Introduction
Osteosarcoma (OS) is the most common type of primary
malignant cancer originating from the bone in children and young
adolescents (1). Considerable
advances have been made in surgical technology and combined
therapeutic strategies, which has significantly increased the
survival rate of patients with OS to 65–75%. However, the survival
rate of OS patients with lung metastasis and advanced clinical
stage is poor, and this is a major cause of mortality in such
patients (2).
Several molecular factors have been explored for
their prognostic significance in OS, including cluster of
differentiation 44, ezrin and papillomavirus binding factor
(3–5).
However, no reliable prognostic factors have yet been established
for OS. Therefore, the identification of novel strong predictors of
tumor progression and survival is of significance in improving
therapeutic strategies against OS.
The role of Ca2+ in global
cancer-associated cell signaling pathways is well known.
Fluctuations in Ca2+ homeostasis may lead to an increase
in cell proliferation (6,7) and even induce differentiation (8) and apoptosis (9). A previous study demonstrated that the
family of transient receptor potential (TRP) channels are essential
mediators of sensory signals with marked effects on cellular
functions and signaling pathways, including calcium homeostasis
(10). Further evidence indicated
that TRP channels are associated with oncogenesis and that these
channels might be potential targets for cancer treatment (11). The transient receptor potential
melastatin member 8 (TRPM8), a member of the TRP family, is a
Ca2+-permeable cation channel also known as the ‘cold
receptor’ as it may be activated by cold temperature and menthol
(12). Since the identification of
the TRPM8 gene in 2001, it has been observed that the abnormal
expression of TRPM8 is associated with the phenotype of cancers
(13). Furthermore, the prognostic
significance of TRPM8 has been described in several cancer types,
including prostate (14), breast
(15), pancreatic (16) and bladder cancer (17). A previous study indicated that TRPM8
is overexpressed in OS and that knockdown of TRPM8 suppresses
cancer malignancy and enhances epirubicin-induced apoptosis in
human OS cells (18), However, little
is known about the expression and clinical significance of TRPM8 in
OS.
The present study assessed the expression profile of
TRPM8 messenger RNA (mRNA) in OS by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
TRPM8 protein expression by western blot analysis and
immunohistochemistry. In addition, the association of TRPM8
expression with clinicopathological parameters and prognosis in
patients with OS receiving curative surgical resection was
investigated.
Materials and methods
Patients and tissue samples
Tumor specimens were collected from two consecutive
cohorts of patients with primary OS. Cohort A consisted of 20
patients treated at the First Affiliated Hospital of China Medical
University (Shenyang, China) between July and October 2014, from
whom fresh tumor samples coupled with adjacent non-tumorous bone
tissues 5–10 cm away from the tumor edge were obtained for later
analysis of TRPM8 mRNA and protein expression. All the fresh
specimens were stored at −80°C until use. Cohort B consisted of 98
OS patients who underwent curative tumor resection at the First
Affiliated Hospital of China Medical University between March 2003
and November 2008. None of the patients had received chemotherapy
or radiotherapy prior to surgery. The study was approved by the
Institutional Review Board of China Medical University. Written
informed consent was obtained from all participants. The clinical
stage of the OS patients was determined according to the
tumor-node-metastasis (TNM) classification of the International
Union Against Cancer (UICC) (19).
The clinicopathological information of the patients is shown in
Table I.
 | Table I.Association of TRPM8 expression with
clinicopathological features of osteosarcoma. |
Table I.
Association of TRPM8 expression with
clinicopathological features of osteosarcoma.
