Introduction
Gastric cancer is characterized by a rapid
progression and decreased survival time, poor response to
conventional chemotherapy and poor prognosis of patients, and is
one of the most common malignancies across the world (1,2).
Worldwide, 952,000 cases of gastric cancer were newly diagnosed,
and 723,000 associated mortalities occurred in 2012 (3). Improving the survival rate of patients
with gastric cancer is currently a major clinical challenge. Tumor
growth, metastasis and prognosis are associated with angiogenesis
(4,5),
which is a multifactorial and multi-step process (6). Inhibition of tumor cell growth,
invasion, migration and metastasis may prevent tumor angiogenesis.
It is therefore accepted that angiogenesis blockade is a novel
treatment for cancer (7,8).
Melatonin (MLT), an indoleamine synthesized in the
pineal gland and other organs, is known to have a wide variety of
physiological functions, including inhibiting inflammation,
regulating circadian rhythms and increasing the activity of
antioxidant enzymes (9,10). Recently, numerous studies have
demonstrated anti-tumor effects of MLT (11–13).
Furthermore, an increased expression of MLT nuclear receptors
RZR/ROR have been demonstrated to contribute to the antitumor
activity of MLT (14,15).
The present study was performed using in vivo
and in vitro experiments and aimed to evaluate the effect of
MLT on gastric cancer growth. The present results demonstrated that
MLT inhibited angiogenesis in gastric cancer, and MLT nuclear
receptor was involved in the inhibition of gastric cancer cell
growth caused by MLT. The data support the theory that MLT may
regulate gastric cancer angiogenesis, which provides novel insights
into the treatment of gastric cancer.
Materials and methods
Human gastric cancer SGC-7901 cell
culture
SGC-7901 cells were purchased from the Shanghai
Institutes for Biological Sciences, Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Invitrogen™; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified
incubator containing 5% CO2. Hypoxia was induced by
adding CoCl2 (final concentration, 100 µM) in the culture medium.
Cells with satisfactory growth were selected for subsequent
experiments.
Microscopic observation of cell
morphology
SGC-7901 cells were seeded onto 6-well plates
(Corning Inc., Corning, NY, USA) at a density of 1×106
cells/well, and incubated in a 37°C incubator in a humidified
atmosphere containing 5% CO2. One day later, the cells
were treated with 1 and 3 mM MLT (Sigma-Aldrich, St. Louis, MO,
USA), and cell morphology was visualized 24 h post-treatment with a
TE2000 inverted fluorescence microscope (Nikon Corporation, Tokyo,
Japan).
Cell proliferation and viability
assays
The SGC-7901 cells were seeded onto 96-well plates
(Corning Inc.) at a density of 5,000 cells/well), and treated with
0, 0.01, 0.1, 1 and 3 mM MLT one day later. Following a further
culture for 0.2, 2, 16 and 24 h, the viability and proliferation of
the SGC-7901 cells was detected using a cell counting kit-8 (CCK-8)
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan),
according to the manufacturer's protocol. Cell density was
determined by measuring the absorbance value (A value) at 450 nm
with a Varioskan™ Flash Multimode Reader (Thermo Fisher Scientific,
Inc.).
In vivo xenograft tumor growth
A total of 60 4-week-old male nude mice of the
BALB/c strain [BALB/cA-nu (nu/nu)] were purchased from Shanghai
Laboratory Animal Center (Shanghai, China), and were maintained in
a specific pathogen-free facility at 20–25°C in a relatively
humidity of 40–60% under a 12 h-light/12 h-dark cycle, with free
access to food and water. The mice were subcutaneously injected in
the flank with SGC-7901 cells at a density of 3×106
cells per mouse, and were randomly assigned into four groups by
concentration of MLT that the mice were treated with: Control (0
mg/kg), low (50 mg/kg), medium (100 mg/kg) and high (150 mg/kg).
The indicated amounts of MLT, which were intraperitoneally injected
into the mice, were not administered until the diameter of the
xenografts reached 2 mm. The long (L) and short (W) axes of the
tumors were measured with a caliper, and the tumor size (V) was
calculated using the following formula: V = 4/3π × l/2 ×
(W/2)2.
Immunohistochemical staining of the
tumors
Mice were sacrificed using the cervical dislocation
method, and tumors were excised 7 days following MLT treatment. The
excised tumors were cut into 5-µm sections, and transferred to
gelatin-coated slides (Sigma-Aldrich). Following a 30-min
incubation in phosphate-buffered saline (PBS) containing 0.3%
Triton X-100 (Sigma-Aldrich), the sections were incubated with a
primary antibody against RZR/ROR receptor [rabbit anti-RZR/RORα
polyclonal antibody (1:500; catalog number ab60134; Abcam,
Cambridge, MA, USA), rabbit anti-RZR/RORβ polyclonal antibody
(1:500; catalog number ab78007; Abcam) or rabbit anti-RZR/RORγ
polyclonal antibody (1:500; catalog number ab188756; Abcam) or
vascular endothelial growth factor (VEGF) (rabbit anti-VEGF
polyclonal antibody (1:200; catalog number ab46154; Abcam) at 37°C
overnight. After washing with 1X-PBS three times, the sections were
incubated with a polyclonal goat anti-rabbit biotinylated antibody
(1:100; catalog number BA1003; Boster Biological Technology Co.,
Pleasanton, CA, USA) for 1 h, and subsequently incubated in
VECTASTAIN® Elite ABC Reagent (Vector Laboratories,
Inc., Burlingame, CA, USA). Subsequently, the sections were stained
with 3,3′-diaminobenzidine (DAB; Sigma-Aldrich). Images were taken
with a Leica DM4000B photo microscope (Leica Microsystems GmbH,
Wetzlar, Germany; magnification, ×400).
