoral inflammation
was classified as follows: Absent, 0; mild, 1; moderate, 2; and
marked, 3.
The present study was performed in accordance with
the Declaration of Helsinki for Human Experimentation and was
approved by the local Bioethics Committee (Nr R-I-002/406/2014).
All participants provided written informed consent prior to
examination.
Micropapillary component analysis
The micropapillary colorectal component was
diagnosed according to 4-µm formalin-fixed and paraffin-embedded
sections that underwent hematoxylin and eosin staining, and
immunohistochemical analysis. To exclude poorly-differentiated
clusters of adenocarcinoma cells in lymphovascular vessels,
immunohistochemical staining was performed with an endothelial
cell-specific monoclonal mouse anti-human podoplanin antibody
(clone D2-40; #05463645001; Roche Diagnostics, Warsaw, Poland;
dilution, 0.12 µg/ml). Reverse polarity, resulting in a
characteristic ‘inside-out’ pattern of IMPC, was identified
following incubation with a monoclonal mouse antibody against
epithelial membrane antigen (EMA) (E29 clone; #05878900001; Roche
Diagnostics; dilution, 0.54 µg/ml). The reaction was performed
using the Novocastra Novolink Polymer Detection system (Novocastra;
Leica Biosystems, Milton Keynes, UK). A color reaction for
peroxidase was developed with chromogene diaminobenzidine. Positive
and negative controls were performed according to the
manufacturer's protocols (Novocastra; Leica Biosystems).
Counterstaining was performed with hematoxylin.
Statistical analysis
Statistical analysis was performed using STATISTICA
10.0 software (StatSoft, Inc., Tulsa, OK, USA). The
χ2-quadrate coefficient test was used to analyze
associations between parameters. P<0.05 was considered to
indicate a statistically significant difference. Cases with missing
data for any variables were removed from the sample.
Results
Patient clinicopathological
characteristics
Pathological analysis confirmed the diagnosis of
colorectal cancer in all 115 cases and stage was determined
according to the World Health Organization classification system
(19). Out of 115 patients,
adenocarcinoma type was diagnosed in 3 patients with IMPC and 99
patients with conventional carcinoma, whereas adenocarcinoma with a
mucous component was diagnosed in 2 individuals with IMPC and 11
with conventional carcinoma. Tumors invaded into the anal canal in
4 cases of IMPC and 57 cases of conventional carcinoma. Tumors were
classified as moderately-differentiated (G2) in 5 patients with
IMPC and 104 patients with conventional carcinoma, and only 6
patients with conventional carcinoma were classified as having
poorly-differentiated (G3) tumors. Only 1 patient with conventional
carcinoma was staged as pT1, 6 patients as pT2, 100 patients as pT3
and 3 patients as pT4, whilst all patients with IMPC were staged as
pT3. In addition, inflammatory infiltrate was absent in 1 patient
and weak in 4 patients with IMPC, and was absent, weak, moderate
and marked in 20, 47, 36 and 7 conventional carcinoma cases,
respectively. The presence of venous invasion was observed in all
patients with IMPC and in 58 patients with conventional carcinoma.
At the time of the diagnosis, 4 out of 5 patients with IMPC and 51
out of 110 patients with conventional carcinoma presented with
metastases at local lymph nodes, whereas only 28 out of 110
conventional carcinoma cases presented with metastases at distant
organs.
Micropapillary component
examination
Among the study group, 5 out of 115 (4.3%) cases
were diagnosed with a micropapillary colorectal component.