|
|
| TRPM8 |
|
|---|
|
|
|
|
|
|---|
| Features | Cases, n | Positive, n (%) | Negative, n (%) | P-valuea |
|---|
| Age at diagnosis |
|
|
| 0.481 |
| <18
years | 47 | 30 (63.8) | 17 (36.2) |
|
| ≥18
years | 51 | 29 (56.9) | 22 (43.1) |
|
| Gender |
|
|
| 0.905 |
|
Female | 42 | 25 (59.5) | 17 (40.5) |
|
| Male | 56 | 34 (60.7) | 22 (39.3) |
|
| Histological
subtype |
|
|
| 0.207 |
|
Osteoblastic | 44 | 22 (50.0) | 22 (50.0) |
|
|
Chondroblastic | 20 | 15 (75.0) | 5
(25.0) |
|
|
Fibroblastic | 14 | 9
(64.3) | 5
(35.7) |
|
|
Mixed | 20 | 14 (70.0) | 6
(30.0) |
|
| Clinical stage |
|
|
| 0.007 |
|
I+IIA | 62 | 31 (50.0) | 31 (50.0) |
|
|
IIB/III | 36 | 28 (77.8) | 8
(22.2) |
|
| Distant
metastasis |
|
|
| 0.030 |
|
Absent | 60 | 31 (51.7) | 29 (48.3) |
|
|
Present | 38 | 28 (73.7) | 10 (26.3) |
|
| Tumor size |
|
|
| 0.429 |
| <5
cm | 53 | 30 (56.6) | 23 (43.4) |
|
| ≥5
cm | 45 | 29 (64.4) | 16 (35.6) |
|
| Anatomic
location |
|
|
| 0.369 |
|
Tibia/femur | 60 | 34 (56.7) | 26 (43.3) |
|
|
Elsewhere | 38 | 25 (65.8) | 13 (34.2) |
|
All 98 OS patients received follow-up for a period
ranging from 72 to 132 months. Ten patients were lost to follow-up.
The median overall survival and disease-free survival time of
patients was 68 and 55 months, respectively.
RT-qPCR
RT-qPCR was performed using the SYBR-Green PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in a total volume of 20 µl on a 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). In
brief, total RNA was extracted from tissues using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The reverse transcription reaction was
performed using a high-capacity cDNA synthesis kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). A dissociation step
was performed to generate melting curves to confirm the specificity
of the amplification. Expression levels of the analyzed genes were
normalized to the expression of GAPDH. The fold change in gene
expression was calculated by the 2−ΔΔCq method (Cq of
TRPM8 - Cq of GAPDH). The sequences of the primer pairs were as
follows: TRPM8 forward, 5′-GAGCTGGATGAGCACAAC-3′; TRPM8 reverse,
5′-GAAGTAAGCGAAGACGATG-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′; and GAPDH reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The primers were synthesized by Sangon
Biotech (Shanghai, China).
Western blot analysis
Total protein from cells was extracted in lysis
buffer (Pierce, Rockford, IL, USA) and quantified using the
Bradford method. Samples were separated by sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, transferred to
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA),
and incubated overnight at 4°C with polyclonal rabbit anti-mouse
TRPM8 (dilution, 1:1,000; cat. no. ab3243; Abcam, Cambridge, MA,
USA) and monoclonal rabbit anti-human GAPDH (dilution, 1:1,000;
cat. no. 2118; Cell Signaling Technology, Inc., Danvers, MA, USA)
antibodies. Following incubation with horseradish
peroxidase-conjugated goat anti-mouse/rabbit IgG (dilution,
1:10,000; cat. no. 7074; Cell Signaling Technology, Inc.) at 37°C
for 2 h, bound proteins were visualized using enhanced
chemiluminescence (Pierce) and detected using a Bioimaging System
(UVP Inc., Upland, CA, USA). Relative protein levels were
quantified using GAPDH as a loading control.
Immunohistochemistry
Tissue sections (4 µm thick) were obtained from
formalin-fixed and paraffin-embedded tissue blocks from the OS
samples. Sections were washed in xylene to remove the paraffin,
rehydrated with serial dilutions of alcohol, and then washed in
phosphate-buffered saline solution. Endogenous peroxidase activity
was blocked by 3% H2O2 at 37°C for 30 min.
Sections were incubated in 10% normal goat serum (Boster Biological
Technology, Ltd., Wuhan, China) to block non-specific protein
binding sites. Sections were then incubated in primary antibodies
against TRPM8 (dilution, 1:200; Abcam) overnight at 4°C. After the
primary antibody was washed off, sections were incubated with
polyclonal goat anti-rabbit biotin-conjugated secondary antibodies
(dilution, 1:1,000; cat. no. E043201; Dako, Glostrup, Denmark) for
30 min at 37°C. Sections were then incubated with streptavidin
horseradish peroxidase for 30 min at 37°C. 3,3′-diaminobenzidine
substrate (Sigma-Aldrich, St. Louis, MO, USA) was applied to the
section, and then sections were counterstained with hematoxylin
(Abcam). Sections in which primary antibodies were omitted were
used as negative control.