Enzyme-linked immunosorbent (ELISA)
assay
SGC-7901 cells were seeded onto dishes (60 mm
diameter) and incubated at 37°C for 24 h. Subsequently, the cells
were incubated in darkness with MLT at various concentrations (0,
50, 100 and 150 mM) and 100 µM CoCl2 (Sigma-Aldrich) at 37°C for 24
h. The cell cultures were transferred to centrifuge tubes and
centrifuged at 4°C, 18,000 × g for 15 min on a 5417 R refrigerated
microcentrifuge (Eppendorf, Hamburg, Germany). The supernatants
were collected and the VEGF level in the supernatant was determined
using a Human VEGF Quantikine ELISA kit (R & D Systems, Inc.,
Minneapolis, MN, USA) by measuring the A value at 450 nm. The cell
count was estimated with a hemocytometer at least three times, and
the standard curve was plotted.
Western blotting assay
Total protein was isolated from the tumor tissues
using a Total Protein Extraction kit (Beijing Transgen Biotech Co.,
Ltd., Beijing, China), and the protein concentration was quantified
with a Bicinchoninic Acid Protein Assay kit (Sigma-Aldrich). The
protein samples were separated by 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (110 V; 1.5 h), and the proteins
were transferred to polyvinylidene difluoride (PVDF) membranes (EMD
Millipore; Billerica, MA, USA) at 4°C (110 V; 1.5 h) using a wet
transfer technique. Subsequently, the membranes were blocked in 5%
skimmed milk in PBS, incubated with a primary antibody against
SUMO-specific protease 1 (SENP1) (rabbit anti-SENP1 polyclonal
antibody; 1:1,000; catalog number SAB2102107; Sigma-Aldrich) or
hypoxia-inducible factor-1α (HIF-1α) (rabbit anti-HIF-1α monoclonal
antibody; 1:1,000; catalog number ab51608; Abcam) at 4°C overnight,
washed in Tris-buffered saline-Tween 20 (TBST), and incubated with
a monoclonal goat anti-mouse IgG secondary antibody (1:4,000;
catalog number, A3562; Sigma-Aldrich) at room temperature for 1.5
h. Subsequently, the PVDF membranes were washed in TBST and the
immunoreactive bands were visualized through exposure to X-ray film
for 1–15 min. The protein purity was evaluated by densitometric
analysis using the Quantity One software version 4.4.2 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The quantity of target
protein was calibrated with respect to β-actin, and control values
and relative intensities were obtained.
Reverse transcription-quantitative
polymerase chain reaction (qPCR)
Total RNA was extracted from the tumor tissues using
TRIzol reagent (Invitrogen™; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The RNA was
reverse-transcribed into first-strand cDNA using the GoScript™
Reverse Transcription System (Promega Corporation, Madison, WI,
USA), which contained M-MLV reverse transcriptase. The primers used
for RT were designed using the Primer Express version 5.0 software
(Applied Biosystems®; Thermo Fisher Scientific, Inc.),
and were synthesized by BioSune Biotechnology Co. (Shanghai,
China). The cycling conditions were as follows: 95°C for 30 sec,
followed by 40 cycles of 95°C for 5 sec and 60°C for 34 sec. The
reaction was conducted on a ABI PRISM 7500 Real-time PCR System
(Applied Biosystems®; Thermo Fisher Scientific, Inc.).
The relative expression of RZR/ROR receptor, SENP1, HIF-1α, VEGF
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) genes was
determined using qPCR with Brilliant III SYBR Green QRT-PCR Master
Mix (Agilent Technologies, Inc., Santa Clara, CA, USA) on a
StepOnePlus® Real-Time PCR system (Applied
Biosystems®; Thermo Fisher Scientific, Inc.). qPCR was
performed using the following primers: RZR/RORγ, forward
5′-GAGGCCATTCAGTACGTGGT-3′ and reverse 5′-GCAATCTCATCCTCGGAAAA-3′;
SENP1, forward 5′-GAGGATGGATGCTGGAGAAG-3′ and reverse
5′-TGTCTGAGGAAGGATTATCTGAG-3′; HIF-1α, forward
5′-ACTCAGGACACAGATTTAGACTTG-3′ and reverse
5′-ATCAGTGGTGGCAGTGGTAG-3′; VEGF, forward 5′-CTTGCCTTGCTGCTCTAC-3′
and reverse 5′-ACCACTTCGTGATGATTCTG-3′; GAPDH, forward
5′-CCGAGAATGGGAAGCTTGTC-3′ and reverse 5′-TTCTCGTGGTTCACACCCATC-3′.
The cycling conditions were as follows: 40 cycles of 95°C for 30
sec, and 60°C for 1 min. Relative mRNA expression levels were
calculated using the 2−∆∆Cq method (16). Melting curve analysis was employed to
check the specificity of the amplification reaction.
Statistical analysis
All experiments were repeated at least three times.
