Introduction
Breast cancer accounts for 22.9% of invasive cancers
in women (1), and 16% of female
cancers in total (2). In 2012, breast
cancer accounted for 25.2% all of cancers diagnosed in women,
making it the most common female cancer (3). Over 18 breast cancer sub-types have been
described, and these are classified primarily by histological
appearance (4). Outcomes for breast
cancer vary depending on the cancer type, extent of disease and
patient age (5). Current treatment
strategies focus on surgery, in combination with radiotherapy and
chemotherapy. However, overall survival and mortality rates, in
particular for late-stage breast cancer patients, remain poor
(6). A deeper understanding of breast
cancer tumorigenesis may aid the development of more effective
treatment measures.
The rapid development of gene therapy for numerous
cancer types has proceeded in recent years. Much progress has been
made towards developing targets within the
phosphatidylinositol-3-kinase/protein kinase B (7) and RAS/mitogen-activated protein kinase
kinase/extracellular signal-regulated kinase (8) pathways. Additionally, the discovery of
the breast cancer stem cell offers an opportunity to potentially
cure breast cancer (9–12). The existence of the breast cancer stem
cell requires that the mechanisms underlying breast cancer
pathogenesis be reconsidered, and doing so may generate novel
therapeutic options for breast cancer (13).
Cancer stem cells are a small group of tumor cells
with the capacity to proliferate continuously and also
differentiate (14). These cells are
predominantly in the G0 or resting phase of the cell cycle, and can
be activated to proliferate only by external stimulation under
optimum conditions (15). The
majority of current antitumor drugs target proliferating tumor
cells (16). Therefore, these drugs
do not kill cancer stem cells (17).
Accurately limiting the growth of cancer stem cells in a specific
state, including the quiescent, proliferative or differential
states, is challenging. This makes the detection of cell
characteristics and development of potential intervention
treatments difficult, particularly for the quiescent cells.
Breast cancer stem cells were cultured using soft or
hard agar as the matrix surface for cell contact in the media.
Cells were cultured with or without stem cell growth factors based
on the adherence features of the growing cells and the growth
maintenance principle of the tumor stem cells (18). Growth indicators of breast cancer stem
cells were monitored, including the growth rate of cancer cell
clone spheres, the cell cycle, levels of proliferating cell nuclear
antigens and telomerase activity. Breast cancer stem cells were
successfully limited in either the quiescent, proliferative or
differential states, providing valuable references to aid future
study of the cell cycle in cancer stem cells.
Materials and methods
Cell culture conditions
The breast cancer MDA-MB-231, MDA-MB-435, and MCF-7
cell lines were obtained from American Type Culture Collection
(Manassas, VA, USA). Cells were cultured in complete medium
consisting of Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), containing high glucose
and pyruvate and supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.). Cells were maintained
at 37°C in a humidified 5% CO2 atmosphere.
Separation of cluster of
differentiation (CD)133-positive breast stem cells
Cells (1×108) were suspended in 0.5 ml
PBE incubation solution, consisting of phosphate-buffered saline
(PBS), 0.5% bovine serum albumin (Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) and 0.08%
ethylenediaminetetraacetic acid (pH 7.2), then incubated with a
rabbit anti-human CD133 polyclonal antibody (20 µg/ml; dilution,
1:2,000; catalog no. NB120-16518; Novus Biologicals LLC, Littleton,
CO, USA) at 4°C for 30 min. Antibody-coated superfine magnetic
beads (Miltenyi Biotec, Bergisch Gladbach, Germany) were then
added, followed by incubation at 10°C for 15 min. The cell
suspension was then added to the separation column and naturally
eluted. The column was rinsed twice with PBS and separated from the
magnetic field, inserted into a fresh tube, and 1–2 ml PBE was
administered along the needle core to remove the CD133-positive
cells. These cells were cultured in neurobasal medium (Invitrogen;
Thermo Fisher Scientific, Inc.) containing 1X B27 (Invitrogen;
Thermo Fisher Scientific, Inc.), 2 mM L-glutamine, 30 units/ml
penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA), 20
ng/ml basic fibroblast growth factor (bFGF; Miltenyi Biotec), and
20 ng/ml epidermal growth factor (EGF; Provitro Biosciences, Mt.
Vernon, WA, USA).
Preparation of agarose gel matrix
medium
Neurobasal medium containing 1X B27 was prepared,
and 0.025 or 3 g low melting point agarose (Nanjing Sunshine
Biotechnology Co., Ltd., Jiangsu, China) was added to 10 ml medium
and mixed. The 0.05% (soft) and 30% (hard) agarose gel matrix media
were stored at room temperature until required. Prior to use, the
media were melted and then cooled to 37–39°C.
