Introduction
Carcinogenesis is a complex, multistage process.
Advances in gene sequencing technology have demonstrated that
certain DNA mutations and chromosomal abnormalities are important
in tumorigenesis and tumor development. Aberrations in RNA, a
central element in gene expression, are also vital in
tumorigenesis, tumor development and malignant growth at the
posttranscriptional and epigenetic level. Posttranscriptional
modifications, including RNA editing and alternative splicing,
render it possible to diversify the transcriptome, while
restricting the size of the genome. Members of the adenosine
deaminase acting on RNA (ADAR) family of enzymes, which catalyze
adenosine-to-inosine (A-to-I) RNA editing, have been associated
with alternative splicing in glioma (1,2). Levels of
A-to-I RNA editing are reportedly reduced in glioma, and
alternative splicing variants (ASVs) of ADAR2 are expressed at
various levels (3). Notably, the
expression of ADAR2 mRNA is unaltered in glioma, and the underlying
mechanism of this phenomenon remains unclear. The present review
discusses alternative splicing and RNA editing in glioma,
specifically in terms of the ADAR2 spliced isoforms.
RNA editing and ADARs
RNA editing was first identified in trypanosomes by
Benne et al (4), who concluded
that alterations in nucleotide sequences occur during or following
transcription of the frameshift gene by a RNA editing process. A
subsequent study identified a RNA duplex unwinding activity when
antisense RNA was injected into fertilized frog eggs (5). The unwinding activity was later
demonstrated to arise from structural alterations in the RNA when
adenosine (A) is converted to inosine (I) (6). The following types of RNA editing have
been identified (7): Uridine (U)
insertion or deletion (8); cytosine
(C) insertion and dinucleotide insertion (9); small nucleolar RNA-mediated nucleotide
modification of ribosomal RNAs (10);
transfer (t)RNA editing (11); C-to-U
editing (12); and A-to-I editing
(13). In mammals, A-to-I editing is
the most common type of RNA editing causing genetic diversity
(14). A-to-I RNA editing occurs at
over one hundred million genomic sites, which are located in the
majority of human genes (15). ADAR
enzymes, responsible for the catalytic conversion of A to I
(14), share a highly conserved
catalytic deaminase domain (DM) at their C-terminal, which binds to
double-stranded DNA (dsRNA) synergistically with the N-terminus
dsRNA-binding domain (dsRBD) (16–18). In
vertebrates, three members of the ADAR family (ADAR1, ADAR2 and
ADAR3) have been identified (19).
There are three dsRBDs for ADAR1, and two for ADAR2 and ADAR3
(18). ADAR1 and ADAR2 are
ubiquitously expressed in humans and exhibit catalytic activity,
whereas ADAR3 is expressed specifically in the brain and has no
catalytic activity (14). ADAR3 has
been revealed to competitively inhibit deaminase activities of
other ADARs by binding to dsRNA (20). The adenosine deaminase reaction
catalyzed by ADAR2 is site-specific (21), as demonstrated in studies of the GluA2
subunit of glutamate AMPA receptor (21–23), in
which the conversion of a glutamine (Q) to an arginine (R) codon is
exclusively mediated by ADAR2 (23).
ADAR2 activity is essential for brain development and function
(24), and >99.9% of RNA editing
occurs at the Q/R site of GluA2 in the human central nervous system
(25). In addition, RNA editing plays
a role in controlling microRNA (miRNA) biogenesis (26). A recent study of the mouse brain
revealed that reproducible alterations in the sequence and
abundance of mature miRNAs are induced by ADAR2 (27). Furthermore, ADAR2-mediated editing is
site-specific, as opposed to sequence-specific, and ADAR2 edits the
coding and noncoding regions of mRNAs (28,29).
Therefore, a single-base modification during the recoding process
during editing may affect the coding potential of the RNA and its
splicing.
Alternative splicing
Studies conducted in 1977 revealed that the coding
regions in DNA sequences are not continuous, and the final tRNA or
mRNA is a spliced product (30,31). In
1978, Gilbert presented ‘Why genes in pieces?’ (32), in which the terms ‘intron’ and ‘exon’
were first introduced. The research by Gilbert led to an additional
study, which confirmed that alternative splicing occurs in
eukaryotic cells (33). In 1994, the
level of alternatively spliced human genes was estimated to be only
5% (34). However, by the 21st
century, data from The International Genome Sequencing Consortium
predicted that ≥50% of human genes are alternatively spliced
(33,35,36). In
2008, Wang et al (37) and Pan
et al (38) demonstrated that
>90% of multi-exon genes are alternatively spliced, and the
majority of these are specifically alternatively spliced tissue.