Immunohistochemistry evaluation and
selection of cutoff score
The immunostaining was examined under a light
microscope (Olympus-IX83; Olympus, Tokyo, Japan) by two
pathologists blinded to the experimental conditions. The agreement
on the scores between the two pathologists was almost 100%. In
cases where the pathologists disagreed on the score, the
immunohistochemical scoring was repeated by the two pathologists
until the same score was achieved. Each section was assigned an
intensity score from 0–3 (0 for no staining, 1 for weak staining, 2
for moderate staining, and 3 for strong staining) and the
proportion of tumor cells for that intensity over the total number
of tumor cells was recorded in 5% increments from a range of 0–100.
A final score (range, 0–300) was achieved by adding the sum of
scores obtained for each intensity and the proportion of the area
stained. Receiver operating characteristic (ROC) curve analysis was
used to determine the cutoff value for TRPM8 expression in the
training set using the 0, 1-criterion. In the TRPM8 score, the
sensitivity and specificity for survival status in the present
study was plotted to generate the ROC curve to determine the cutoff
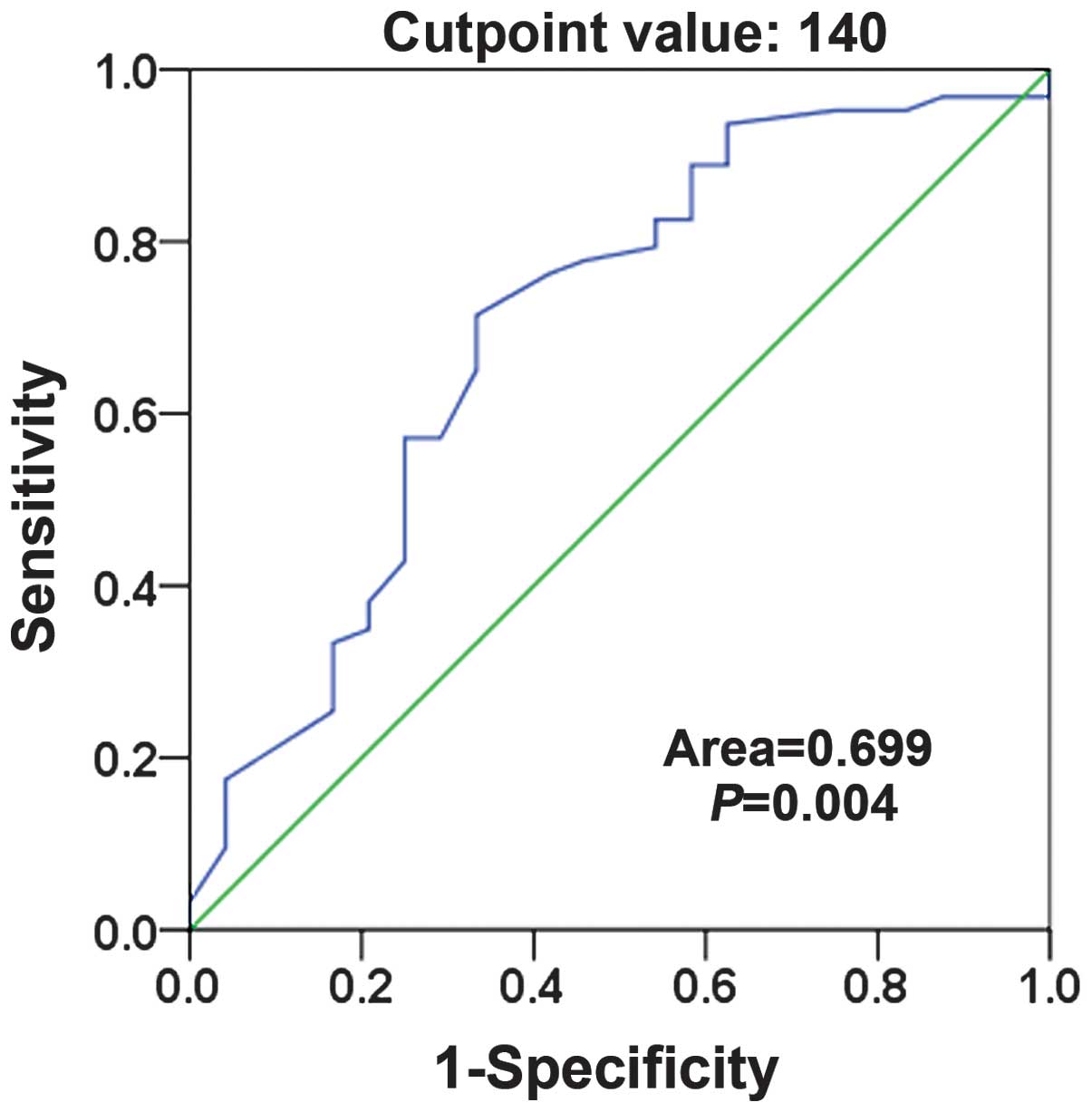
score for positive expression of TRPM8 in osteosarcoma (Fig. 1). The score closest to the points of
maximum sensitivity and maximum specificity (140) was selected as
the cutoff value. The tumors designated as having negative
expression of TRPM8 were those with scores below the cutoff value.