All data are expressed as the mean ± standard error of the mean,
and all statistical analyses were performed with SPSS version 16.0
software (SPSS, Inc., Chicago, IL, USA). Data were analyzed by
one-way analysis of variance, followed by Fisher's least
significant difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
MLT suppresses gastric cancer cell
growth and VEGF secretion
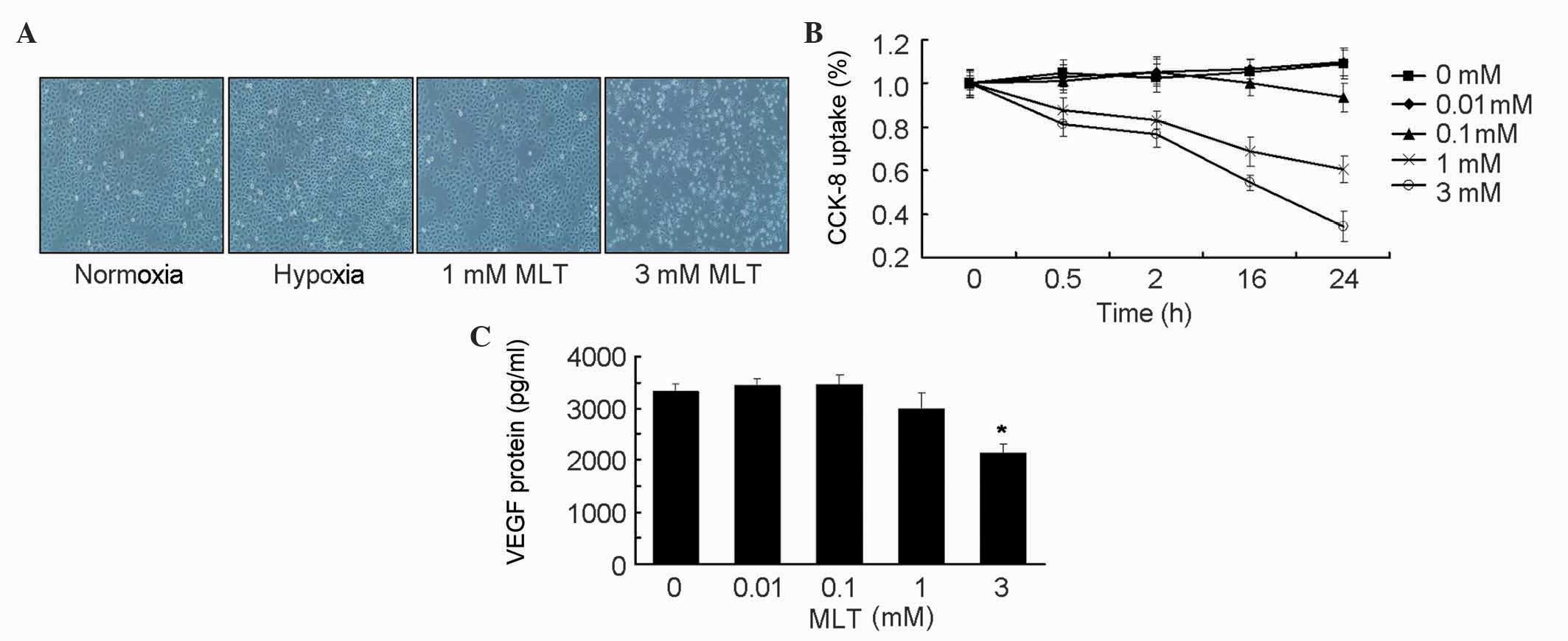
To examine the effects of MLT treatment on SGC-7901
cells, cell morphology and cellular viability in response to MLT
stimuli was examined. Microscopy revealed a significantly reduced
number of SGC-7901 cells in the MLT treatment groups compared to
the control cells. In addition, MLT treatment inhibited the growth
of SGC-7901 cells in a time- and dose-dependent manner (Fig. 1A and B). Subsequently, whether
angiogenesis was impaired following MLT treatment was assessed by
measuring VEGF protein content in the supernatant of the cells. The
present findings demonstrated that treatment with low
concentrations of MLT had no remarkable effect on VEGF secretion,
while 3 mM MLT inhibited VEGF production in SGC-7901 cells
(P=0.023), which was in agreement with the growth inhibitory
effects (Fig. 1C).
