Introduction
Leukemia is a malignant clonal disorder of the
hematopoietic system with high heterogeneity and poor prognosis. In
recent years, although the incidence of leukemia has increased
gradually (1), the exact oncogenic
mechanisms underlying leukemia have not been fully characterized.
In leukemia, cancer cells with multidrug resistance (MDR) to
chemotherapeutic drugs significantly reduce the efficacy of cancer
chemotherapy (2). Various mechanisms
are involved in the MDR of cancer, including the induction of the
anti-apoptotic machinery, an increase in intracellular drug efflux
and a reduction in drug uptake (3).
Overexpression of ATP-binding cassette (ABC) transporters,
particularly ABCB1, ABCC1 and ABCG2, are one of most common reasons
for the development of MDR in cancer cells (4–7).
Long non-coding RNAs (lncRNAs) are defined as
transcripts containing >200 nucleotides that are typically
transcribed by RNA polymerase II (8).
Although the existence of lncRNAs has been known for several
decades, the multiple functions of the lncRNA components of the
noncoding genome have only been determined in the last 10 years.
lncRNAs have important roles in maintaining cellular homeostasis
during cell or tissue development, and are also critical factors in
the pathophysiology of cancer (8–13). The
molecular mechanisms of action of lncRNAs are highly variable; they
function as molecular scaffolds for stabilizing protein-protein and
protein-DNA interactions, and also act as decoys and guides that
facilitate proximal and distal macromolecular interactions that
typically occur on a genome template (9,10).
Nuclear paraspeckle assembly transcript 1 (NEAT1), a
structural scaffold for the formation of paraspeckles, has been
identified as a nuclear-restricted lncRNA and is known to exist as
two isoforms: 3.7 kb NEAT1_1 (MENε) and 23 kb NEAT1_2 (MENβ)
(14,15). NEAT1_1 is generally polyadenylated,
while NEAT1_2 lacks a polyA tail (14). This lncRNA is an architectural
component of a subnuclear structure termed the paraspeckle, which
is suggested to be involved in the regulation of gene expression by
retaining messenger RNAs (mRNAs) for nuclear editing (15,16).
However, the expression and function of NEAT1 in leukemia is still
unclear.
The aim of the present study was to characterize the
involvement of NEAT1 in MDR associated with leukemia. NEAT1 was
highly deregulated in samples from patients with leukemia compared
with those from healthy donors, indicating a tumor suppressor
function of NEAT1 in leukemia. Further evidence indicated that
NEAT1 expression was repressed in leukemia cell lines, K562, THP-1,
HL-60 and Jurkat. The transfection of a NEAT1 overexpression
plasmid into K562 and THP-1 leukemia cells reduced MDR conferred by
cytotoxic drugs, such as Alisertib and Bortezomib, which further
enhanced the sensitivity of these cell lines to cytotoxic cell
death.
Materials and methods
Evolutionary analysis
A previous study by Zeng et al reported that
NEAT1 lncRNA is comprised of two isoforms, NEAT1_1 and NEAT1_2
(17). Therefore, the present study
compared conservative sequences of the two isoforms using rVista
2.0 (http://rvista.dcode.org/) by performing
evolutionary analysis of transcription factor binding sites.
Patient samples
A total of 36 patients (17 males and 19 females;
median age, 53.1 years) with leukemia and 15 healthy donors (5
males and 11 females; median age, 26.0 years) from Weihai Maternal
and Child Health Hospital (Weihai, China) were enrolled in the
present study during February 2012 to December 2014. Written
informed consent was provided by all patients, and the study
protocol was approved by the Institutional Research Ethics Board of
Weihai Maternal and Child Health Hospital. Inclusion criteria for
patients were age 20–70 years, primary leukemia without other
diseases and familial inherited diseases. The diagnosis of leukemia
was determined by a combination of clinical, morphological,
laboratory and immunophenotypic criteria, as defined by the World
Health Organization classification (18). Peripheral white blood cell (PWBC)
samples from 5 ml whole blood of the patients or healthy donors
were isolated with the Ficoll-Paque method, and then used for RNA
extraction and subsequent analysis of NEAT1_1 and NEAT1_2, or
stored at −80°C.
