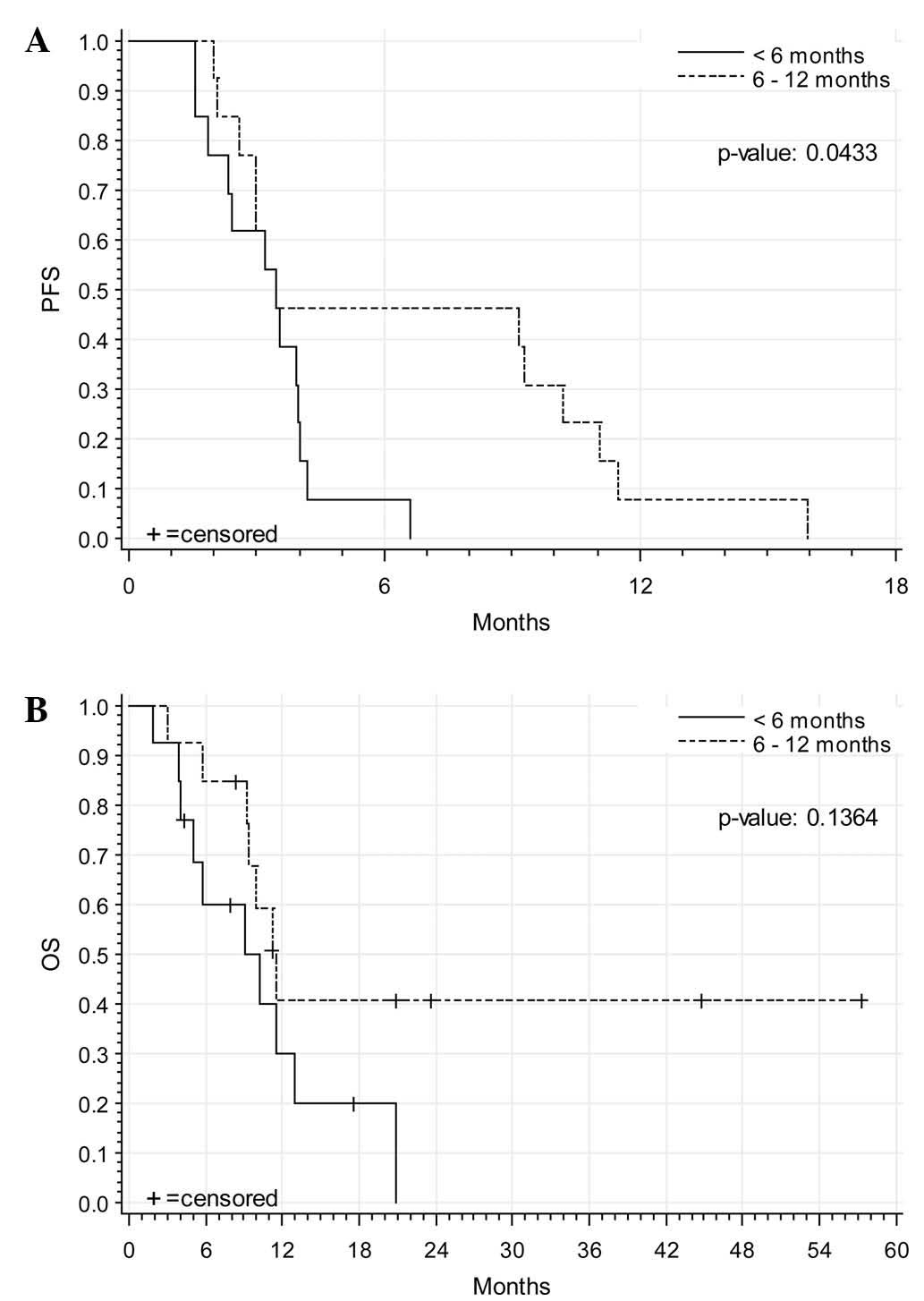
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennessy BT, Colemann RL and Markmann M:
Ovarian Cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong DK: Relapsed ovarian cancer:
Challenges and management strategies for a chronic disease.
Oncologist. 7(Suppl 5): 20–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piccart MJ, Du Bois A, Gore ME, Neijt JP,
Pecorelli S and Pujade-Lauraine E: A new standard of care for
treatment of ovarian cancer. Eur J Cancer. 36:10–12. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bookman MA: Standard treatment in advanced
ovarian cancer in 2015 The state of the art. Int J Gynecol Cancer.