Micropapillary components accounted for 2–10% of the tumor volume,
and were situated in the center of the tumor mass in 2 cases, and
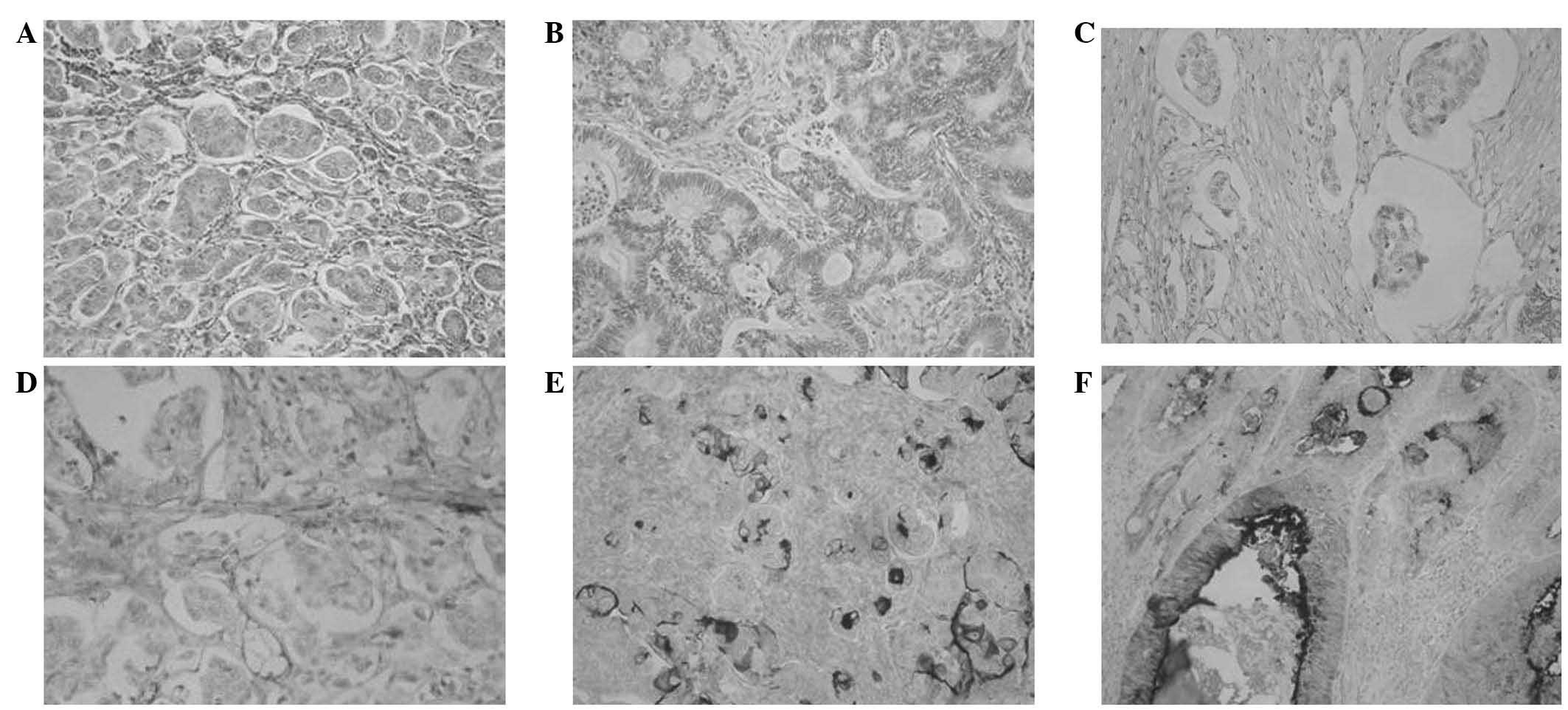
at the invasive front in 3 (Fig. 1A).
Conventional adenocarcinoma was observed in 95.7% of CRC cases
(Fig. 1B). Negative reactivity for
podoplanin confirmed the presence of a micropapillary component
(Fig. 1C), whereas positive
reactivity for this protein was present in the endothelium
(Fig. 1D). In patients with IMPC,
positive EMA expression was observed on the outer membrane of
epithelial cells (Fig.1E), whilst in
normal glandular cells, EMA was expressed in luminal regions
(Fig. 1F).
Associations between IMPC and selected
clinicopathological parameters
The presence of IMPC was observed to positively
correlate with histopathological type (P=0.001) and tumor invasion
into venous vessels (P=0.033). Furthermore, the presence and number
of lymph node metastases was greater in IMPC cases compared with
conventional carcinoma cases, but the difference was not
statistically significant (P=0.087 and P=0.094, respectively). IMPC
demonstrated a negative association with the presence of
peritumoral inflammatory infiltrate (P=0.098). No statistically
significant differences were observed between IMPC and the
remaining clinicopathological parameters, including age, gender,
tumor localization, pT status, stage and distant organ metastasis.
The results are presented in Table
I.
 | Table I.Associations between IMPC and non-IMPC
and selected clinicopathological parameters. |
Table I.