Cell culture
K562 chronic myelogenous leukemia cells, THP-1 acute
monocytic leukemia cells, HL-60 primary myeloid leukemia cells and
Jurkat T lymphocytic leukemia cells were purchased from Jinan
CN-Cell Biotechnology, Co, Ltd. (Jinan, China). All cell lines were
cultured in RPMI-1640 complete medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.) at 37°C, 5% CO2
and saturated humidity. Human PWBCs isolated from the healthy
donors were cultured in RMPI-1640 complete medium and served as the
negative control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the blood samples and
cell lines using a TRIzol total RNA extraction kit purchased from
Tiangen Biotech Co., Ltd. (Beijing, China), according to the
manufacturer's protocol. RNA was eluted with 100 µl RNase-free
water and stored at −80°C. RT-qPCR was conducted using an iTaq
Universal SYBR Green One-Step kit (BioRad Laboratories, Inc.,
Hercules, CA, USA) in a StepOnePlus™ Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The cycling conditions
for the synthesis of complementary DNA were 25°C for 15 min, 42°C
for 60 min and 80°C for 5 min, while the qPCR cycling conditions
were 50°C (2 min), 95°C (10 min), and 40 cycles of 95°C (15 sec)
and 60°C (1 min). The primers used were as follows: Forward:
5′-AATTCTGTTACGTCATGT-3′ and reverse,
5′-TTTCTAATGAGTTTAGAACTCAAAC-3′ for NEAT1_1; forward,
5′-CCTATCCGTTGGTTTGTG-3′ and reverse, 5′-GAGGGTTGGGAACTTGTC-3′ for
NEAT1_2; and forward, 5′-CTGGGCTACACTGAGCACC-3′ and reverse,
5′-AAGTGGTCGTTGAGGGCAATG-3′ for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH). Housekeeping GAPDH mRNA was used as the
control for comparing relative expression of RNAs. mRNA expression
was quantified using the quantification cycle method (19).
Western blotting
Cells were washed twice in phosphate-buffered saline
and lysed on ice for 30 min in radioimmunoprecipitation assay
buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). Protein
extracts were separated by 8% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis. Proteins were then transferred to a
polyvinylidenedifluoride membrane, and probed with mouse anti-human
ABCG2 monoclonal antibody (dilution, 1:500; cat no. sc-58222; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) and a goat anti-mouse
polyclonal antibody against β-actin (dilution, 1:2,000; cat no.
sc-1616; Santa Cruz Biotechnology). Western blot signals were
visualized using a SuperSignal West Pico Chemiluminescent Substrate
kit (Pierce; Thermo Fisher Scientific, Inc.).
Transfection
A NEAT1 overexpression plasmid, pcDNA3.1-NEAT1, was
commercially constructed by Genechem Co., Ltd. (Shanghai, China),
and empty pcDNA3.1 vector (which was a gift from Dr Tao Li,
Department of Biology, Zhejiang Normal University, Jinhua, China)
was used as the control. To establish cell lines with transient
overexpression of NEAT1, K562 and THP-1 cells (1×107/ml)
were transfected with 10 µg pcDNA3.1-NEAT1 plasmid or control
pcDNA3.1 vector in a 0.2 cm cuvette (BTX Instrument Division;
Harvard Apparatus, Inc., Holliston, MA, USA) using a ECM 830
Electroporator (BTX Instrument Division; Harvard Apparatus, Inc.)
at 130 V for 20 msec.
Half maximal inhibitory concentration
(IC50) detection
Apoptosis-inducing agents Alisertib (Celgene
Corporation, Summit, NJ, USA) and Bortezomib (Shanghai Haoran
Biotechnology, Co, Ltd., Shanghai, China) were utilized to
determine the percentage of apoptotic cells using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, K562 and THP-1 cell lines were collected in the
logarithmic phase of growth and stained with trypan blue to
determine cell viability using an inverted phase contrast
microscope (CKX41; Olympus Corporation, Tokyo, Japan). Thereafter,
the cell concentration was adjusted to 1×105 cells/ml.
Each well of the 96-well culture plate was seeded with 100 µl cells
or with no cells (control groups). Following transfection with
NEAT1 or control vectors, cells were cultured for 12 h with
Alisertib (0.1 µg/ml) or Bortezomib (0.1 µg/ml). MTT reagent (5
mg/ml, 10 µl/well) was added 4 h before termination of culture.
Following incubation, cells were centrifuged at 153 × g for 5 min
at 4°C. The supernatant in each well was then removed, and the
crystals were solubilized with 150 µl dimethyl sulfoxide. Plates
were shaken for 1 min and optical density (OD) was measured at 490
nm using a standard plate reader. The inhibition rate of cell
proliferation was calculated as follows: Inhibition rate (%) = (1 -
OD of the test / OD of the control) × 100%. The IC50 was calculated
by mid-efficacy analysis in K562 and THP-1 cells using the LOGIT
method.