Wang et al (37) also
described various types of alternative splicing, including exon
skipping, intron retention, alternative 5′ splice sites,
alternative 3′ splice sites, mutually exclusive exons, mutually
exclusive 5′ untranslated regions (UTRs), mutually exclusive 3′
UTRs and tandem UTRs. Alternative splicing results in the
expression of diverse proteins and affects transcription factors,
cell signaling, transmembrane proteins and secreted extracellular
proteins (39). Consequently, the
structural and functional alterations in these proteins and
signaling pathways may be involved in carcinogenesis (39).
Alternative splicing of human ADAR2
mRNA
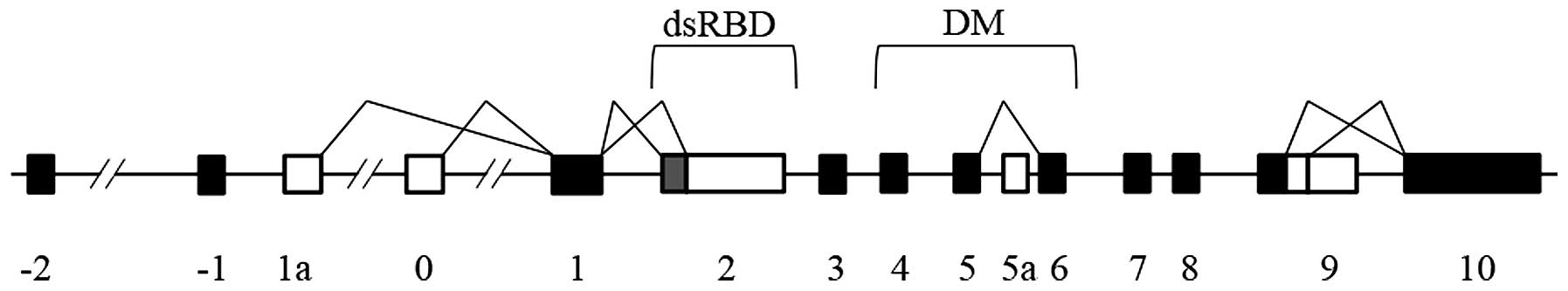
The human ADAR2 gene is located on the long arm of
chromosome 21 (21q22.3) and spans ~153 kbp (40–42). A
study by Slavov and Gardiner (42)
revealed that the genomic structure of the human ADAR2 gene
consists of 15 exons. The structure of ADAR2 mRNA is illustrated in
Fig. 1. Kawahara et al
(43) followed the exon and intron
identifiers reported by Slavov and Gardiner to divide ADAR2 mRNA
into four regions based on alternative splicing sites: The first
region includes exons 2-1, in which two ASVs occur; the second
region includes exons 2-3, in which three ASVs occur; the third
region includes exons 4–8, in which two ASVs occur; and the fourth
region includes exons 9–10. So far, a total of nine splicing sites
in ADAR2 mRNA have been confirmed (42–48).
Alternative splicing at these sites occurs independently, resulting
in dozens of ADAR2 spliced isoforms. This renders it challenging to
analyze tissue-specific and developmental-stage-dependent
properties of the ADAR2 ASVs in vivo (49). Studies have identified that
alternative splicing occurs at exon 2 and exon 4–6, which are dsRBD
and DM coding regions, and these affect the enzyme activity of
ADAR2 (43,44,46). In
addition, inclusion of exon 5a results in the generation of a
protein, which has ~50% reduction in activity (44). The exception is the splicing variants,
which have a distinctive truncated shorter C-terminal structure,
and exhibit no editing activity, if splicing occurs at exon 9
(45). Agranat et al (48) described a splicing event that the 93
nucleotide sequence located in intron 7 was included as exon 7a.