15:(Suppl 3). S212–S220. 2005. View Article : Google Scholar
|
|
6
|
du Bois A, Herrstedt J, Hardy-Bessard AC,
Müller HH, Harter P, Kristensen G, Joly F, Huober J,
Avall-Lundqvist E, Weber B, et al: Phase III trial of carboplatin
plus paclitaxel with or without gemcitabine in first-line treatment
of epithelial ovarian cancer. J Clin Oncol. 28:4162–4169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naumann RW and Coleman RL: Management
strategies for recurrent platinum-resistant ovarian cancer. Drugs.
71:1397–1412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pfisterer J and Ledermann JA: Management
of platinum-sensitive recurrent ovarian cancer. Semin Oncol. 33(2
Suppl 6): S12–S16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cormio G, Loizzi V, Gissi F, Camporeale A,
De Mitri P, Leone L, Putignano G and Selvaggi L: Long-term
topotecan therapy in recurrent or persistent ovarian cancer. Eur J
Gynaecol Oncol. 32:153–155. 2011.PubMed/NCBI
|
|
10
|
Markman M: Pegylated liposomal
doxorubicin: Appraisal of its current role in the management of
epithelial ovarian cancer. Cancer Manag Res. 3:219–225. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gordon AN, Granai CO, Rose PG, Hainsworth
J, Lopez A, Weissman C, Rosales R and Sharpington T: Phase II study
of liposomal doxorubicin in platinum-and paclitaxel-refractory
epithelial ovarian cancer. J Clin Oncol. 18:3093–3100.
2000.PubMed/NCBI
|
|
12
|
Muggia FM, Hainsworth JD, Jeffers S,
Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia
A, et al: Phase II study of liposomal doxorubicin in refractory
ovarian cancer: Antitumor activity ad toxicity modification by
liposomal encapsulation. J Clin Oncol. 15:987–993. 1997.PubMed/NCBI
|
|
13
|
Israel VP, Garcia AA, Roman L, Muderspach
L, Burnett A, Jeffers S and Muggia FM: Phase II study of liposomal
doxorubicin in advanced gynecological cancers. Gynecol Oncol.
78:143–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Campos SM, Penson RT, Mays AR, Berkowitz
RS, Fuller AF, Goodman A, Matulonis UA, Muzikansky A and Seiden MV:
The clinical utility of liposomal doxorubicin in recurrent ovarian
cancer. Gynecol Oncol. 81:206–212. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Markman M, Kennedy A, Webster K, Peterson
G, Kulp B and Belinson J: Phase 2 trial of liposomal doxorubicin
(40 mg/m(2)) in platinum/paclitaxel-refractory ovarian and
fallopian tube cancers and primary carcinoma of the peritoneum.
Gynecol Oncol. 78:369–372. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernardi D, Errante D, Stefani M and
Salvagno L: Non-pegylated liposomal doxorubicin in metastatic
breast cancer patients: A valuable therapeutic option requiring
caution. Breast. 19:549–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lorusso D, Di Stefano A, Carone V, Fagotti
A, Pisconti S and Scambia G: Pegylated liposomal
doxorubicin-related palmar-plantar erythrodysesthesia (‘hand-foot’
syndrome). Ann Oncol. 18:1159–1164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miolo G, Baldo P, Bidoli E, Lombardi D,
Scalone S, Sorio R and Veronesi A: Incidence of palmar-plantar
erythrodysesthesia in pretreated and unpretreated patients
receiving pegylated liposomal doxorubicin. Tumori. 95:687–690.
2009.PubMed/NCBI
|
|
19
|
Meier W, Römisch M and Hepp H: The value
of reoperation in the treatment of ovarian cancer. Geburtshilfe
Frauenheilkd. 53:30–34. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
World Health Organization: WHO handbook
for reporting results of cancer treatment. WHO offset publication.
No. 48. World Health Organization; Geneva, Switzerland: 1979
|
|
21
|
Cancer Therapy Evaluation Program. Common
toxicity criteria for adverse events. Version 3.0. National Cancer
Institute, National Institutes of Health; Bethseda, MD: 2006
|
|
22
|
Food and Drug Administration:
International Conference on Harmonisation. Good Clinical Practice
Consolidated Guideline. 1997.https://www.gpo.gov/fdsys/pkg/FR-1997-05-09/pdf/97-12138.pdfAccessed.
May 25–2016
|
|
23
|
Heintz AP, Odicino F, Maisonneuve P,
Beller U, Benedet JL, Creasman WT, Ngan HY, Sideri M and Pecorelli
S: Carcinoma of the ovary. J Epidemiol Biostat. 6:107–138.
2001.PubMed/NCBI
|
|
24
|
Bokemeyer C and Lipp HP: Allergic
reaction. Compendium of Oncology Standards in Diagnosis and
Therapy. Schmoll HJ, Höffken K and Possinger K: 4th.
Springer-Verlag GmbH; Heidelberg: pp. pp1947–1952. 2006, (In
German).
|