Flow cytometry assay
An Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining kit (BD Biosciences,
San Jose, CA, USA) was used to detect apoptosis, according to the
manufacturer's instructions. In brief, cells were transfected with
different plasmids and treated with Alisertib or Bortezomib for 48
h. Subsequently, the cells were collected via centrifugation at 153
× g for 10 min at 4 °C, stained with Annexin V-FITC/PI for 15 min
at room temperature and subjected to flow cytometric analysis (BD
LSRFortessa flow cytometer; BD Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS
version 11.0 for Windows (SPSS, Inc., Chicago, IL, USA). Mean
differences between groups were compared with Student's t-test,
while comparison of the different treatment groups to the control
group was analyzed with analysis of variance. Data are presented as
the mean ± standard deviation of ≥3 independent experiments, unless
otherwise stated. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of NEAT1 mRNA is suppressed
in patients with leukemia
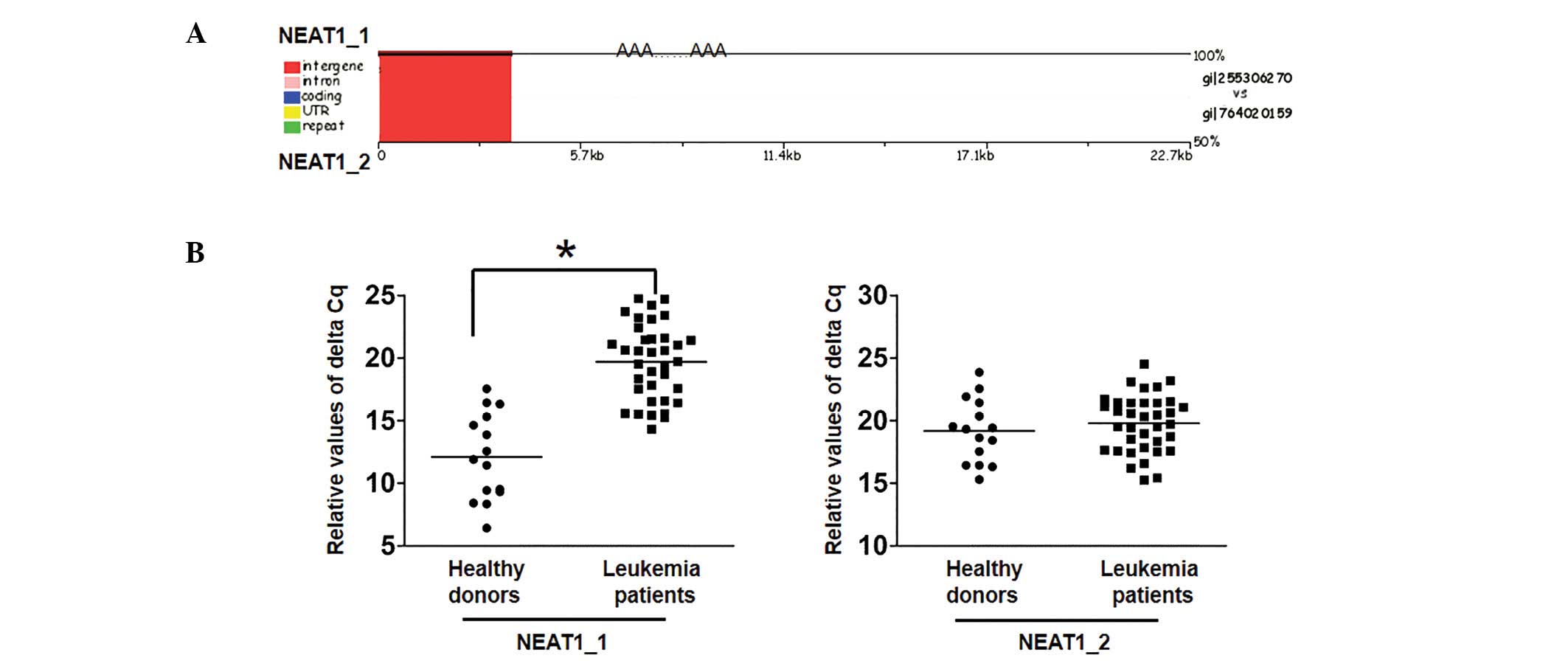
The present study compared conservative sequences of
the two isoforms by performing evolutionary analysis of
transcription factor binding sites. The sequence alignment results
demonstrated that there was a conserved sequence between the two
isoforms (Fig. 1A). Furthermore,
RT-qPCR was used to analyze the transcription level of NEAT1
isoforms in sera from patients with leukemia, and revealed that
expression of the NEAT1_1 isoform was decreased compared with
healthy donors while NEAT1_2 expression was not significantly
different between the two cohorts (P<0.05; Fig. 1B). The results suggest that NEAT1_1
may have a role as a tumor suppressor in the oncogenesis of
leukemia.
NEAT1 mRNA is downregulated in
leukemia cell lines
To further explore the detailed molecular functions
of NEAT1, its expression was determined in four different leukemia
cell lines, K562, THP-1, HL-60 and Jurkat, as well as human
peripheral white blood cells (PWBC) isolated from healthy donors
(negative control). The results showed that NEAT1 maintained
relatively high mRNA expression levels in PWBCs, but was
significantly suppressed in leukemia cells compared with the
control PWBCs (Fig. 2A).