MLT inhibits tumorigenesis of gastric
cancer cells in vivo
Since the antitumor activity of MLT has been
extensively described previously, the present study sought to
determine the effective concentration of MLT that was required to
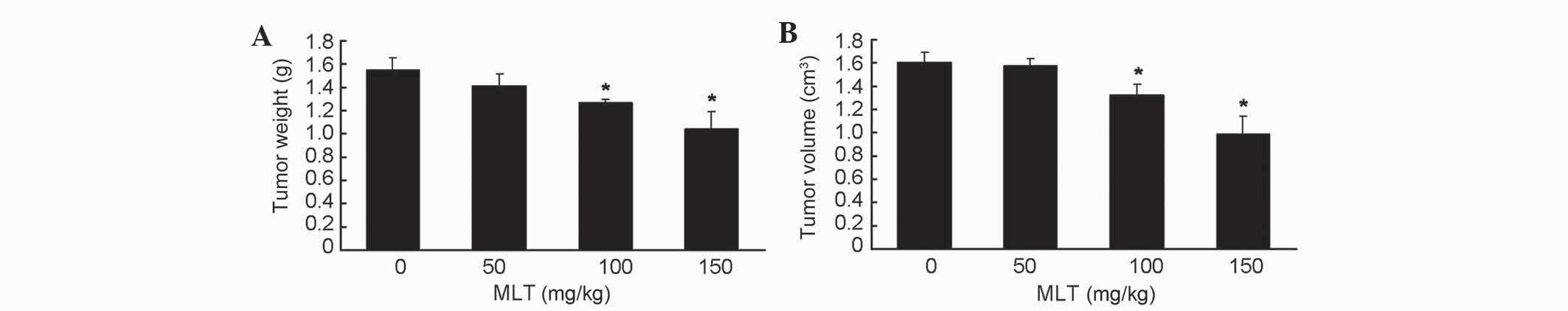
inhibit the in vivo xenograft tumor growth of gastric cancer
in nude mice. Tumor weight and volume (Fig. 2A and B, respectively) were decreased
in tumor-bearing mice treated with low (50 mg/kg) doses of MLT, and
significantly decreased in tumor-bearing mice treated with medium
(100 mg/kg; P=0.043 and 0.041 in Fig. 2A
and B, respectively) and high (150 mg/kg; P=0.039 and 0.031 in
Fig. 2A and B, respectively) doses of
MLT compared with untreated mice. These results additionally
confirmed the antitumor activity of MLT against gastric cancer
cells.
Nuclear receptor RZR/RORγ is expressed
and MLT decreases VEGF expression in gastric cancer tissue
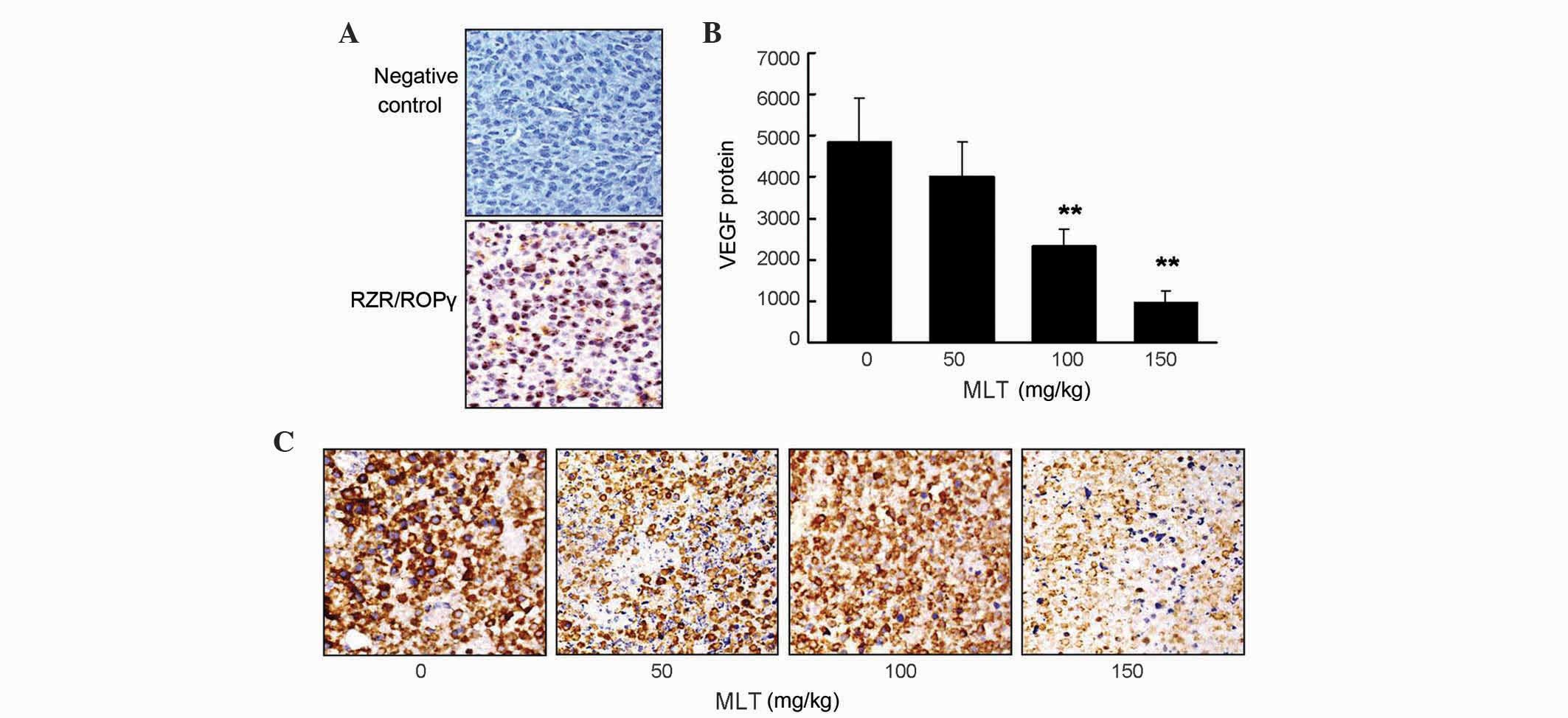
Immunohistochemistry staining results revealed that
RZR/RORγ was expressed in gastric cancer tissues (Fig. 3A), while the expression of RZR/RORα or
RZR/RORβ was not detected (data not shown). To investigate the
effect of effective concentrations of MLT on VEGF expression in
gastric cancer tissue, immunohistochemical staining was performed
to detect VEGF protein expression. The present findings
demonstrated that there was a significantly reduced VEGF protein
expression in the tumor tissues collected from MLT-treated
tumor-bearing mice compared with control mice (P=0.009 and 0.003
for 100 and 150 mg/kg MLT, respectively; Fig. 3B and C).