This also occurs outside ADAR2 function domain coding regions, but
does not lead to a catalytic activity product. Alternative exon 7a
is expressed tissue-specifically, with high levels in the skeletal
muscle, heart and testis, and low levels in the brain (48). Alternative splicing sites in human
ADAR2 mRNA are summarized in Table I,
which is adapted from a previous study by the present authors
(50).
 | Table I.Summary of alternative splicing sites
in human ADAR2 mRNA. |
Table I.
Summary of alternative splicing sites
in human ADAR2 mRNA.
| Author, year | ASV, exon | Effect on ADAR2
transcript | Effect on ADAR2
protein | Effect on catalytic
activity | (Ref.) |
|---|
| Slavov and
Gardiner, 2002 | −1-1 | Inclusion of exon
1a | 28-amino-acid
N-terminal extension | Unknown | (42) |
| Kawahara et
al, 2005 | 1–3 | Skipping of exon
2 | Generation of a
12-amino-acid protein | None | (43) |
| Kawahara et
al, 2005 | 9–10 | Inclusion of intron
9 | Unknown | Unknown | (43) |
| Kawahara et
al, 2005 | 9 | Splices exon 9, 83
nt downstream from stop codon | Unknown | Unknown | (43) |
| Gerber et
al, 1997 | 5–6 | Inclusion of exon
5a | Insertion of AluJ
cassette in the catalytic domain | Decreased | (44) |
| Lai et al,
1997 | 9 | Truncates 3′ end of
the coding region | Replacement of 29
C-terminal residues with 2 amino acids | None | (45) |
| Rueter et
al, 1999 | 1–2 | Addition of 47 nt
to 5′ end of exon 2 | Generation of a
9-kDa protein | Decreased | (46) |
| Maas and Gommans,
2009 | −1-1 | Inclusion of exon
0 | 49-amino-acid
N-terminal extension | Unknown | (47) |
| Agranat et
al, 2010 | 7–8 | Inclusion of exon
7a | Nonsense-mediated
mRNA decay | None | (48) |
Reduced RNA editing in glioma
Although extensive sequencing and analysis of the
human genome have revealed a clear association between genes and
cancer, numerous questions have also been raised. Currently, in the
postgenomic era, the field of epigenetics has received much
attention. Epigenetic alterations, including alternative splicing
and ADAR-mediated A-to-I RNA editing, have been associated with
several types of cancer, including breast cancer, neuroblastoma and
hepatocellular carcinoma, and there is a particularly clear
association with glioma (3,39,51).
Glioma is the most common type of tumor in the
central nervous system, and is classified into four grades that
reflect the degree of malignancy. Glioblastoma multiforme (grade
IV) is the most aggressive type of glioma and is fatal (1). An association between RNA editing and
glioma, particularly the pathogenesis of glioblastoma, was first
identified by Maas et al (1),
who demonstrated that there was reduced editing at the Q/R site of
GluR-B, with no corresponding alteration in ADAR2 expression and no
difference in the alternative splicing of ADAR2 mRNA in tumor and
normal tissues. Cellular mechanisms that regulate ADAR2 catalytic
activity are unknown, but may involve posttranslational
modification or controlled subcellular localization of ADAR2
(1). Another study demonstrated that
a reduction in editing levels was associated with the grade of
malignancy in pediatric astrocytomas, which was attributed to
altered ADAR2 catalytic activity (2).
Furthermore, that study reported that ADAR2 overexpression
inhibited cell proliferation and migration in vitro. In
addition, alternative splicing within exon 2 of the ADAR1 pre-mRNA
in high-grade tumors generated a 110-kDa protein, as opposed to the
full-length 150-kDa protein. Overexpressed ASVs of ADAR1 are
hypothesized to form heterodimers with ADAR2, disrupting the
balance between ADAR1, ADAR2 and ADAR3, and competing for specific
ADAR2 editing activity at the Q/R site of GluR-B (2). These findings suggest that the
alternative splicing events in ADAR1 regulate ADAR2-mediated RNA
editing.
A previous study demonstrated a significant loss of
ADAR2 editing activity in newly diagnosed and recurrent pediatric
high-grade astrocytoma (52).