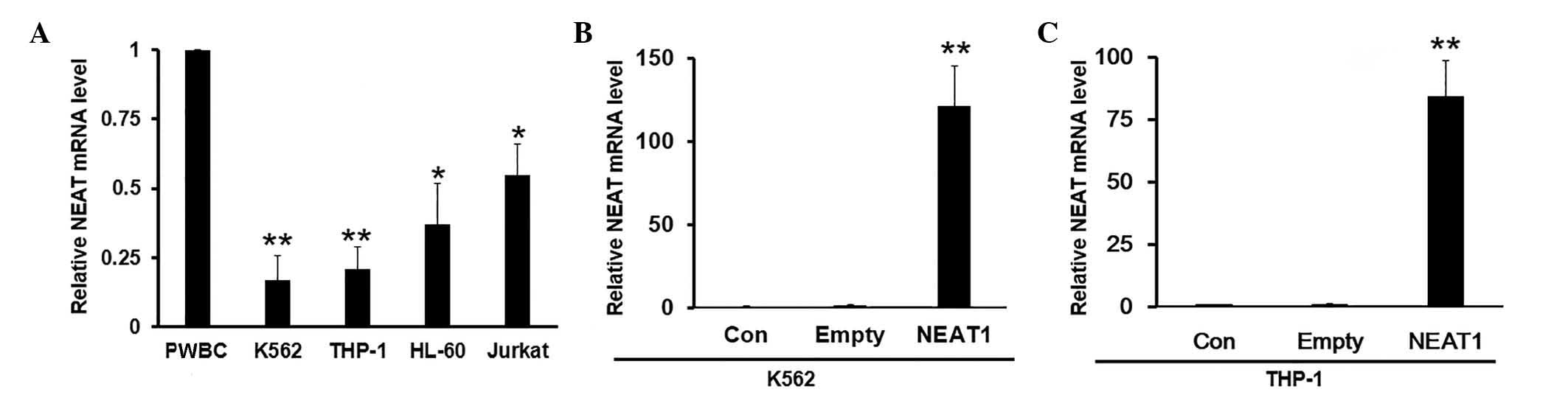 | Figure 2.NEAT1 mRNA levels are decreased in a
panel of leukemia cell lines. (A) The mRNA expression levels of
NEAT1 were detected in K562, THP-1, HL-60 and Jurkat cells by
reverse transcription-polymerase chain reaction (RT-qPCR). PWBC
cells from healthy donors were used as the control. Values were
normalized to GAPDH. (B and C) Representative experimental data
showing the transfection efficiency of empty vector or NEAT1
overexpression plasmid in (B) K562 and (C) THP-1 cells, as verified
by RT-qPCR. Data are presented as mean ± standard deviation from
three independent experiments. *P<0.05, **P<0.01 for K562,
THP-1, HL-60 and Jurkat cell lines compared with PWBC control, or
NEAT1 compared with empty vector. PWBC, peripheral white blood
cells; Con, control; NEAT1, nuclear paraspeckle assembly transcript
1. |
Overexpression of NEAT1 overcomes MDR
and enhances drug sensitivity
In order to validate whether the tumor suppressor
function of NEAT1 is repressed during the transformation stages,
overexpression of NEAT1 was induced by transfection with
full-length NEAT1 plasmid. The efficiency of overexpression was
validated by RT-qPCR. Notably, NEAT1 mRNA was upregulated at least
100-fold compared with cells transfected with empty vector
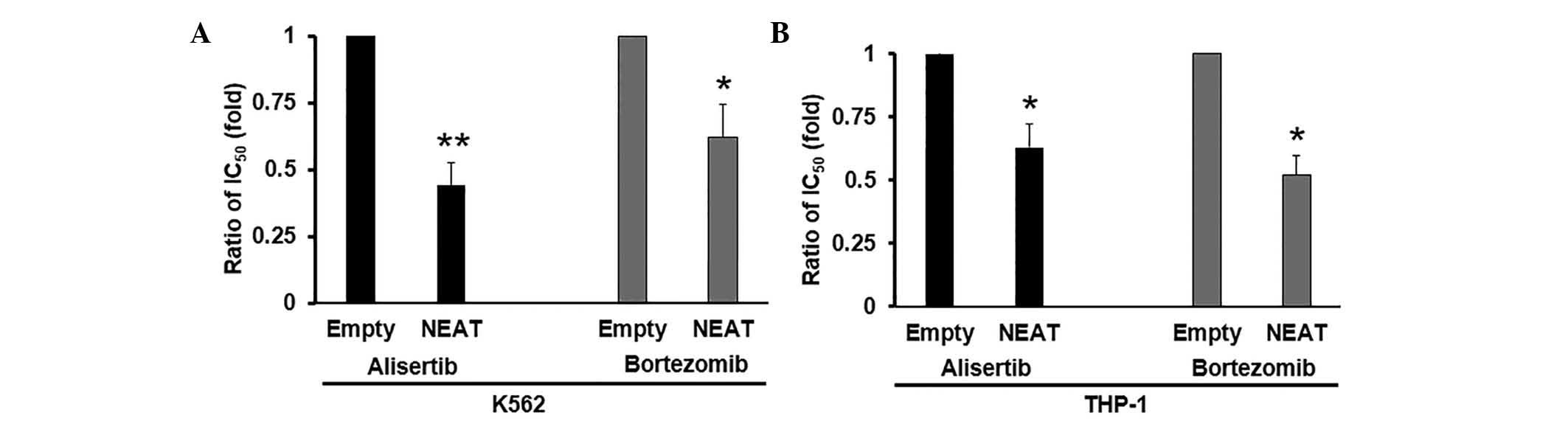
(Fig. 2B and C). Next, in order to
investigate the effects of NEAT1 on MDR in leukemia cells,
cytotoxicity assays were performed with Alisertib and Bortezomib in
K562 and THP-1 cells. The mean IC50 values are shown in Table I. Compared with K562 and THP-1 cells
transfected with empty vector, cells overexpressed with NEAT1
exhibited significantly higher sensitivity to Alisertib and
Bortezomib. NEAT1 overexpression decreased the IC50 values of
Alisertib and Bortezomib in K562 (P<0.01 and P<0.05,
respectively; Fig. 3A) and THP-1
(P<0.05; Fig. 3B) cells. Taken
together, the current results suggest that NEAT1 overexpression
significantly enhances the sensitivity of anti-leukemic drugs in
culture.