Effects of MLT on RZR/RORγ, SENP1,
HIF-1α and VEGF expression at mRNA and protein levels in gastric
cancer tissue
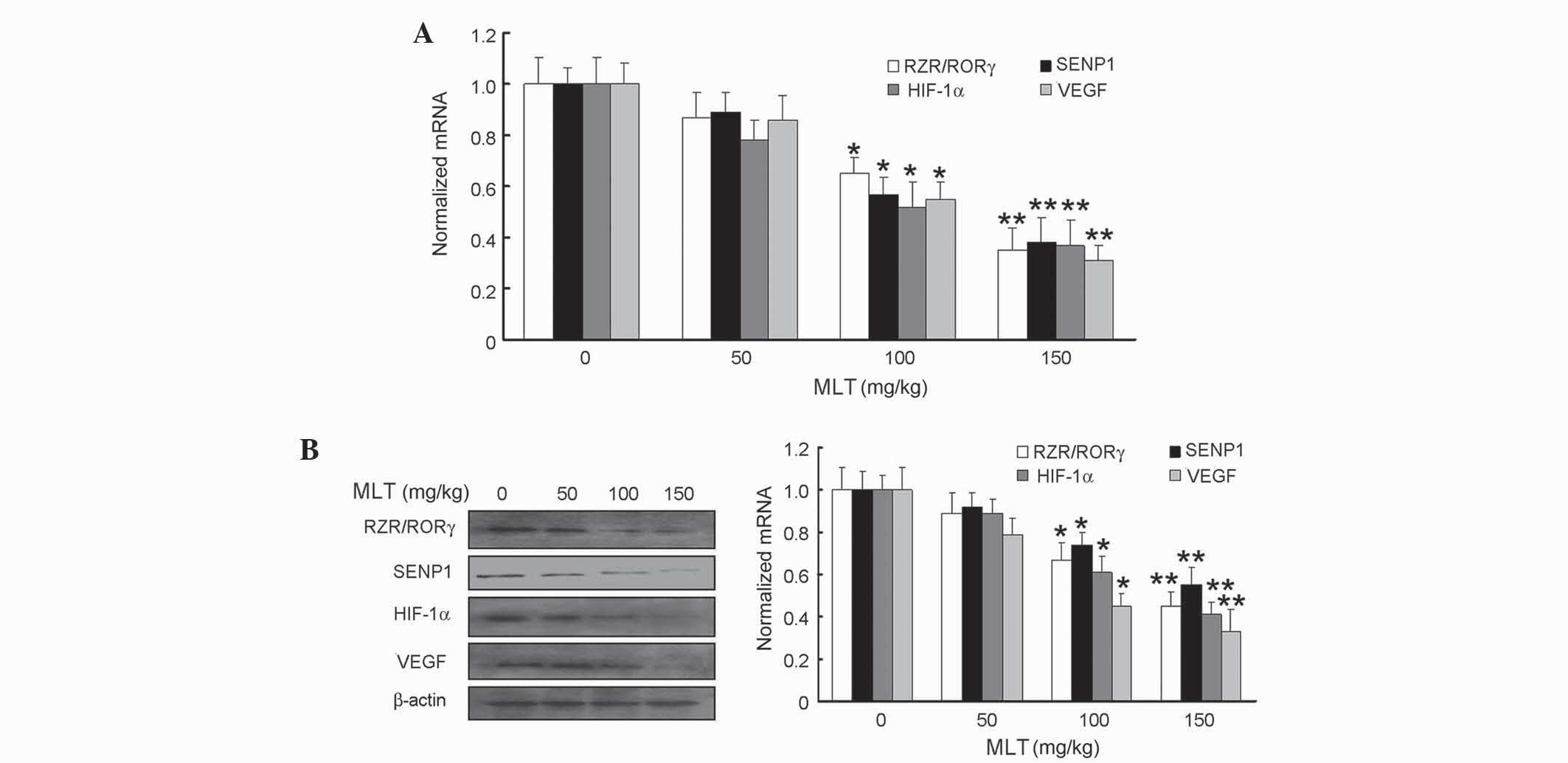
To explore the underlying mechanisms of the
anti-tumor activity of MLT in gastric cancer tissue, the present
study sought to determine the expression of RZR/RORγ, SENP1, HIF-1α
and VEGF following treatment with MLT. The transcriptional and
translational expression of these molecules was determined using
qPCR and western blot analysis, respectively. The present findings
revelaed significantly reduced mRNA and protein levels of RZR/ROR
receptor, SENP1, HIF-1α and VEGF in the tumor tissues derived from
the MLT-treated tumor-bearing mice compared with untreated mice
(Fig. 4A and B). The P-values
obtained for 100 mg/kg MLT in regards to mRNA expression were as
follows: P=0.038 for RZR/RORγ, P=0.035 for SENP1, P=0.033 for
HIF-1α and P=0.034 for VEGF, while for 150 mg/kg MLT, the results
revealed P=0.005 for RZR/RORγ, P=0.007 for SENP1, P=0.006 for
HIF-1α and P=0.004 for VEGF (Fig.