Those deemed to have positive expression were those with scores
above or equal to the cutoff value.
Statistical analysis
Analyses were performed using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). The t-test was used to analyze data from
OS tissues and matched normal bone tissues detected by RT-qPCR and
western blot analysis. The association between TRPM8 expression and
clinicopathological parameters was evaluated by Pearson's
χ2 test or Fisher's exact probability test. Survival
probabilities were estimated by the Kaplan-Meier method and
assessed by a log-rank test. Univariate and multivariate Cox
proportional hazard regression models were used for assessing the
association between potential confounding variables and prognosis
(overall survival or disease-free survival). P<0.05 was
considered to indicate a statistically significant difference.
Results
Overexpression of TRPM8 mRNA in OS
tissues
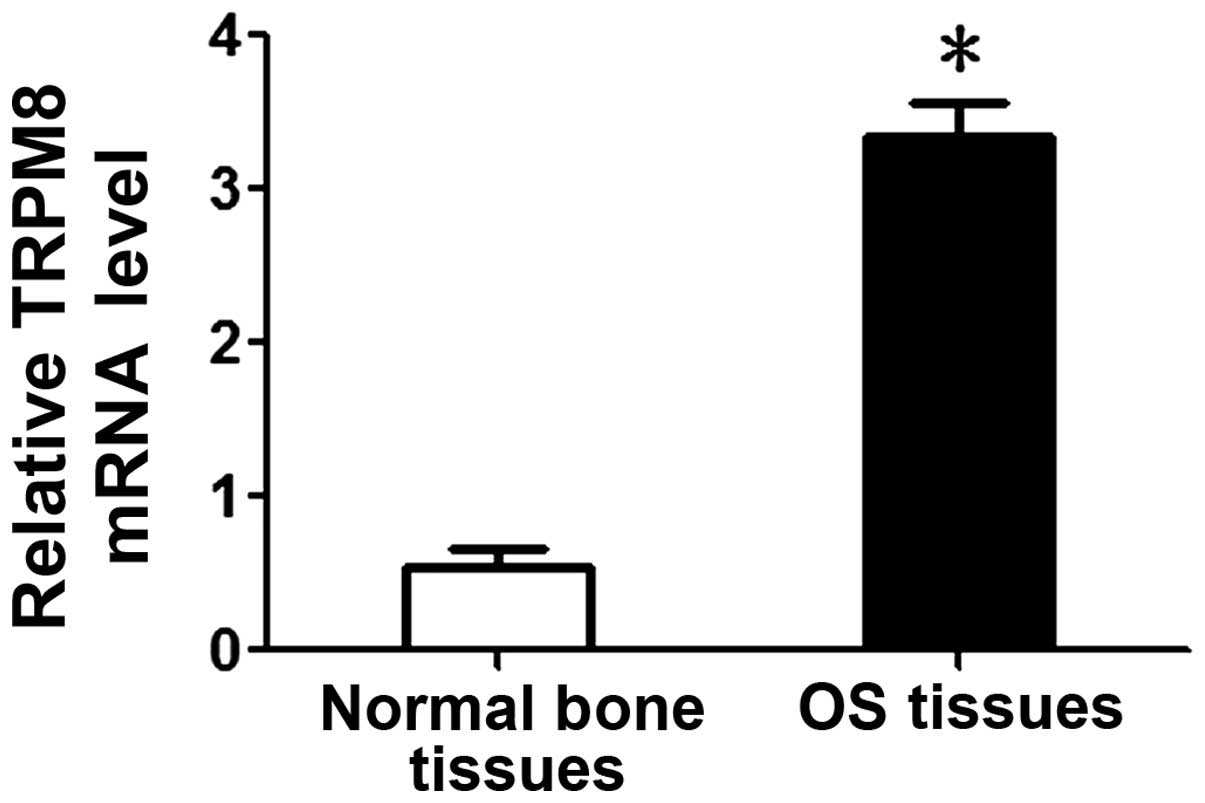
TRPM8 mRNA levels were investigated by qPCR in 20
cases of frozen OS and paired normal bone tissues. It was observed
that the mean TRPM8 mRNA level in OS tissues was significantly
higher than that in normal bone tissues (3.34±0.23 vs. 0.55±0.12;
P<0.05; Fig. 2).