Associations between IMPC and non-IMPC
and selected clinicopathological parameters.
| Parameter | IMPC | Non-IMPC | R | P-value |
|---|
| Gender |
|
| −0.059 | 0.528 |
|
Female | 3 | 50 |
|
|
| Male | 2 | 60 |
|
|
| Age, years |
|
| 0.129 | 0.168 |
|
<60 | 0 | 31 |
|
|
|
>60 | 5 | 79 |
|
|
| Localization |
|
| −0.090 | 0.336 |
|
Colon | 4 | 64 |
|
|
|
Rectum | 1 | 46 |
|
|
| Histological
type |
|
| 0.293 | 0.001a |
|
Non-mucinous | 3 | 64 |
|
|
|
Mucinous | 2 | 46 |
|
|
| pT anal invasion |
|
| 0.195 | 0.153 |
|
Present | 4 | 57 |
|
|
|
Absent | 1 | 53 |
|
|
| pT stage |
|
| 0.035 | 0.710 |
| 1 | 0 | 1 |
|
|
| 2 | 0 | 6 |
|
|
| 3 | 5 | 100 |
|
|
| 4 | 0 | 3 |
|
|
| Malignancy stage |
|
| −0.045 | 0.628 |
| G2 | 5 | 104 |
|
|
| G3 | 0 | 6 |
|
|
| Inflammation at
invasive tumor front |
|
| −0.225 | 0.098 |
| 0 | 1 | 20 |
|
|
| 1 | 4 | 47 |
|
|
| 2 | 0 | 36 |
|
|
| 3 | 0 | 7 |
|
|
| Venous
invasion |
|
| 0.288 | 0.033a |
|
Present | 5 | 58 |
|
|
|
Absent | 0 | 51 |
|
|
| Lymph node
involvement |
|
| 0.233 | 0.087 |
|
Present | 4 | 51 |
|
|
|
Absent | 1 | 59 |
|
|
| Lymph node status,
no. |
|
| 0.228 | 0.094 |
|
<5 | 2 | 45 |
|
|
|
>5 | 3 | 6 |
|
|
| Distant
metastasis |
|
| −0.121 | 0.198 |
|
Present | 0 | 28 |
|
|
|
Absent | 5 | 82 |
|
|
Discussion
Micropapillary components are rare in forms of
gastrointestinal cancer and typically constitute one of several
histological types (6). In colon
cancer, a micropapillary structure has been observed in 9–19% of
all malignancies affecting the organ (7,20,21). The present study identified only 5
patients with IMPC accounting for 4.3% of all study cases. The area
occupied by the micropapillary component may be between 5–95% of
the tumor volume and is most commonly located in the invasive front
of the tumor (3,6,7). Verdú
et al (6) described the
coexistence of an early cancer of the sigmoid with IMPC in a
pedunculated polyp obtained during colonoscopy, the component being
the major tumor morphological feature. Furthermore, Kondo et
al (2) reported of a case of
tubulovillous adenoma case in which a small area of IMPC developed.
Lino-Silva (8) described a patient
that presented with pure rectal IMPC, in which the micropapillary
structure occupied >95% of the lesion (8). In the current study, the micropapillary
components accounted for 2–10% of the lesions and were located in
the center of the tumor mass in 2 patients and in the invasive
front in 3 cases. In accordance with previously reported cases, all
IMPC cases in the present study coexisted with a
moderately-differentiated form of cancer invading the subserosal
layer through the muscular layer (1,3,7,20,21).
Statistical analysis performed in the current study
demonstrated that IMPCs accompany non-mucinous adenocarcinomas more
frequently than mucinous carcinoma (P=0.001). Similarly, Kim et
al (20) described the presence
of micropapillary components in a case of intestinal non-mucinous
adenocarcinoma. These observations are also consistent with
findings reported by Vendu et al (6), Haupt et al (7), Lino-Silva (8) and Xu et al (22), in which IMPC coexisted with
adenocarcinoma of the colon. Colorectal adenocarcinomas consist of
a phenotypically heterogenic population of cancer cells derived
from glandular epithelium, which may be affected by epigenetic
lesions and mutations (19). As a
result, cancer cells acquire features of high-grade malignancy and
manifest these in a specific morphological manner, for example,
through the formation of micropapillary structures or mucus
production capacity. This may explain the rare co-occurrence of
IMPC and mucinous adenocarcinoma.