 | Table I.Mean IC50 values in K562
and THP-1 cells. |
Table I.
Mean IC50 values in K562
and THP-1 cells.
|
|
| IC50,
µg/ml |
|---|
|
|
|
|
|---|
| Cell line | Group | Alisertib | Bortezomib |
|---|
| K562 | Empty | 0.21±0.03 | 0.45±0.06 |
|
| NEAT1 |
0.09±0.02b |
0.28±0.04a |
| THP-1 | Empty | 0.35±0.05 | 0.63±0.08 |
|
| NEAT1 |
0.22±0.04a |
0.33±0.07a |
NEAT1 overexpression inhibits ABCG2
transporter protein expression
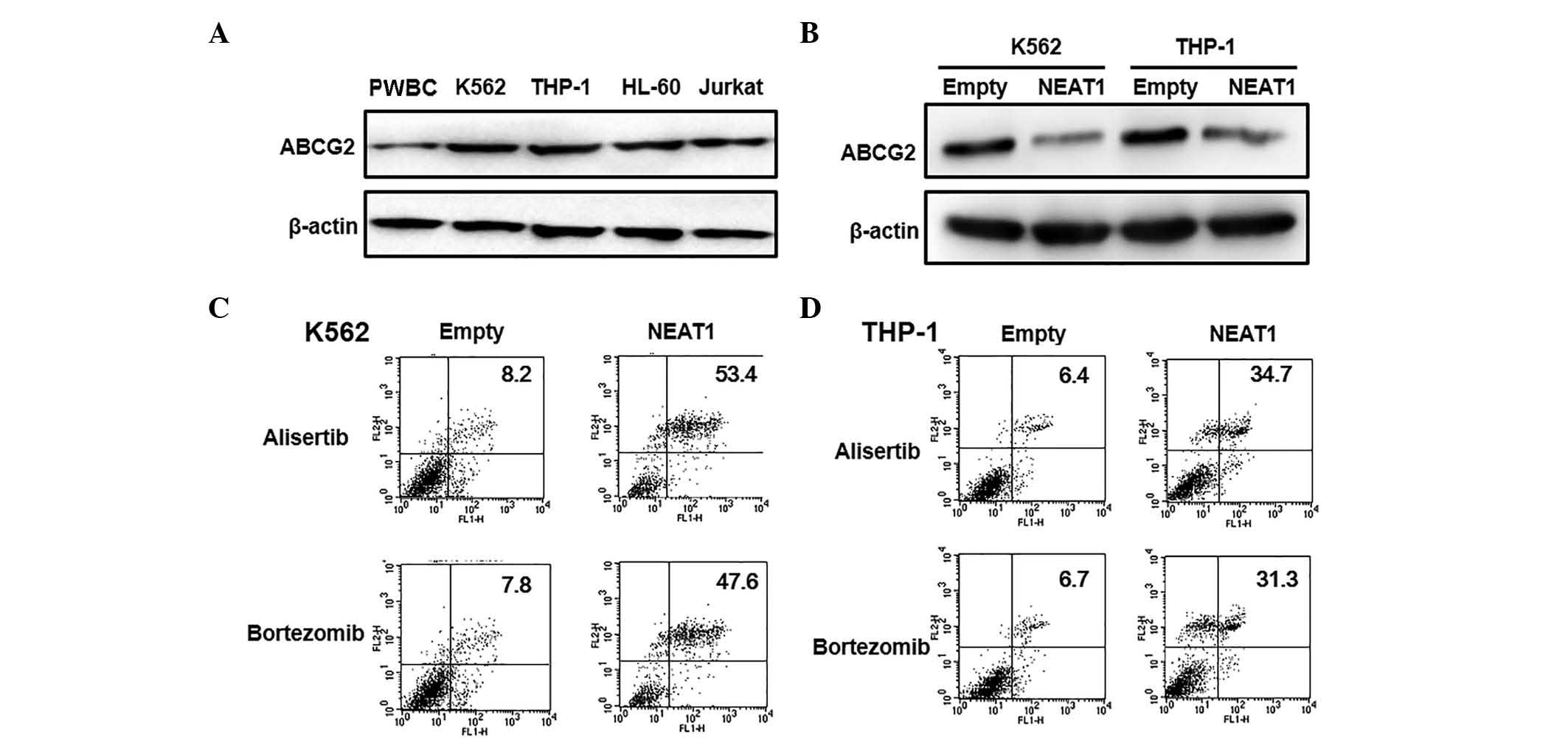
It is well known that ABCG2 has important roles in
MDR in leukemia (20,21). Therefore, to evaluate the effects of
NEAT1 on the MDR of leukemic cells, the expression level of ABCG2
was detected by western blotting. First, the ABCG2 expression level
was verified in four different types of leukemia cell and normal
PWBC cells. The expression of ABCG2 was markedly increased in
leukemia cells compared with the control cells (Fig. 4A). Furthermore, when NEAT1 was
overexpressed in K562 and THP-1 cells, the expression level of
ABCG2 was significantly decreased compared with control cells
transfected with empty vector, consistent with the changes in IC50
values observed in leukemia cells (Fig.