Notably, ADAR2 editing activity was substantially rescued in the
only patient with prolonged survival, suggesting that ADAR2
activity/expression is a possible prognostic marker. Other results
suggest attenuated A-to-I editing of miRNA-376a* promotes
invasiveness of glioblastoma cells in vitro and orthotopic
xenograft mouse models (53). In
addition, a recent study demonstrated that ADAR2 editing activity
inhibits glioblastoma growth by modulating the cell division cycle
14B/S-phase kinase-associated protein 2/p21/p27 axis (54).
ADAR2 ASVs regulate RNA editing in
glioma
Reduced RNA editing without a significant alteration
in ADAR2 expression has been widely documented (1–3,55); however, the underlying mechanism
remains unclear. Due to the large number of ADAR2 ASVs, ADAR2
expression may not associate fully with its editing activity. This
raises the question of whether alternative splicing is another
regulatory factor that may interfere with ADAR2-mediated RNA
editing. However, the precise association between RNA editing and
alternative splicing is complex and remains unclear.
Rueter et al (46) demonstrated that the addition of 47
nucleotides to the 5′ end of exon 2 occurs subsequent to RNA
editing within intron 1, which reduces ADAR2 activity in
vivo. A previous study by the present authors revealed the
expression levels of this ADAR2 ASV in human glioma tissues and
glioma-derived U251 and BT325 cell lines (55); the ADAR2 ASV was expressed in 10% of
low-grade astrocytomas, 16.7% of oligodendrogliomas, 12.5% of
anaplastic astrocytomas and 25% of glioblastomas multiforme. In
addition, the increased expression of this self-editing-induced ASV
corresponded to the increasing malignancy of the glioma, and its
expression appeared to be associated with the malignant features of
glioma, as identified in the glioblastoma multiforme group of ADAR2
ASV+ patients, who had more severe peritumoral brain
edema, tumor invasion in more brain lobes and a shorter median
survival time compared with ADAR2 ASV− patients.
Therefore, it appears that ADAR2 mRNA levels are not altered in
glioma-derived cell lines or glioma tissues, the self-editing of
the ADAR2 pre-mRNA generates an ADAR2 ASV in glioma-derived cell
lines and glioma tissues, and the expression of this ADAR2 ASV may
be associated with the malignancy of glioma.
Early studies by the present authors lead to a
hypothesis that ADAR2 splicing isoforms may affect its enzyme
activity; therefore leading to investigations concerning the
association between splicing isoform expression and the clinical
features of glioma. However, the identified percentage of ADAR2
ASVs (10–25%) was not enough to theoretically explain the reduced
RNA editing of the GluA2 Q/R site. Hideyama and Kwak (56) demonstrated that expression of Q/R
site-unedited GluA2 requires >50% reduction of ADAR2 activity;
therefore, the present authors analyzed the differences in the
alternative splicing patterns of ADAR2 in glioma U87, U251 and A172
cell lines and normal human astrocytes HA1800 cells (50). Quantitative polymerase chain reaction
identified no significant differences in the ADAR2 pre-mRNA
splicing patterns at exon 1a or 2 between the glioma-derived cell
lines and normal human astrocytes. However, transcripts including
exon 5a were predominantly expressed in the glioma-derived cell
lines, and transcripts without exon 5a were relative to its
expression in normal human astrocytes. Taken together, these
findings indicate that alternative splicing in glioma cells causes
an abnormal increase in the expression of exon 5a, leading to the
active suppression of ADAR2 activity and a reduction in A-to-I RNA
editing.
Therefore, A-to-I RNA editing is regulated by the
pattern of ADAR2 alternative splicing in glioma. Collectively,
in vitro results suggest an association between the
increased expression of abnormal ADAR2 isoforms or specific ADAR2
ASVs and the malignant characteristics of astrocytoma. Further
studies concerning human glioma tissues are required to support
in vitro findings, using normal brain white matter as a
control. The primary aim of such a study would be to determine
whether the alterations in ADAR2 ASV expression may be used as a
novel marker for the molecular classification of glioma and to
monitor tumor progression in patients.