The presence of IMPC in patients with colorectal
cancer has been previously demonstrated to positively correlate
with lymph node invasion by cancer cells. Vendu et al
(6) noted a that invasion of blood
vessels was significantly more frequent in patients with IMPC
compared with patients with conventional adenocarcinoma. However,
differences have been reported between respective studies regarding
the involvement of blood and lymphatic vessels in cases of IMPC in
colorectal cancer. Vessels were invaded in the majority of all
single colorectal cancer cases accompanied by IMPC described in the
literature (1–3,18), whilst
studies analyzing larger groups report that vascular involvement
was noted in 40–45% of patients with colorectal cancer and IMPC
(7,20). By contrast, Lino-Silva (8) and Xu et al (22) observed neoplastic cell emboli in the
blood and lymphatic vessels in approximately one third of patients.
These reports and the results of the present study suggest that
IMPC in colorectal cancer may be responsible for high malignancy
and cancer cell invasiveness. Furthermore, the present study
observed a higher incidence of lymph node metastases in patients
with IMPC, and according to the literature, lymph node involvement
is estimated to occur in 63–100% of all cases (6,17). Kim
et al (20) confirmed the
significance of intestinal cancer IMPC in pathomorphological
diagnostics. The authors observed the presence of local lymph node
involvement in 2 out of 3 patients with an IMPC tumor invading the
submucosal membrane (pT1). These findings indicate the importance
of early diagnosis through the analysis of biopsy and surgical
specimens, as the lesion may determine whether the patient has a
high risk of developing metastases. A number of studies reported
that IMPC invasion was observed in up to 90% of resected lymph
nodes and was the only histological component of the metastases
that had formed (3,5,20). The
invasive properties of IMPC structures are associated with
respective adhesion proteins: Outer membrane expression of MUC1 was
confirmed to indicate cancer cell adhesion disorders and induce
disturbances in their association with the extracellular matrix
(23). Furthermore, positive
expression of E-cadherin was identified in the cytoplasm of cells
with a micropapillary structure as compared with neoplastic
glandular ducts and normal ducts (24).
The present study also observed that IMPC lesions in
colorectal cancer were less frequently accompanied by peritumoral
inflammatory infiltrate compared with conventional adenocarcinoma,
although the differences were not significant. This association has
never been confirmed in any previously reported IMPC colorectal
cancer cases. It may be hypothesized that IMPC may induce
suppression of the immune system, thus creating an optimal
microenvironment for the primary tumor to grow and metastasize. In
the digestive system, massive inflammatory infiltrate composed of
neutrophilic granulocytes, which form clusters in the stroma and
focal endothelial microabscesses, have only been identified in
patients with pancreatic IMPC (24).
In conclusion, the associations identified by the
present study indicate the highly aggressive and invasive nature of
IMPC. Therefore, detection of these structures in the colon is
histopathologically and prognostically significant. However,
considering the relatively rare occurrence of IMPCs, these
structures continue to present a diagnostic challenge for
pathomorphologists, and require thorough analysis for an accurate
diagnosis and prognosis.
References
|
1
|
Sonoo H, Kameyama M, Inatugi N, Nonomura A
and Enomoto Y: Pedunculated polyp of early sigmoid colon cancer
with invasive micropapillary carcinoma. Jpn J Clin Oncol.
39:523–527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kondo T: Colon invasive micropapillary
carcinoma arising in tubulovillous adenoma. Pol J Pathol.
59:183–185. 2008.PubMed/NCBI
|
|
3
|
Sakamoto K, Watanabe M, De La Cruz C,
Honda H, Ise H, Mitsui K, Namiki K, Mikami Y, Moriya T and Sasano
H: Primary invasive micropapillary carcinoma of the colon.
Histopathology. 47:479–484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ushiku T, Matsusaka K, Iwasaki Y, Tateishi
Y, Funata N, Seto Y and Fukayama M: Gastric carcinoma with invasive
micropapillary pattern and its association with lymph node
metastasis. Histopathology. 59:1081–1089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wen P, Xu Y, Frankel WL and Shen R:
Invasive micropapillary carcinoma of the sigmoid colon: Distinct
morphology and aggressive behavior. Int J Clin Exp Pathol.
1:457–460. 2008.PubMed/NCBI
|
|
6
|
Verdú M, Román R, Calvo M, Rodón N, García
B, González M, Vidal A and Puig X: Clinicopathological and
molecular characterization of colorectal micropapillary carcinoma.