4B). Next, the role of NEAT1 in regulating MDR was determined
by inducing apoptosis in K562 and THP-1 cells. The results
demonstrated that low doses of Alisertib or Bortezomib did not
induce apoptosis in either cell line transfected with empty vector.
However, apoptosis induced by Alisertib (53.4 vs 8.2% for K562
cells and 47.6 vs 7.8% for THP-4 cells) and Bortezomib (34.7 vs
6.4% for K562 cells and 31.3 vs 6.7% for THP-4 cells) increased in
both cell lines following overexpression of NEAT1 (Fig. 4C and D). Taken together, these data
suggest that lncRNA NEAT1 may regulate apoptosis and MDR-associated
ABCG2 protein expression in leukemia.
Discussion
The human transcriptome is composed of a large set
of protein-coding mRNAs, as well as numerous non-coding transcripts
that have structural, regulatory or unknown functions. Over the
last decade, much attention has been focused on microRNAs (miRNAs),
a class of small non-coding RNAs that are involved in various
biological and pathological processes (22,23). More
recently, lncRNAs, generally defined as non-coding RNAs of >200
nt in length without known protein-coding function (24), have risen to prominence with central
roles in a diverse range of functions (25,26). A
small number of studies suggest that lncRNAs derived from multiple
tumors are overexpressed or underexpressed, influencing cancer
growth, survival and migration or invasion (26–28).
Furthermore, lncRNAs have been shown to be dysregulated in various
types of cancer and several lncRNAs have been functionally
associated with cancer cell differentiation (14,29,30).
Although an increasing number of lncRNAs have been characterized in
epigenetic regulation and the prognosis of solid tumors, they have
not been explored extensively in hematological malignancies, such
as leukemia.
NEAT1 is a critical component of the paraspeckle
structure, which is formed from a single, intergenic exon with two
smaller conserved regions in mammalian lineage; however, it is not
easily identified in other vertebrates (31). NEAT1 is proposed to be involved in the
regulation of genetic expression through controlling the nuclear
retention of mRNAs containing long inverted repeats. These mRNAs
are capable of forming intramolecular double-stranded RNAs and
causing translation repression as a result of adenosine-to-inosine
editing (16,32). In the current study, the expression
and function of NEAT1 was explored in patients with leukemia, as
well as in a panel of leukemia cell lines. NEAT1 mRNA expression
was significantly decreased in sera from patients with leukemia and
in leukemia cell lines compared with the high levels observed in
normal healthy donor sera and PWBCs, respectively. Several studies
have proposed that the expression of isoforms NEAT1_1 and NEAT1_2
are equally distributed in numerous tissues, such as the prostate,
colon, pancreas and ovaries (31,33).
During the differentiation of embryonic stem cells and muscle
cells, NEAT1 lncRNAs have been found to be upregulated (14,16). A
previous study reported that estrogen receptor α-regulated NEAT1
lncRNA acted as a transcriptional regulator and promoted
tumorigenesis leading to prostate adenocarcinoma in experimental
animal models (34). Furthermore,
NEAT1 expression was associated with oncogenic activity and
metastatic progression in lung cancer (35,36).
However, downregulation of NEAT1 has been observed in the several
other malignancies, including those affecting the liver, retina and
esophagus (37). Furthermore,
anomalous NEAT1 expression has been reported in various human
malignancies, including acute promyelocytic leukemia, where NEAT1
repression was predominant in patients with leukemia caused by
promyelocytic leukemia-retinoic acid receptor α (RARα) gene
translocation (17). In addition,
time-lapse imaging experiments with NEAT1 constructs demonstrated
the integral role of NEAT1 expression and paraspeckles formation at
the NEAT1 locus (38). Despite a
small number of studies revealing the composition and organization
of the paraspeckle structure, its role in the pathogenesis of many
malignancies are still unexplored.