Culture of breast cancer stem cell
clones
In total, 3 experimental groups were defined: Soft
gel+EGF+bFGF; hard gel+EGF+bFGF; and hard gel+2% fetal bovine serum
(FBS) as a control. Agarose gel medium was placed in the bottom of
each well in a 48-well plate. Following a 2-h equilibration, the
stem cell maintenance factors EGF and bFGF were added to the
surface of the gel, and the stem cell maintenance factors were
replaced by 2% FBS onto the surface of the hard gel in the control
group. CD133-positive MDA-MB-231, MDA-MB-435 or MCF-7 cells with a
sphere diameter of ~25 µm were then added to wells. The average
sphere diameter was then measured at 0, 2, 4, 6 and 8 days for
analysis of sphere growth.
Telomerase activity
The telomerase activity detection kit (Roche
Diagnostics, Indianapolis, IN, USA) was used according to
manufacturer's instructions. Briefly, 100 µl cell lysate from each
sample was incubated for 1 h at 4°C and centrifuged at 12,000 × g
for 15 min. Total messenger RNA (mRNA) was obtained from the upper
aqueous phase and 5 µl cell extract was used for each polymerase
chain reaction (PCR). The PCR conditions were: Primer extension at
25°C for 30 min, telomerase inactivation at 94°C for 5 min,
amplification by 32 cycles of denaturation at 94°C for 30 sec,
annealing at 50°C for 30s, and extension at 72°C for 90 sec, and a
final extension at 72°C for 10 min. Finally, 5 µl amplification
product was mixed with working liquid and substrate following
hybridization. The distribution of reaction product bands was
analyzed using 1% agarose gel electrophoresis and the results were
visualized under ultraviolet light.
Immunocytochemistry
Immunofluorescence staining of Oct-4 and Ki67 was
performed on cell spheres at 72 h subsequent to inoculation. Cells
in 96-well plates were fixed with 2% formalin at room temperature,
and blocked with 5% goat serum antigen (Zhongshan Golden Bridge
Biotechnology Co., Ltd.). Primary antibodies, mouse anti-human
Oct-4 monoclonal antibody (catalog no. sc-5279; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and mouse anti-human ki-67
monoclonal antibody (catalog no. H00004288-M01; OriGene
Technologies, Inc., Beijing, China), were added at a 1:1,000
dilution. Incubation was performed in a moisture box at 4°C
overnight. Texas-Red (catalog no. sc-474354) or fluorescein
isothiocyanate-labeled secondary antibody (dilution, 1:500; Santa
Cruz Biotechnology, Inc.) was then added, and
4′,6-diamidino-2-phenylindole was used to stain cell nuclei.
Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA)
was used for image analysis.
Flow cytometry cell-cycle
analysis
Cell spheres were harvested 72 h post-inoculation
and formed into a single cell suspension. Cells were rinsed and
fixed. Following staining with propidium iodide for 30 min, the
cell-cycle status was determined by flow cytometry. Flow cytometric
analysis was performed by using a FACSCalibur Flow Cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The cell proliferation index
(PIx) was calculated using the formula: PIx =
(S + G2M) / (G0G1 + S +
G2M). At least 10,000 PI-negative events were collected
for analysis. Acquired data were analyzed using CELLQuest 3.3
software (BD Biosciences).
Western blotting
Cell spheres were cultured in the required media for
72 h. Protein lysates (15 µg) were fractionated in 4–20% sodium
dodecyl sulfate polyacrylamide gel electrophoresis gels,
transferred to nitrocellulose membranes and blocked with 5% skimmed
milk and 0.1% Tris-buffered saline with Tween 20 (TBST). Membranes
were then incubated with primary antibodies, rabbit anti-human
cyclin E1 polyclonal antibody (dilution, 1:2,000; catalog no.
sc-198; Santa Cruz Biotechnology, Inc.), rabbit anti-human cyclin
D1 polyclonal antibody (dilution, 1:1,000; catalog no. sc-718;
Santa Cruz Biotechnology, Inc.) and rabbit anti-human cyclin B1
polyclonal antibody (dilution, 1:2,000; catalog no. sc-752; Santa
Cruz Biotechnology, Inc.). Next, membranes were washed five times
in 0.1% TBST and incubated for 1 h, followed by incubation with
secondary chicken anti-rabbit immunoglobulin (Ig)G horeradish
peroxidase (HRP) (dilution, 1:2,000; catalog no. sc-516087; Santa
Cruz Biotechnology, Inc.) or chicken anti-goat IgG-HRP (dilution,
1:2,000; catalog no. sc-516086; Santa Cruz Biotechnology, Inc.).