Mod Pathol. 24:729–738. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Haupt B, Ro JY, Schwartz MR and Shen SS:
Colorectal adenocarcinoma with micropapillary pattern and its
association with lymph node metastasis. Mod Pathol. 20:729–733.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lino-Silva LS: Pure micropapillary rectal
carcinoma with CK7 and CK20 coexpression and loss of CDX2
reactivity. Int J Morphol. 30:25–29. 2012. View Article : Google Scholar
|
|
9
|
Ohtsuki Y, Kuroda N, Yunoki S, Murakami S,
Mizukami Y, Okada Y, Iguchi M, Lee GH and Furihata M:
Immunohistochemical analysis of invasive micropapillary carcinoma
pattern in four cases of gastric cancer. Med Mol Morphol.
46:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seidman JD and Kurman RJ:
Subclassification of serous borderline tumors of the ovary into
benign and malignant types. A clinicopathologic study of 65
advanced stage cases. Am J Surg Pathol. 20:1331–1345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luna-Moré S, Gonzalez B, Acedo C, Rodrigo
I and Luna C: Invasive micropapillary carcinoma of the breast. A
new special type of invasive mammary carcinoma. Pathol Res Pract.
190:668–674. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YS, Kaneko M, Sakamoto DG, Takeshima Y
and Inai K: The reversed apical pattern of MUC1 expression is
characteristics of invasive micropapillary carcinoma of the breast.
Breast Cancer. 13:58–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siriaunkgul S and Tavassoli FA: Invasive
micropapillary carcinoma of the breast. Mod Pathol. 6:660–662.
1993.PubMed/NCBI
|
|
14
|
Amin MB, Ro JY, el-Sharkawy T, Lee KM,
Troncoso P, Silva EG, Ordóñez NG and Ayala AG: Micropapillary
variant of transitional cell carcinoma of the urinary bladder.
Histologic pattern resembling ovarian papillary serous carcinoma.
Am J Surg Pathol. 18:1224–1232. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amin MB, Tamboli P, Merchant SH, Ordóñez
NG, Ro J, Ayala AG and Ro JY: Micropapillary component in lung
adenocarcinoma: A distinctive histologic feature with possible
prognostic significance. Am J Surg Pathol. 26:358–364. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagao T, Gaffey TA, Visscher DW, Kay PA,
Minato H, Serizawa H and Lewis JE: Invasive micropapillary salivary
duct carcinoma: A distinct histologic variant with biologic
significance. Am J Surg Pathol. 28:319–326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laurent I, Uzan C, Gouy S, Pautier P,
Duvillard P and Morice P: Results after conservative treatment of
serous borderline tumors of the ovary with a micropapillary
pattern. Ann Surg Oncol. 15:3561–3566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuroda N, Oonishi K, Ohara M, Hirouchi T,
Mizuno K, Hayashi Y and Lee GH: Invasive micropapillary carcinoma
of the colon: An immunohistochemical study. Med Mol Morphol.
40:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamilton SR, Rubio CA, Vogelstein B, Sobin
LH, Kudo S, Fogt F, Riboli E, Winawer SJ, Nakamura S, Goldgar DE,
et al: Tumours of the colon and rectum: Carcinoma of the colon and
rectum. World Health Organization Classification of Tumours -
Pathology and Genetics of Tumours of the Digestive System. Hamilton
SR and Aaltonen LA: IARC Press; Lyon, France: pp. 104–110. 2000
|
|
20
|
Kim MJ, Hong SM, Jang SJ, Yu E, Kim JS,
Kim KR, Gong G and Ro JY: Invasive colorectal micropapillary
carcinoma: An aggressive variant of adenocarcinoma. Hum Pathol.
37:809–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lino-Silva LS, Salcedo-Hernández RA and
Caro-Sánchez CH: Colonic micropapillary carcinoma, a recently
recognized subtype associated with histological adverse factors:
Clinicopathological analysis of 15 cases. Colorectal Dis.
14:e567–e572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu F, Xu J, Lou Z, Di M, Wang F, Hu H and
Lai M: Micropapillary component in colorectal carcinoma is
associated with lymph node metastasis in T1 and T2 Stages and
decreased survival time in TNM stages I and II. Am J Surg Pathol.
33:1287–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wesseling J, van der Valk SW, Vos HL,
Sonnenberg A and Hilkens J: Episialin (MUC1) overexpression
inhibits integrin-mediated cell adhesion to extracellular matrix
components. J Cell Biol. 129:255–265. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khayyata S, Basturk O and Adsay NV:
Invasive micropapillary carcinomas of the ampullo-pancreatobiliary
region and their association with tumor-infiltrating neutrophils.
Mod Pathol. 18:1504–1511. 2005. View Article : Google Scholar : PubMed/NCBI
|