Conclusion
In summary, abnormal expression and activity of
ADAR2 contributes to abnormal RNA editing. Additional study into
specific ADAR2 ASVs is required to identify the specific RNA
abnormalities that are associated with tumorigenesis and tumor
development, and to determine the complex associations between
ADAR-mediated A-to-I editing and the alternative splicing of
pre-mRNA ADAR2 in specific diseases. Furthermore, additional study
is required to establish the precise regulation of A-to-I editing
by ADAR2 ASVs and how they contribute to glioma genesis and
progression. Overall, future aims would be to identify novel ADAR2
target genes, investigate the rescue potential of ADAR2 editing
activity and define the underlying rules of ADAR2 ASVs differential
expression. Identification of subtle alterations in the
transcriptome introduced by A-to-I RNA editing and ADAR2 isoforms
with differential activity generated by alternative splicing may
continue to lead to additional and notable findings.
Acknowledgements
The authors wish to acknowledge the funding agencies
that supported certain original work cited in this review: The
National Science Foundation of China (Beijing, China; grant no.
30672159) and the Doctoral Program of Higher Education Research
Fund (Beijing, China; grant no. 20110061110070).
Glossary
Abbreviations
Abbreviations:
|
A-to-I
|
adenosine-to-inosine
|
|
ADAR
|
adenosine deaminase acting on RNA
|
|
ASV
|
alternative splicing variant
|
|
dsRBD
|
dsRNA-binding domain
|
References
|
1
|
Maas S, Patt S, Schrey M and Rich A:
Underediting of glutamate receptor GluR-B mRNA in malignant
gliomas. Proc Natl Acad Sci USA. 98:14687–14692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cenci C, Barzotti R, Galeano F, Corbelli
S, Rota R, Massimi L, Di Rocco C, O'Connell MA and Gallo A:
Down-regulation of RNA editing in pediatric astrocytomas: ADAR2
editing activity inhibits cell migration and proliferation. J Biol
Chem. 283:7251–7260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Z, Tian Y, Tian N, Zhao X, Du C, Han L
and Zhang H: Aberrant alternative splicing pattern of ADAR2
downregulates adenosine-to-inosine editing in glioma. Oncol Rep.
33:2845–2852. 2015.PubMed/NCBI
|
|
4
|
Benne R, Van Den Burg J, Brakenhoff JP,
Sloof P, Van Boom JH and Tromp MC: Major transcript of the
frameshifted coxll gene from trypanosome mitochondria contains four
nucleotides that are not encoded in the DNA. Cell. 46:819–826.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rebagliati MR and Melton DA: Antisense RNA
injections in fertilized frog eggs reveal an RNA duplex unwinding
activity. Cell. 48:599–605. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagner RW, Smith JE, Cooperman BS and
Nishikura K: A double-stranded RNA unwinding activity introduces
structural alterations by means of adenosine to inosine conversions
in mammalian cells and Xenopus eggs. Proc Natl Acad Sci USA.
86:2647–2651. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang W, Fei Y and Page M: Biological
significance of RNA editing in cells. Mol Biotechnol. 52:91–100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osato D, Rogers K, Guo Q, Li F, Richmond
G, Klug F and Simpson L: Uridine insertion/deletion RNA editing in
trypanosomatid mitochondria: In search of the editosome. RNA.
15:1338–1344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Visomirski-Robic LM and Gott JM:
Insertional editing in isolated Physarum mitochondria is linked to
RNA synthesis. RNA. 3:821–837. 1997.PubMed/NCBI
|
|
10
|
Doe CM, Relkovic D, Garfield AS, Dalley
JW, Theobald DE, Humby T, Wilkinson LS and Isles AR: Loss of the
imprinted snoRNA mbii-52 leads to increased 5htr2c pre-RNA editing
and altered 5HT2CR-mediated behaviour. Hum Mol Genet. 18:2140–2148.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klipcan L, Moor N, Kessler N and Safro MG:
Eukaryotic cytosolic and mitochondrial phenylalanyl-tRNA
synthetases catalyze the charging of tRNA with the meta-tyrosine.
Proc Natl Acad Sci USA. 106:11045–11048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lynch M, Koskella B and Schaack S:
Mutation pressure and the evolution of organelle genomic
architecture. Science. 311:1727–1730. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sommer B, Kohler M, Sprengel R and Seeburg
PH: RNA editing in brain controls a determinant of ion flow in
glutamate-gated channels. Cell. 67:11–19. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishikura K: Functions and regulation of
RNA editing by ADAR deaminases. Annu Rev Biochem. 79:321–349. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bazak L, Haviv A, Barak M, Jacob-Hirsch J,
Deng P, Zhang R, Isaacs FJ, Rechavi G, Li JB, Eisenberg E and
Levanon EY: A-to-I RNA editing occurs at over a hundred million
genomic sites, located in a majority of human genes. Genome Res.