The specific protein was detected using a Super Signal protein
detection kit (Pierce; Thermo Fisher Scientific, Inc.). The
membrane was then stripped and re-probed with a goat anti-human
β-actin polyclonal primary antibody (dilution, 1:1,000; catalo no.
sc-1616; Santa Cruz Biotechnology, Inc.).
Statistical analysis
Data are expressed as the mean ± standard error.
Statistical analysis was performed using analysis of variance,
χ2 test or Student's t-test on the SPSS 11.0
software (SPSS, Inc., Chicago, IL, USA). Statistically significant
differences were indicated by P<0.05 or P<0.01.
Results
Culture of CD133-positive breast
cancer stem cell clone spheres
CD133-negative cells were separated by flow
cytometry. These cells exhibited no significant change after
culture for two weeks and could not form spheres until four weeks
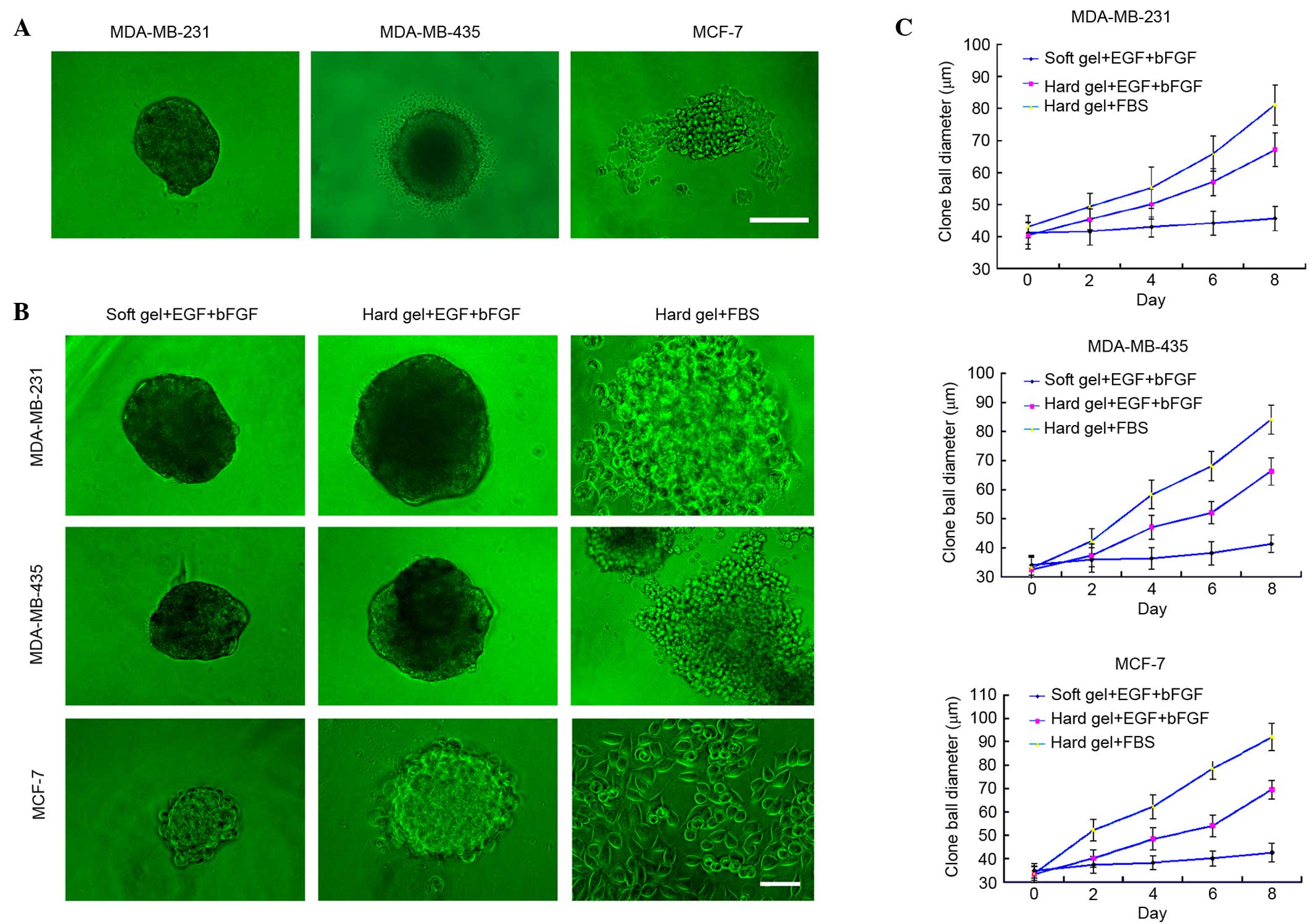
(data not shown). CD133-positive breast cancer cells formed evident
spheres and gradually grew larger (Fig.