Overexpression of TRPM8 protein in OS
tissues
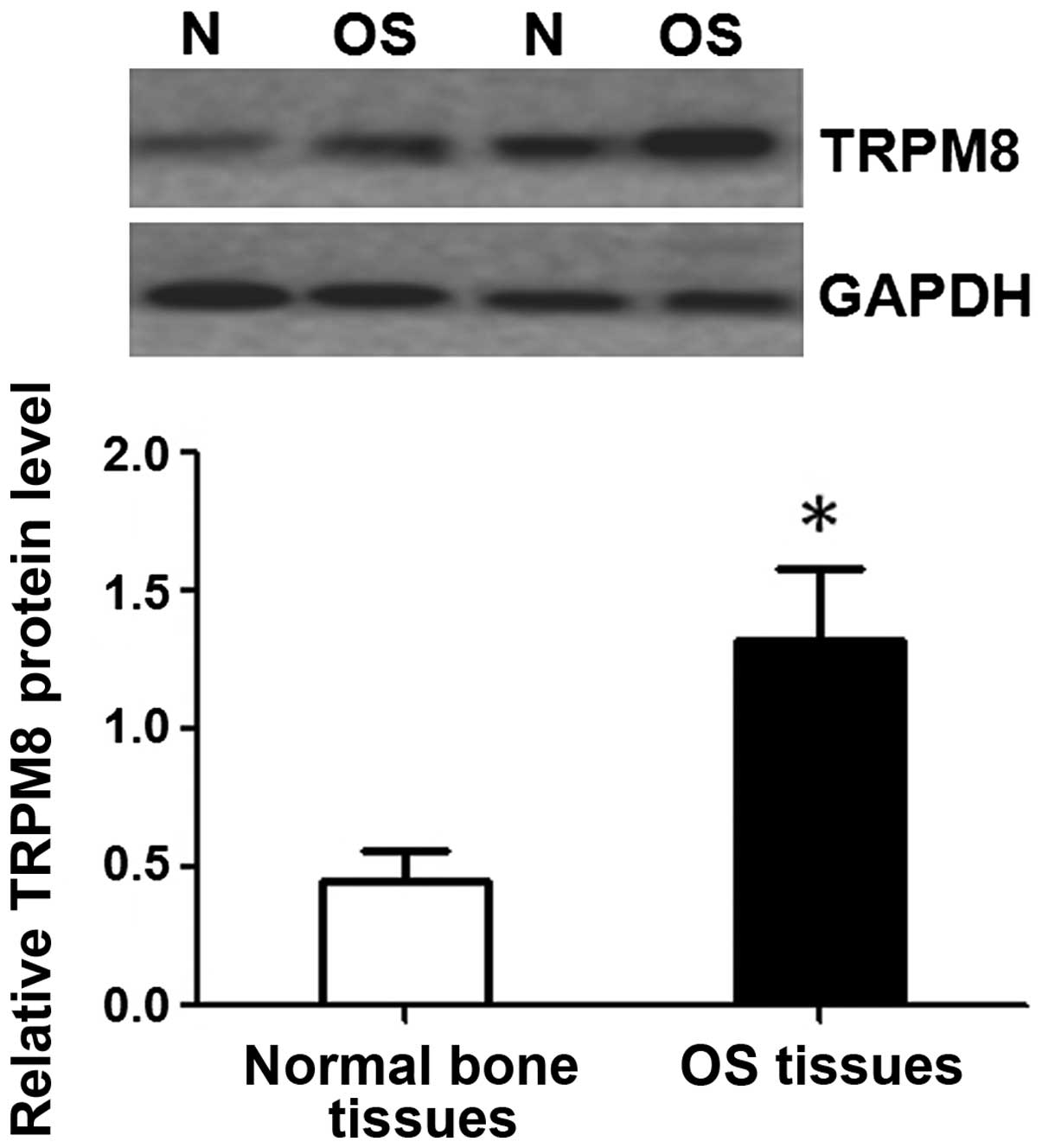
To investigate whether TRPM8 was also elevated at
the protein level, western blot analysis was performed on the same
specimens that were used in the detection of TRPM8 mRNA. Western
blot also demonstrated that the expression of TRPM8 protein was
significantly higher in OS tissues compared with that in normal
bone tissues (1.32±0.26 vs. 0.45±0.11; P<0.05; Fig. 3).