4A). In terms of protein expression, 100 mg/kg MLT resulted in
P=0.040 for RZR/RORγ, P=0.042 for SENP1, P=0.038 for HIF-1α and
P=0.028 for VEGF, while 150 mg/kg MLT led to P=0.006 for RZR/RORγ,
P=0.009 for SENP1, P=0.005 for HIF-1α and P 0.004 for VEGF
(Fig. 4B).
Discussion
The physiological adjustment in response to
alterations in oxygen tension is reported to be critical for normal
embryonic development and the pathophysiology of ischemic vascular
disorders (17–19). Low oxygen induces the stabilization
and activity of HIF-1α, a critical transcription factor for the
adaptation to the hypoxic tumor microenvironment. Indeed, it has
been demonstrated that HIF-1α is involved in the progression of
solid tumors through the stimulation of VEGF transcription under a
hypoxic microenvironment (19–21).
Antibodies and soluble proteins targeting VEGF and its receptors
have therefore been developed and tested for their anti-angiogenic
efficacy against solid tumors, and satisfactory anti-angiogenic
activity was achieved (22–24).
MLT, which was firstly identified as a major
secretory product from the pineal gland of bovines, is a critical
regulator in the coordination of circadian rhythms and seasonal
reproduction, and exhibits antioxidant, oncostatic and
anti-proliferative actions (12,25–27). It
has been recently demonstrated that MLT markedly reduces the risk
of mortality and adverse events of multiple malignancies, notably
in breast cancer, colon cancer, melanoma, lung cancer and malignant
glioblastoma (28–30). Previous studies performed by the
present authors demonstrated that MLT inhibited the growth of
murine foregastric carcinoma cells in vivo and in
vitro (31,32). The findings of the current study
reveal that MLT inhibits the proliferation of SGC-7901 cells in a
dose- and time-dependent manner, and treatment with 3 mM MLT
suppresses VEGF protein secretion in SGC-7901 cells exposed to
CoCl2. As expected, MLT treatment caused an inhibition
on tumor growth and formation of novel blood vessels, and
suppressed tumor angiogenesis in nude mice, as shown by a decrease
in VEGF expression. The results suggest that MLT functions as an
antitumor agent due to its anti-angiogenesis activity. Therefore,
it is hypothesized that MLT may alter tumor adaptation to hypoxia
and inhibit angiogenesis of solid tumors to exhibit anticancer
activity.
It has been proposed that the putative nuclear MLT
receptor belongs to a novel subclass of orphan nuclear receptors,
and it was also suggested that the immunomodulatory and antitumor
effects of MLT was dependent on its intracellular action in
response to nuclear signaling (14,15). In
addition, the actions of MLT had been reported to be mediated by
mechanisms that are either receptor-dependent or independent. The
present study investigated whether MLT inhibited HIF-1α protein
expression in a receptor-dependent manner. Previously, MLT nuclear
receptors were cloned and termed retinoid Z receptor (RZR) and
retinoid acid receptor-related orphan receptor (ROR). The RZR/ROR
family consists of three subtypes (α, β and γ) with four splicing
variants of the α-subtype (33). The
present study revealed that RZR/RORγ receptors were highly
expressed in gastric cancer tissue from tumor-bearing nude mice.
HIF-1α is reported to be critical for hypoxia-dependent
angiogenesis during tumor progression, and its expression is
associated with a poor prognosis in patients with solid tumors
(34). Therefore, it appears that MLT
may act as a HIF-1α inhibitor in tumor microenvironments, including
hypoxia. Consequently, it may be assumed that MLT effectively
decreases HIF-1α expression through the removal of MLT nuclear
receptors during tumor progression.
Hypoxia induces nuclear translocation and
SUMOylation of HIF-1α, which binds to von Hippel-Lindau molecule in
a hydroxyl proline-independent manner, which finally leads to its
ubiquitination and proteasomal degradation (35). SENP1, which is predominately a nuclear
protein, is well-positioned to regulate the activity and stability
of HIF-1α in the nucleus by removing SUMO (36–38). Thus,
SENP1 is critical in maintaining HIF-1α stability during hypoxia
and is involved in hypoxia-dependent angiogenesis during tumor
progression. Several inhibitors and oncolytic adenoviruses, which
function during HIF-1α modification or in the downstream signaling
pathways, have been previously developed (39,40). MLT
has been reported to suppress tumor angiogenesis by inhibiting
HIF-1α stabilization under hypoxic conditions (41,42). The
current study demonstrated that MLT effectively decreased the
levels of RZR/RORγ, SENP1, HIF-1α and VEGF at mRNA and protein
levels in developing gastric cancer. It is hypothesized that MLT
reduces HIF-1α expression by removing RZR/ROR receptors and SENP1
during tumor progression. In addition, MLT has been identified to
lower HIF-1α expression by inhibiting its stability and
accumulation (43). The present
findings demonstrate that MLT suppressed HIF-1α accumulation via
RZR/ROR receptor and SENP1 inactivation, and MLT may act as a
promising agent for treatment of gastric cancer.