1A). However, not all CD133-positive breast cancer cells grew
into typical clone spheres. MDA-MB-231 cells formed round spheres,
whereas MDA-MB-435 cells had distinct lace-like extensions
surrounding the spheres. MCF-7 cells did not form readily
identifiable spheres (Fig. 1A).
Analysis of breast cancer stem cell
sphere growth curves in a limited culture environment
The aforementioned clone spheres were transferred to
limited culture medium, in which they exhibited distinct cellular
morphologies and growth velocities (Fig.
1B and C). When cultured on a soft agar surface supplemented
with EGF and bFGF, breast cancer stem cells from all 3 cell lines
formed typical spheres, which increased in diameter with incubation
time. The diameter of the breast cancer stem cell spheres cultured
on hard agar with EGF and bFGF increased relatively more rapidly
from 2 days post-inoculation. Additionally, spheres cultured on the
hard gel surface with FBS rapidly spread out and expanded, but lost
their 3-dimensional structure.
Breast cancer stem cell spheres
proliferate in limited culture conditions
The MDS-MB-231 cell line was selected for further
follow-up experiments based on the formation of round spheres.
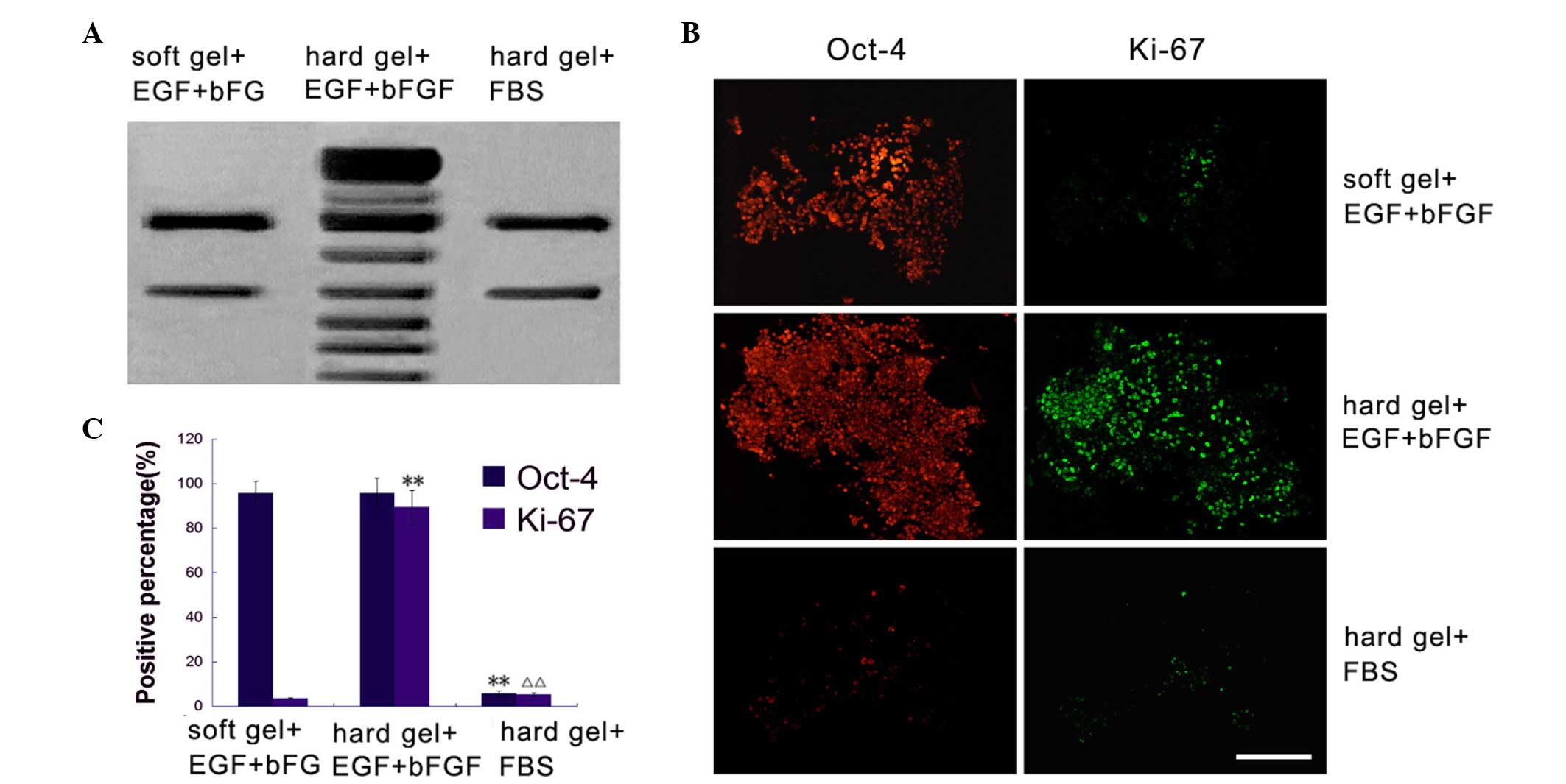
Telomerase activity can indirectly indicate cell proliferation
(19). Spheres cultured on a soft
agar surface supplemented with EGF and bFGF or a hard agar surface
with 2% FBS possessed minimal telomerase activity (Fig. 2A). However, spheres cultured on hard
agar with EGF and bFGF possessed relatively more telomerase
activity (Fig. 2A).
When cultured on a soft agar medium with EGF and