Understanding the mechanisms involved in
chemoresistance is crucial to developing therapies targeted at
diseases that respond poorly to chemotherapy, such as like
leukemia. Drugs, including tyrosine kinase inhibitors, are known to
cause lysosomal degradation of ABCG2 and reverse the stemness
properties of leukemic stem cells (39,40). In a
recent study, Liu et al reported that lncRNA maternally
expressed gene 3 is significantly decreased in human lung
adenocarcinoma and partially contributes to the cisplatin
resistance of lung adenocarcinoma cells (41). Zhang et al showed that lncRNA
plasmacytoma variant translocation-1 (PVT-1) was highly expressed
in gastric cancer tissues from cisplatin-resistant patients and
cisplatin-resistant gastric cancer cells; and overexpression of
PVT1 in gastric carcinoma promoted the development of MDR (42). Furthermore, Hang et al
demonstrated that high expression of lncRNA AK022798 induced by
Notch1 resulted in the formation of cisplatin-resistant SGC7901 and
BGC823 cells via regulating the expression of ABCC1 and ABCB1
(43). The current study utilized the
overexpression of NEAT1 in K562 and THP-1 cell lines to determine
the MDR function in response to two different anti-leukemic drugs.
The results suggested that NEAT1 has a crucial role in decreasing
MDR induced by Alisertib and Bortezomib, and sensitizing cells to
anti-cancer drugs. Furthermore, western blot analysis supported
that overexpression of NEAT1 could inhibit ABCG2 expression in
leukemia cells. There is no evidence in the literature currently
linking MDR and NEAT1 in leukemia. However, RARα overexpression and
associated MDR upregulation was reported to enhance differentiation
of various leukemia cell lines (44).
Taken together, these findings further highlight the importance of
NEAT1 dysregulation in hematological malignancies, including
leukemia.
In conclusion, the present study provides novel
insights into the mechanisms regulating MDR in leukemia cell lines,
and identified NEAT1 as a potential therapeutic target that could
be manipulated to mitigate the occurrence of leukemia. The results
of the current study indicate that, as NEAT1 is repressed in
leukemic patients and cell lines, overexpression of NEAT1 may be a
tangible strategy to inhibit ABCG2 and, thus, overcome
chemoresistance associated with anti-leukemic drugs.
References
|
1
|
Coombs CC, Tavakkoli M and Tallman MS:
Acute promyelocytic leukemia: Where did we start, where are we now
and the future. Blood Cancer J. 5:e3042015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fung KL and Gottesman MM: A synonymous
polymorphism in a common MDR1 (ABCB1) haplotype shapes protein
function. Biochim Biophys Acta. 1794:860–871. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CJ, Chin JE, Ueda K, Clark DP, Pastan
I, Gottesman MM and Roninson IB: Internal duplication and homology
with bacterial transport proteins in the mdr1 (P-glycoprotein) gene
from multidrug-resistant human cells. Cell. 47:381–389. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tatsuta T, Naito M, Oh-hara T, Sugawara I
and Tsuruo T: Functional involvement of P-glycoprotein in
blood-brain barrier. J Biol Chem. 267:20383–20391. 1992.PubMed/NCBI
|
|
7
|
Thiebaut F, Tsuruo T, Hamada H, Gottesman
MM, Pastan I and Willingham MC: Cellular localization of the
multidrug-resistance gene product P-glycoprotein in normal human
tissues. Proc Natl Acad Sci USA. 84:7735–7738. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flynn RA and Chang HY: Long noncoding RNAs
in cell-fate programming and reprogramming. Cell Stem Cell.
14:752–761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rinn JL and Chang HY: Genome regulation by
long noncoding RNAs. Annu Rev Biochem. 81:145–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huarte M and Rinn JL: Large non-coding
RNAs: Missing links in cancer? Hum Mol Genet. 19:R152–R161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JT: Epigenetic regulation by long
noncoding RNAs. Science. 338:1435–1439. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Batista PJ and Chang HY: Long noncoding
RNAs: Cellular address codes in development and disease. Cell.
152:1298–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sunwoo H, Dinger ME, Wilusz JE, Amaral PP,
Mattick JS and Spector DL: MEN epsilon/beta nuclear-retained
non-coding RNAs are up-regulated upon muscle differentiation and
are essential components of paraspeckles. Genome Res. 19:347–359.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naganuma T and Hirose T: Paraspeckle
formation during the biogenesis of long non-coding RNAs. RNA Biol.
10:456–461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen LL and Carmichael GG: Altered nuclear
retention of mRNAs containing inverted repeats in human embryonic
stem cells: Functional role of a nuclear noncoding RNA. Mol Cell.