24:365–376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Macbeth MR, Schubert HL, Vandemark AP,
Lingam AT, Hill CP and Bass BL: Inositol hexakisphosphate is bound
in the ADAR2 core and required for RNA editing. Science.
309:1534–1539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryter JM and Schultz SC: Molecular basis
of double-stranded RNA-protein interactions: Structure of a
dsRNA-binding domain complexed with dsRNA. EMBO J. 17:7505–7513.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuttan A and Bass BL: Mechanistic insights
into editing-site specificity of ADARs. Proc Natl Acad Sci USA.
109:E3295–E3304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Slotkin W and Nishikura K:
Adenosine-to-inosine RNA editing and human disease. Genome Med.
5:1052013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen CX, Cho DS, Wang Q, Lai F, Carter KC
and Nishikura K: A third member of the RNA-specific adenosine
deaminase gene family, ADAR3, contains both single- and
double-stranded RNA binding domains. RNA. 6:755–767. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Levanon EY, Eisenberg E, Yelin R, Nemzer
S, Hallegger M, Shemesh R, Fligelman ZY, Shoshan A, Pollock SR,
Sztybel D, et al: Systematic identification of abundant A-to-I
editing sites in the human transcriptome. Nat Biotechnol.
22:1001–1005. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Collingridge GL, Olsen RW, Peters J and
Spedding M: A nomenclature for ligand-gated ion channels.
Neuropharmacology. 56:2–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higuchi MSF, Köhler M, Sommer B, Sprengel
R and Seeburg PH: RNA editing of AMPA receptor subunit GluR-B: A
basepaired intron-exon structure determines position and
efficiency. Cell. 75:1361–1370. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barresi S, Tomaselli S, Athanasiadis A,
Galeano F, Locatelli F, Bertini E, Zanni G and Gallo A:
Oligophrenin-1 (OPHN1), a gene involved in X-linked intellectual
disability, undergoes RNA editing and alternative splicing during
human brain development. PLoS One. 9:e913512014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hogg M, Paro S, Keegan LP and O'Connell
MA: RNA editing by mammalian ADARs. Adv Genet. 73:87–120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang W, Chendrimada TP, Wang Q, Higuchi M,
Seeburg PH, Shiekhattar R and Nishikura K: Modulation of microRNA
processing and expression through RNA editing by ADAR deaminases.
Nat Struct Mol Biol. 13:13–21. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vesely C, Tauber S, Sedlazeck FJ, Tajaddod
M, Haeseler AV and Jantsch MF: ADAR2 induces reproducible changes
in sequence and abundance of mature microRNAs in the mouse brain.
Nucleic Acids Res. 42:12155–12168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang Y, Zhou X and Jin Y: ADAR-mediated
RNA editing in non-coding RNA sequences. Sci China Life Sci.
56:944–952. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hundley HA and Bass BL: ADAR editing in
double-stranded UTRs and other noncoding RNA sequences. Trends
Biochem Sci. 35:377–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berget SM, Moore C and Sharp PA: Spliced
segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl
Acad Sci USA. 74:3171–3175. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sambrook J: Adenovirus amazes at Cold
Spring Harbor. Nature. 268:101–104. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gilbert W: Why genes in pieces? Nature.
271:5011978. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brett D, Pospisil H, Valcarcel J, Reich J
and Bork P: Alternative splicing and genome complexity. Nat Genet.
30:29–30. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sharp PA: Split genes and RNA splicing.
Cell. 77:805–815. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lander ES, Linton LM, Birren B, Nusbaum C,
Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al:
Initial sequencing and analysis of the human genome. Nature.
409:860–921. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharp PA: The discovery of split genes and
RNA splicing. Trends Biochem Sci. 30:279–281. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang ET, Sandberg R, Luo S, Khrebtukova I,
Zhang L, Mayr C, Kingsmore SF, Schroth GP and Burge CB: Alternative
isoform regulation in human tissue transcriptomes. Nature.