bFGF, breast cancer stem cell spheres exhibited low proliferative
activity. The Ki67-positive expression rate was 3.67±0.24%, and the
majority of cells maintained Oct-4 expression (95.79±5.31%). When
cultured on hard agar with EGF and bFGF, breast cancer stem cells
exhibited a relatively high proliferative activity, with a
Ki67-positive expression rate of 89.39±7.45% (F=0.013 vs. soft gel
+ EGF + bFGF). Only a small proportion of cells were maintained in
the stemness state, with an Oct-4-positive rate of 95.71±6.85%
(F=0.455 vs. soft gel + EGF + bFGF). When cultured on hard agar
plus 2% FBS, breast cancer stem cells exhibited similarly low
proliferative activity, with a Ki67-positive expression rate of
5.29±0.69% (F=0.034 vs. soft gel + EGF + bFGF; and F=0.019 vs. hard
gel + EGF + bFGF). Oct-4 was expressed in only 5.77±1.24% of cells
(F=0.014 vs. soft gel + EGF + bFGF; and F=0.017 vs. hard gel + EGF
+ bFGF) (Fig. 2B and C).
Breast cancer stem cells are held in a
specific phase of the cell cycle when cultured in limiting
conditions
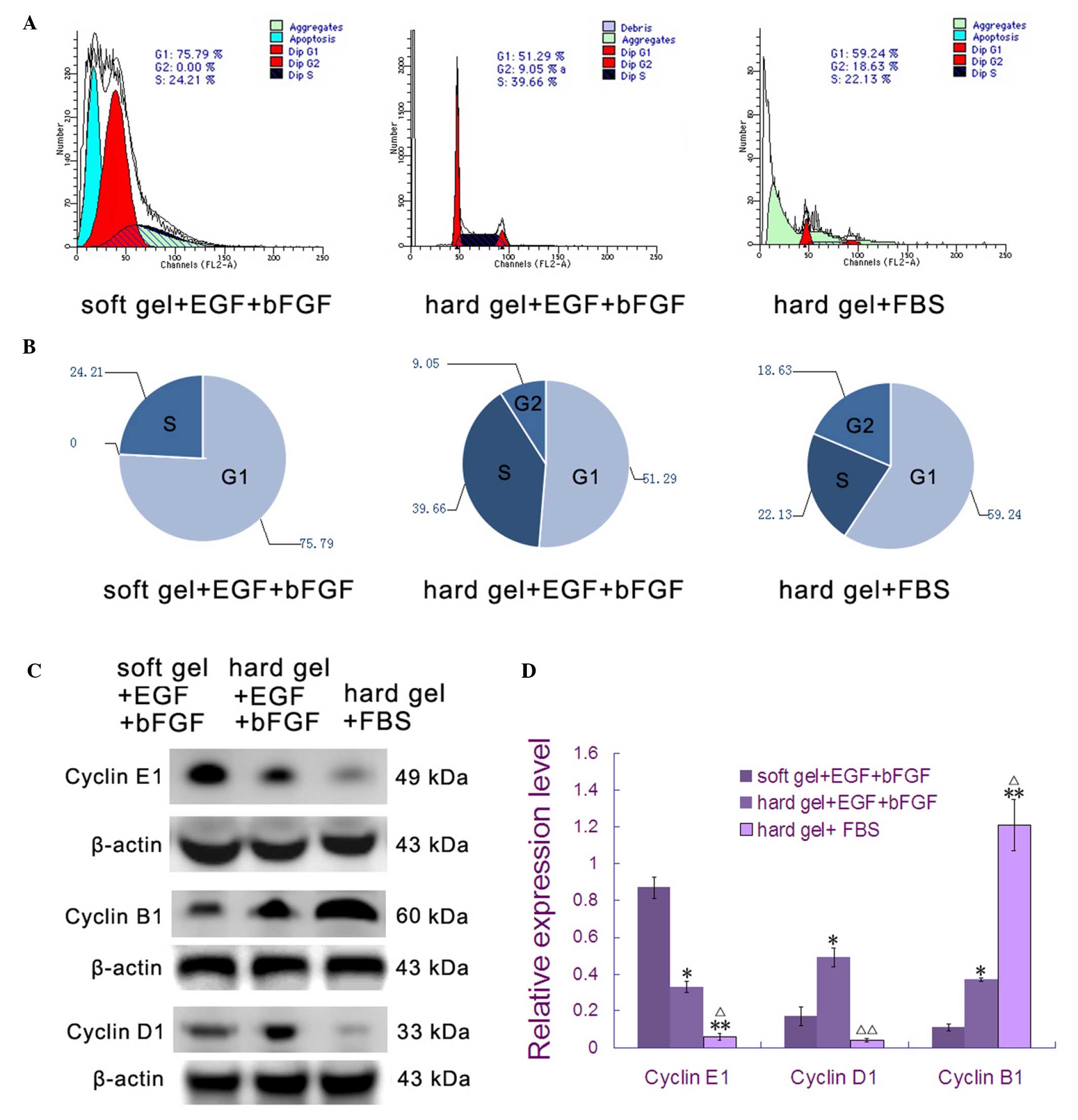
Flow cytometry revealed that when cultured in soft
agar medium with EGF and bFGF, the majority of breast cancer stem
cells (75.79%) were in a non-proliferative phase (G0 or G1;
Fig. 3A and B). When cultured in hard
agar medium with EGF and bFGF, 48.71% (F=0.031 vs. soft gel + EGF +
bFGF) of the breast cancer stem cells were in proliferative states
(S or G2/M; Fig. 3A and B). While
22.13% (F=0.037 vs. soft gel + EGF + bFGF; and F=0.029 vs. hard gel
+ EGF + bFGF) of breast cancer stem cells were in the S phase when
cultured on the hard agar surface with 2% FBS, there were more