35:467–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L,
Chen S and Li Y: Inhibition of long non-coding RNA NEAT1 impairs
myeloid differentiation in acute promyelocytic leukemia cells. BMC
Cancer. 14:6932014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wadleigh M and Tefferi A: Classification
and diagnosis of myeloproliferative neoplasms according to the 2008
World Health Organization criteria. Int J Hematol. 91:174–179.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahgozar S, Moafi A, Abedi M,
Entezar-E-Ghaem M, Moshtaghian J, Ghaedi K, Esmaeili A and
Montazeri F: mRNA expression profile of multidrug-resistant genes
in acute lymphoblastic leukemia of children, a prognostic value for
ABCA3 and ABCA2. Cancer Biol Ther. 15:35–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gromicho M, Dinis J, Magalhães M,
Fernandes AR, Tavares P, Laires A, Rueff J and Rodrigues AS:
Development of imatinib and dasatinib resistance: Dynamics of
expression of drug transporters ABCB1, ABCC1, ABCG2, MVP and
SLC22A1. Leuk Lymphoma. 52:1980–1990. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saxena A and Carninci P: Long non-coding
RNA modifies chromatin: Epigenetic silencing by long non-coding
RNAs. Bioessays. 33:830–839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaikkonen MU, Lam MT and Glass CK:
Non-coding RNAs as regulators of gene expression and epigenetics.
Cardiovasc Res. 90:430–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rinn JL, Kertesz M, Wang JK, Squazzo SL,
Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E and
Chang HY: Functional demarcation of active and silent chromatin
domains in human HOX loci by noncoding RNAs. Cell. 129:1311–1323.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta RA, Shah N, Wang KC, Kim J, Horlings
HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Chen Z, Wang X, Huang Z, He Z and
Chen Y: Long non-coding RNA: A new player in cancer. J Hematol
Oncol. 6:372013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhuang Y, Wang X, Nguyen HT, Zhuo Y, Cui
X, Fewell C, Flemington EK and Shan B: Induction of long intergenic
non-coding RNA HOTAIR in lung cancer cells by type I collagen. J
Hematol Oncol. 6:352013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hutchinson JN, Ensminger AW, Clemson CM,
Lynch CR, Lawrence JB and Chess A: A screen for nuclear transcripts
identifies two linked noncoding RNAs associated with SC35 splicing
domains. BMC Genomics. 8:392007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Naganuma T, Nakagawa S, Tanigawa A, Sasaki
YF, Goshima N and Hirose T: Alternative 3′-end processing of long
noncoding RNA initiates construction of nuclear paraspeckles. EMBO
J. 31:4020–4034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sasaki YT, Ideue T, Sano M, Mituyama T and
Hirose T: MENepsilon/beta noncoding RNAs are essential for
structural integrity of nuclear paraspeckles. Proc Natl Acad Sci
USA. 106:2525–2530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chakravarty D, Sboner A, Nair SS,
Giannopoulou E, Li R, Hennig S, Mosquera JM, Pauwels J, Park K,
Kossai M, et al: The oestrogen receptor alpha-regulated lncRNA
NEAT1 is a critical modulator of prostate cancer. Nat Commun.
5:53832014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pan LJ, Zhong TF, Tang RX, Li P, Dang YW,
Huang SN and Chen G: Upregulation and clinicopathological
significance of long non-coding NEAT1 RNA in NSCLC tissues. Asian
Pac J Cancer Prev. 16:2851–2855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao W, An Y, Liang Y and Xie XW: Role of
HOTAIR long noncoding RNA in metastatic progression of lung cancer.
Eur Rev Med Pharmacol Sci. 18:1930–1936. 2014.PubMed/NCBI
|
|
37
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ and Lam WL: Human cancer long non-coding RNA
transcriptomes. PloS One. 6:e259152011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bergmann JH and Spector DL: Long
non-coding RNAs: Modulators of nuclear structure and function. Curr
Opin Cell Biol. 26:10–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang F, Wang XK, Shi CJ, Zhang H, Hu YP,
Chen YF and Fu LW: Nilotinib enhances the efficacy of conventional
chemotherapeutic drugs in CD34+CD38− stem
cells and ABC transporter overexpressing leukemia cells. Molecules.
19:3356–3375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang XK, He JH, Xu JH, Ye S, Wang F, Zhang
H, Huang ZC, To KK and Fu LW: Afatinib enhances the efficacy of
conventional chemotherapeutic agents by eradicating cancer
stem-like cells. Cancer Res. 74:4431–4445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu J, Wan L, Lu K, Sun M, Pan X, Zhang P,
Lu B, Liu G and Wang Z: The long noncoding RNA MEG3 contributes to
cisplatin resistance of human lung adenocarcinoma. PloS One.
10:e01145862015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang XW, Bu P, Liu L, Zhang XZ and Li J:
Overexpression of long non-coding RNA PVT1 in gastric cancer cells
promotes the development of multidrug resistance. Biochem Biophys
Res Commun. 462:227–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hang Q, Sun R, Jiang C and Li Y: Notch 1
promotes cisplatin-resistant gastric cancer formation by
upregulating lncRNA AK022798 expression. Anticancer Drugs.
26:632–640. 2015.PubMed/NCBI
|
|
44
|
Stromskaya TP, Rybalkina EY, Zabotina TN,
Shishkin AA and Stavrovskaya AA: Influence of RARalpha gene on MDR1
expression and P-glycoprotein function in human leukemic cells.
Cancer Cell Int. 5:152005. View Article : Google Scholar : PubMed/NCBI
|