In summary, the results of the current study
demonstrate the anti-angiogenesis and antitumor activity of MLT
against gastric cancer by targeting HIF-1α, which stabilizes the
tumor microenvironment and stimulates tumor angiogenesis. In
addition, MLT effectively decreased the expression of RZR/RORγ,
SENP1, HIF-1α and VEGF at mRNA and protein levels in developing
gastric cancer. These findings suggest that MLT may be vital in
tumor suppression by acting through the RZR/RORγ, SENP1, HIF-1α,
VEGF signaling pathway, and reduces angiogenesis in the developing
tumor. Therefore, MLT nuclear receptor RZR/RORγ may be important in
inhibiting gastric cancer angiogenesis and tumor growth. The
present findings provide a novel option to support the treatment of
gastric cancer. Additional clinical trials to evaluate the efficacy
and safety of MLT against gastric cancer appear to be
justified.
Acknowledgements
This present study was supported by the National
Natural Science Foundation of China (grant nos. 30971541 and
81302601), Key Project of Fujian Provincial Department of Science
& Technology (grant no. 2012Y0033), Natural Science Foundation
of Fujian Provincial Department of Science & Technology (grant
no. 2016J01535) and Major Science & Technology Project of
Fujian Medical University (grant no. 09ZD018).
References
|
1
|
Keighley MR: Gastrointestinal cancers in
Europe. Aliment Pharmacol Ther. 18(Suppl 3): S7–S30. 2003.
View Article : Google Scholar
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziche M and Gullino PM: Angiogenesis and
neoplastic progression in vitro. J Natl Cancer Inst. 69:483–487.
1982.PubMed/NCBI
|
|
5
|
Langsenlehner U, Hofmann G, Renner W,
Gerger A, Krenn-Pilko S, Thurner EM, Krippl P and Langsenlehner T:
Association of vascular endothelial growth factor-a gene
polymorphisms and haplotypes with breast cancer metastases. Acta
Oncol. 54:368–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Risau W: Mechanisms of angiogenesis.
Nature. 386:671–674. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luan X, Gao YG, Guan YY, Xu JR, Lu Q, Zhao
M, Liu YR, Liu HJ, Fang C and Chen HZ: Platycodin D inhibits tumor
growth by antiangiogenic activity via blocking VEGFR2-mediated
signaling pathway. Toxicol Appl Pharmacol. 281:118–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prevete N, Liotti F, Visciano C, Marone G,
Melillo RM and de Paulis A: The formyl peptide receptor 1 exerts a
tumor suppressor function in human gastric cancer by inhibiting
angiogenesis. Oncogene. 34:3826–3838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noche RR, Lu PN, Goldstein-Kral L, Glasgow
E and Liang JO: Circadian rhythms in the pineal organ persist in
zebrafish larvae that lack ventral brain. BMC Neurosci. 12:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y, Sun Y, Yi W, Li Y, Fan C, Xin Z,
Jiang S, Di S, Qu Y, Reiter RJ and Yi D: A review of melatonin as a
suitable antioxidant against myocardial ischemia-reperfusion injury
and clinical heart diseases. J Pineal Res. 57:357–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
León J, Casado J, Jiménez Ruiz SM, Zurita
MS, González-Puga C, Rejón JD, Gila A, de Muñoz Rueda P, Pavón EJ,
Reiter RJ, et al: Melatonin reduces endothelin-1 expression and
secretion in colon cancer cells through the inactivation of FoxO-1
and NF-κB. J Pineal Res. 56:415–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paroni R, Terraneo L, Bonomini F, Finati
E, Virgili E, Bianciardi P, Favero G, Fraschini F, Reiter RJ,
Rezzani R and Samaja M: Antitumour activity of melatonin in a mouse
model of human prostate cancer: Relationship with hypoxia
signalling. J Pineal Res. 57:43–52. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Proietti S, Cucina A, Dobrowolny G,
D'Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A,
Morini V, Reiter RJ and Bizzarri M: Melatonin down-regulates MDM2
gene expression and enhances p53 acetylation in MCF-7 cells. J
Pineal Res. 57:120–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karasek M, Carrillo-Vico A, Guerrero JM,
Winczyk K and Pawlikowski M: Expression of melatonin MT(1) and
MT(2) receptors and ROR alpha(1) receptor in transplantable murine
Colon 38 cancer. Neuro Endocrinol Lett. 23(Suppl 1): S55–S60.
2002.
|
|
15
|
Winczyk K, Pawlikowski M, Guerrero JM and
Karasek M: Possible involvement of the nuclear RZR/ROR-alpha
receptor in the antitumor action of melatonin on murine Colon 38
cancer. Tumour Biol. 23:298–302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-timequantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Höckel M and Vaupel P: Tumor hypoxia:
Definitions and current clinical, biologic, and molecular aspects.
J Natl Cancer Inst. 93:266–276. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Briançon-Marjollet A, Pépin JL, Weiss JW,
Levy P and Tamisier R: Intermittent hypoxia upregulates serum VEGF.