cells in the G2/M phase (18.63%; F=0.035 vs. soft gel + EGF + bFGF;
and F=0.027 vs. hard gel + EGF + bFGF) compared with either of the
two other groups.
Western blotting was used to better identify the
cell cycle phase distribution. Proteins identified were: Cyclin E1,
which accumulates at the G1-S phase boundary and is degraded as
cells progress through S phase (20);
cyclin D1, which is synthesized rapidly, accumulates in the nucleus
during the G1 phase and is degraded as the cell enters S phase
(21); and cyclin B1, which is
expressed predominantly during the G2/M phase (22). Cyclin E1 expression was at the lowest
level when MDS-MB-231 stem cells were cultured in soft agar medium
with EGF and bFGF (Fig. 3C and D).
However, the expression peak of cyclin D1 appeared when cells were
cultured in hard agar medium with EGF and bFGF. Peak cyclin B1
protein levels were present when cells were cultured on the hard
agar surface with 2% FBS (Fig. 3C and
D). These results are consistent with those from the flow
cytometry analysis.
Discussion
Previous studies show that multiple tumor types
possess a small population of stem cells that retain the capacity
for self-renewal and can differentiate into multiple cell types
(9,11). These cells also possess the properties
of unlimited proliferation, low differentiation, high invasion and
immune evasion (9,10,13,14).
Additionally, cancer stem cells can escape death mediated by
conventional radiation and chemotherapy treatments, as the majority
of cells are in a dormant state (G0). These cells are a source of
recurrence following tumor resection surgery (15,17).