456:470–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pan Q, Shai O, Lee LJ, Frey BJ and
Blencowe BJ: Deep surveying of alternative splicing complexity in
the human transcriptome by high-throughput sequencing. Nat Genet.
40:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Venables JP: Aberrant and alternative
splicing in cancer. Cancer Res. 64:7647–7654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tomaselli S, Bonamassa B, Alisi A, Nobili
V, Locatelli F and Gallo A: ADAR enzyme and miRNA story: A
nucleotide that can make the difference. Int J Mol Sci.
14:22796–22816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mittaz L, Scott HS, Rossier C, Seeburg PH,
Higuchi M and Antonarakis SE: Cloning of a human RNA editing
deaminase (ADARB1) of glutamate receptors that maps to chromosome
21q22.3. Genomics. 41:210–217. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Slavov D and Gardiner K: Phylogenetic
comparison of the pre-mRNA adenosine deaminase ADAR2 genes and
transcripts: Conservation and diversity in editing site sequence
and alternative splicing patterns. Gene. 299:83–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kawahara Y, Ito K, Ito M, Tsuji S and Kwak
S: Novel splice variants of human ADAR2 mRNA: Skipping of the exon
encoding the dsRNA-binding domains and multiple C-terminal splice
sites. Gene. 363:193–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gerber A, O'Connell MA and Keller W: Two
forms of human double-stranded RNA-specific editase 1 (hRED1)
generated by the insertion of an Alu cassette. RNA. 3:453–463.
1997.PubMed/NCBI
|
|
45
|
Lai F, Chen CX, Carter KC and Nishikura K:
Editing of glutamate receptor B subunit ion channel RNAs by four
alternatively spliced DRADA2 double-stranded RNA adenosine
deaminases. Mol Cell Biol. 17:2413–2424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rueter SM, Dawson TR and Emeson RB:
Regulation of alternative splicing by RNA editing. Nature.
399:75–80. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Maas S and Gommans WM: Novel exon of
mammalian ADAR2 extends open reading frame. PLoS One. 4:e42252009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Agranat L, Sperling J and Sperling R: A
novel tissue-specific alternatively spliced form of the A-to-I RNA
editing enzyme ADAR2. RNA Biol. 7:253–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Solomon O, Oren S, Safran M, Deshet-Unger
N, Akiva P, Jacob-Hirsch J, Cesarkas K, Kabesa R, Amariglio N,
Unger R, et al: Global regulation of alternative splicing by
adenosine deaminase acting on RNA (ADAR). RNA. 19:591–604. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Galeano F, Tomaselli S, Locatelli F and
Gallo A: A-to-I RNA editing: The ‘ADAR’ side of human cancer. Semin
Cell Dev Biol. 23:244–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dominissini D, Moshitch-Moshkovitz S,
Amariglio N and Rechavi G: Adenosine-to-inosine RNA editing meets
cancer. Carcinogenesis. 32:1569–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tomaselli S, Galeano F, Massimi L, Di
Rocco C, Lauriola L, Mastronuzzi A, Locatelli F and Gallo A: ADAR2
editing activity in newly diagnosed versus relapsed pediatric
high-grade astrocytomas. BMC Cancer. 13:2552013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Choudhury Y, Tay FC, Lam DH, Sandanaraj E,
Tang C, Ang BT and Wang S: Attenuated adenosine-to-inosine editing
of microRNA-376a* promotes invasiveness of glioblastoma cells. J
Clin Invest. 122:4059–4076. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Galeano F, Rossetti C, Tomaselli S,
Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco
CM, Locatelli F and Gallo A: ADAR2-editing activity inhibits
glioblastoma growth through the modulation of the
CDC14B/Skp2/p21/p27 axis. Oncogene. 32:998–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wei J, Li ZH, Du C, Qi B, Zhao X, Wang L,
Bi L, Wang G, Zhang X, Su X, et al: Abnormal expression of an ADAR2
alternative splicing variant in gliomas downregulates
adenosine-to-inosine RNA editing. Acta Neurochir (Wien).
156:1135–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hideyama T and Kwak S: When Does ALS
Start? ADAR2-GluA2 Hypothesis for the Etiology of Sporadic ALS.
Front Mol Neurosci. 4:332011. View Article : Google Scholar : PubMed/NCBI
|