Sleep Med. 15:1425–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu S, Bai R, Zhao Z, Zhang Z, Zhang G,
Wang Y, Wang Y, Jiang D and Zhu D: Overexpression of
hypoxia-inducible factor-1α and vascular endothelial growth factor
in sacral giant cell tumors and the correlation with tumor
microvessel density. Exp Ther Med. 8:1453–1458. 2014.PubMed/NCBI
|
|
20
|
Qi L, Xing LN, Wei X and Song SG: Effects
of VEGF suppression by small hairpin RNA interference combined with
radiotherapy on the growth of cervical cancer. Genet Mol Res.
13:5094–5106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Warfel NA and El-Deiry WS: HIF-1 signaling
in drug resistance to chemotherapy. Curr Med Chem. 21:3021–3028.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fellows A, Mierke DF and Nichols RC:
AUF1-RGG peptides up-regulate the VEGF antagonist, soluble VEGF
receptor-1 (sFlt-1). Cytokine. 64:337–342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang SW, Lien JC, Kuo SC and Huang TF:
PPemd26, an anthraquinone derivative, suppresses angiogenesis via
inhibiting VEGFR2 signaling. Br J Pharmacol. 171:5728–5742. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen K, Ji L, Lu B, Xu C, Gong C, Morahan
G and Wang Z: Andrographolide inhibits tumor angiogenesis via
blocking VEGFA/VEGFR2-MAPKs signaling cascade. Chem Biol Interact.
218:99–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lerner AB, Case JD and Takahashi Y:
Isolation of melatonin and 5-methoxyindole-3-acetic acid from
bovine pineal glands. J Biol Chem. 235:1992–1997. 1960.PubMed/NCBI
|
|
26
|
Ngo TL: Review of the effects of
mindfulness meditation on mental and physical health and its
mechanisms of action. Sante Ment Que. 38:19–34. 2013.(In French).
PubMed/NCBI
|
|
27
|
Chen WY, Giobbie-Hurder A, Gantman K,
Savoie J, Scheib R, Parker LM and Schernhammer ES: A randomized,
placebo-controlled trial of melatonin on breast cancer survivors:
Impact on sleep, mood, and hot flashes. Breast Cancer Res Treat.
145:381–388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim W, Jeong JW and Kim JE: CCAR2
deficiency augments genotoxic stress-induced apoptosis in the
presence of melatonin in non-small cell lung cancer cells. Tumour
Biol. 35:10919–10929. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mao L, Yuan L, Xiang S, Zeringue SB,
Dauchy RT, Blask DE, Hauch A and Hill SM: Molecular deficiency
(ies) in MT1 melatonin signaling pathway underlies the
melatonin-unresponsive phenotype in MDA-MB-231 human breast cancer
cells. J Pineal Res. 56:246–253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin V, Sanchez-Sanchez AM,
Puente-Moncada N, Gomez-Lobo M, Alvarez-Vega MA, Antolín I and
Rodriguez C: Involvement of autophagy in melatonin-induced
cytotoxicity in glioma-initiating cells. J Pineal Res. 57:308–316.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oluwole OO, DePaz HA, Adeyeri A, Jin MX,
Hardy MA and Oluwole SF: Role of CD41CD251 regulatory T cells from
naive host thymus in the induction of acquired transplant tolerance
by immunization with allo-major histocompatibility complex peptide.
Transplantation. 75:1136–1142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu L, Liu H, Zhang H, Wang RX, Song J and
Zhou RX: Growth-inhibitory activity of melatonin on murine
foregastric carcinoma cells in vitro and the underlying molecular
mechanism. Anat Rec (Hoboken). 296:914–920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carlberg C and Wiesenberg I: The orphan
receptor family RZR/ROR, melatonin and 5-lipoxygenase: An
unexpected relationship. J Pineal Res. 18:171–178. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruas JL and Poellinger L:
Hypoxia-dependent activation of HIF into a transcriptional
regulator. Semin Cell Dev Biol. 16:514–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geoffroy MC and Hay RT: An additional role
for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol.
10:564–568. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Q, Xia N, Li T, Xu Y, Zou Y, Zuo Y,
Fan Q, Bawa-Khalfe T, Yeh ET and Cheng J: SUMO-specific protease 1
promotes prostate cancer progression and metastasis. Oncogene.
32:2493–2498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gu J, Fan Y, Liu X, Zhou L, Cheng J, Cai R
and Xue S: SENP1 protects against myocardial ischaemia/reperfusion
injury via a HIF1α-dependent pathway. Cardiovasc Res. 104:83–92.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Helbig L, Koi L, Brüchner K, Gurtner K,
Hess-Stumpp H, Unterschemmann K, Baumann M, Zips D and Yaromina A:
BAY 87–2243, a novel inhibitor of hypoxia-induced gene activation,
improves local tumor control after fractionated irradiation in a
schedule-dependent manner in head and neck human xenografts. Radiat
Oncol. 9:2072014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee SH, Jee JG, Bae JS, Liu KH and Lee YM:
A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α
synthesis and decreases its stability via inhibition of the
PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol.
230:853–862. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park JW, Hwang MS, Suh SI and Baek WK:
Melatonin down-regulates HIF-1 alpha expression through inhibition
of protein translation in prostate cancer cells. J Pineal Res.
46:415–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, García-Palomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai M, Cui P, Yu M, Han J, Li H and Xiu R:
Melatonin modulates the expression of VEGF and HIF-1 alpha induced
by CoCl2 in cultured cancer cells. J Pineal Res.
44:121–126. 2008. View Article : Google Scholar : PubMed/NCBI
|