While numerous studies of cancer stem cells exist,
few reports have examined the limitation of cancer stem cells to
specific phases of the cell cycle, including the quiescent,
proliferative and differential states. Typically, bFGF and EGF are
added to the culture medium to maintain the ‘stemness’ properties
of cancer stem cells (23). However,
cultured stem cells are commonly in a state of rapid proliferation,
and cells in a quiescent state are challenging to observe and
detect (24). Park et al have
shown that the hardness of the cell adhesion material can affect
stem cell proliferation and differentiation (25). Additionally, stem cells exhibit slow
expansion speed, fewer stress fibers and a lower growth rate when
cultured on the surface of a soft matrix (26). Therefore, the present study attempted
to maintain stem cell growth under adherent conditions.
Breast cancer stem cells were cultured on soft and
hard agar contact surfaces using culture medium with or without
stem cell growth factors added. CD133-positive cancer stem cell
spheres were obtained from the MDA-MB-231, MDA-MB-435 and MCF-7
breast cancer cell lines. Inconsistent with the classical theory
(9), not all CD133-positive breast
cancer cells could form typical clone spheres. Only the MDA-MB-231
stem cells were found to form typical round spheres, while MCF-7
stem cells did not adopt a spherical structure. Therefore,
improvements to medium supplementation and novel methods for the
culture of cancer stem cells are required.
Breast cancer stem cells grew constantly with
incubation time only when cultured on the hard agar surface with
the addition of bFGF and EGF. Breast cancer stem cell spheres grew
slowly on the soft agar surface, even in the presence of EGF and
bFGF. While spheres cultured on the hard agar surface with 2% FBS
were slightly enlarged, it is possible that the enlarged tumor cell
spheres were as a result of a single differentiated cell of
increased size, rather than an increased number of individual tumor
stem cells. This would be consistent with a previous report
(27).
Breast cancer stem cells cultured on soft agar plus
EGF and bFGF exhibited almost no telomerase activity. These cells
were in the G1 phase and had low Ki67 expression and increased
expression of the typical tumor stem cell marker protein, Oct-4
(28). As flow cytometry cannot
distinguish G0 from G1, and G0 may be regarded as an extended G1
state (29), these breast cancer stem
cells can be assumed to be in a quiescent state. Contrastingly,
breast cancer stem cells cultured on hard agar plus EGF and bFGF
exhibited high telomerase activity. These cells were in a
proliferative state, expressing high levels of Ki67, Oct-4 and
cyclin D1. Breast cancer stem cells cultured on a hard agar surface
with 2% FBS had low telomerase activity, and few cells were in a
proliferative state. These cells had decreased expression levels of
Ki67 and Oct-4, but increased expression levels of cyclin B1,
indicating a differentiated state.
Breast cancer stem cells may be held in a state of
quiescence, proliferation or differentiation depending upon
adherent growth and the maintenance of the stem cell growth
factors. The findings of the present study enhance the basic
knowledge of cancer stem cells, particularly those in a quiescent
state.
Acknowledgements
The present study was supported by the China
National Natural Scientific Fund, Beijing, China (grant no.,
81471175) and the Tianjin Health Bureau Science and Technology
Projects, Tianjin, China (grant no., 2014KY23).
References
|
1
|
Rothschild E and Banerjee D: Subverting
subversion: A review on the breast cancer microenvironment and
therapeutic opportunities. Breast Cancer (Auckl). 9(Suppl 2):
S7–S15. 2015.
|
|
2
|
Wen KY, Fang CY and Ma GX: Breast cancer
experience and survivorship among Asian Americans: A systematic
review. J Cancer Surviv. 8:94–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta A, Shridhar K and Dhillon PK: A
review of breast cancer awareness among women in India: Cancer
literate or awareness deficit? Eur J Cancer. 51:2058–2066. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alizadeh F, Bolhassani A, Khavari A,
Bathaie SZ, Naji T and Bidgoli SA: Retinoids and their biological
effects against cancer. Int Immunopharmacol. 18:43–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerber B, Marx M, Untch M and Faridi A:
Breast reconstruction following cancer treatment. Dtsch Arztebl
Int. 112:593–600. 2015.PubMed/NCBI
|
|
6
|
Danilak M and Chambers CR: Adherence to
adjuvant endocrine therapy in women with breast cancer. J Oncol
Pharm Pract. 19:105–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krastev TK, Jonasse Y and Kon M:
Oncological safety of autologous lipoaspirate grafting in breast
cancer patients: A systematic review. Ann Surg Oncol. 20:111–119.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alkatout I, Order B, Klapper W, Weigel MT,
Jonat W, Schaefer FK, Mundhenke C and Wenners A: Surgical impact of
new treatments in breast cancer. Minerva Ginecol. 65:363–383.
2013.PubMed/NCBI
|
|
9
|
Hong IS, Jang GB, Lee HY and Nam JS:
Targeting cancer stem cells by using the nanoparticles. Int J
Nanomedicine. 10:251–260. 2015.PubMed/NCBI
|
|
10
|
Bao B, Azmi AS, Ali S, Zaiem F and Sarkar
FH: Metformin may function as anti-cancer agent via targeting
cancer stem cells: The potential biological significance of
tumor-associated miRNAs in breast and pancreatic cancers. Ann
Transl Med. 2:592014.PubMed/NCBI
|
|
11
|
Patel S, Shah K, Mirza S, Daga A and Rawal
R: Epigenetic regulators governing cancer stem cells and
epithelial-mesenchymal transition in oral squamous cell carcinoma.
Curr Stem Cell Res Ther. 10:140–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Menendez JA and Alarcón T: Metabostemness:
A new cancer hallmark. Front Oncol. 4:2622014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anfuso B, Tiribelli C and Sukowati CH:
Recent insights into hepatic cancer stem cells. Hepatol Int.
8(Suppl 2): S458–S463. 2014. View Article : Google Scholar
|
|
14
|
Cherciu I, Bărbălan A, Pirici D,
Mărgăritescu C and Săftoiu A: Stem cells, colorectal cancer and
cancer stem cell markers correlations. Curr Health Sci J.
40:153–161. 2014.PubMed/NCBI
|
|
15
|
Adorno-Cruz V, Kibria G, Liu X, Doherty M,
Junk DJ, Guan D, Hubert C, Venere M, Mulkearns-Hubert E, Sinyuk M,
et al: Cancer stem cells: Targeting the roots of cancer, seeds of
metastasis and sources of therapy resistance. Cancer Res.
75:924–929. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stecklein SR, Jensen RA and Pal A: Genetic
and epigenetic signatures of breast cancer subtypes. Front Biosci
(Elite Ed). 4:934–949. 2012.PubMed/NCBI
|
|
17
|
Mannelli G and Gallo O: Cancer stem cells
hypothesis and stem cells in head and neck cancers. Cancer Treat
Rev. 38:515–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Podberezin M, Wen J and Chang CC: Cancer
stem cells: A review of potential clinical applications. Arch
Pathol Lab Med. 137:1111–1116. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zvereva MI, Shcherbakova DM and Dontsova
OA: Telomerase: Structure, functions, and activity regulation.
Biochemistry (Mosc). 75:1563–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blomme J, Inzé D and Gonzalez N: The
cell-cycle interactome: A source of growth regulators? J Exp Bot.
65:2715–2730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheppard KE and McArthur GA: The
cell-cycle regulator CDK4: An emerging therapeutic target in
melanoma. Clin Cancer Res. 19:5320–5328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poss ZC, Ebmeier CC and Taatjes DJ: The
Mediator complex and transcription regulation. Crit Rev Biochem Mol
Biol. 48:575–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Safa AR, Saadatzadeh MR, Cohen-Gadol AA,
Pollok KE and Bijangi-Vishehsaraei K: Glioblastoma stem cells
(GSCs) epigenetic plasticity and interconversion between
differentiated non-GSCs and GSCs. Genes Dis. 2:152–163. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suhardja A and Hoffman H: Role of growth
factors and their receptors in proliferation of microvascular
endothelial cells. Microsc Res Tech. 60:70–75. 2013. View Article : Google Scholar
|
|
25
|
Park J, Kim HN, Kim DH, Levchenko A and
Suh KY: Quantitative analysis of the combined effect of substrate
rigidity and topographic guidance on cell morphology. IEEE Trans
Nanobioscience. 11:28–36. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin L, Huang J, Xiong C, Zhang Y and Fang
J: Dynamical stress characterization and energy evaluation of
single cardiac myocyteactuating on flexible substrate. Biochem
Biophys Res Commun. 360:352–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar P, Kadakol A, Shasthrula PK, Mundhe
NA, Jamdade VS, Barua CC and Gaikwad AB: Curcumin as an adjuvant to
breast cancer treatment. Anticancer Agents Med Chem. 15:647–656.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeineddine D, Hammoud AA, Mortada M and
Boeuf H: The Oct4 protein: More than a magic stemness marker. Am J
Stem Cells. 3:74–82. 2014.PubMed/NCBI
|
|
29
|
Lyons AB, Blake SJ and Doherty KV: Flow
cytometric analysis of cell division by dilution of CFSE and
related dyes. Curr Protoc Cytom. 9:Unit9.112013.PubMed/NCBI
|