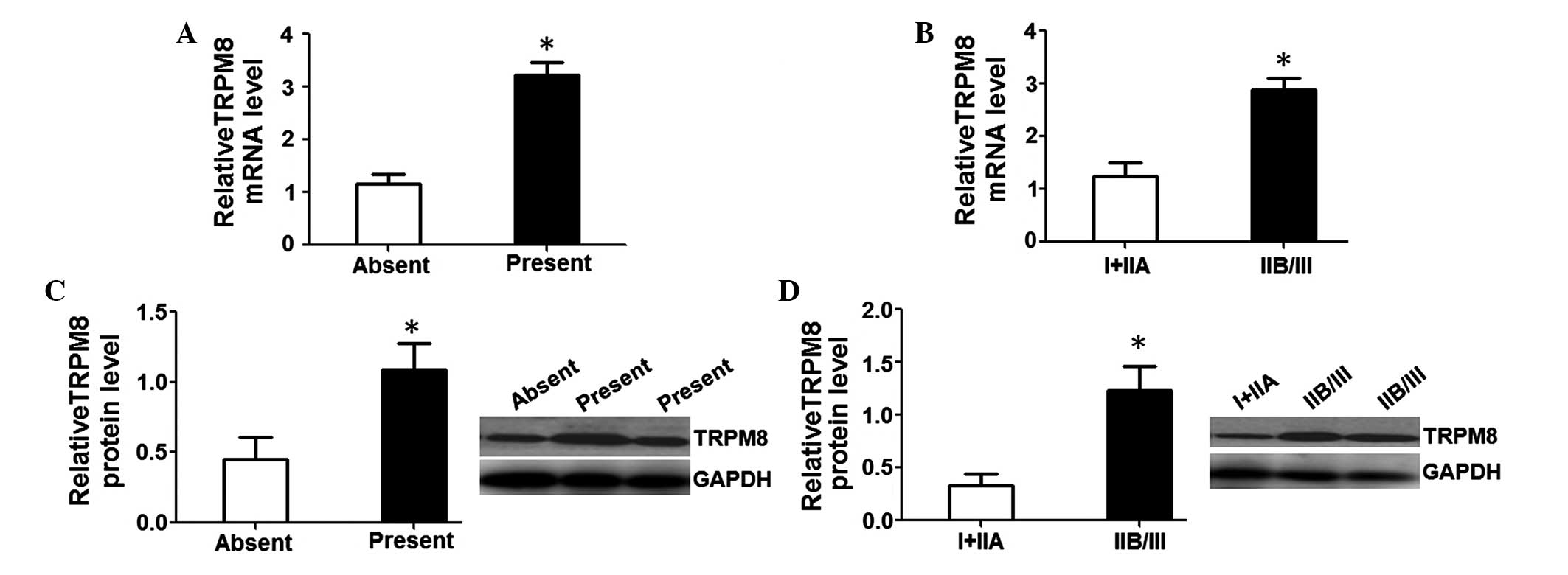
Elevated TRPM8 expression contributes
to higher aggressiveness in patients with OS
To determine the underlying function of TRPM8 in the
occurrence and development of OS, TRPM8 mRNA and protein levels
were assessed in patients with distant metastasis or without
metastasis as well as in patients with various clinical stages. The
results revealed that relative TRPM8 mRNA (3.23±0.23) and protein
(1.09±0.19) levels in patients with distant metastasis were
markedly higher than in those without metastasis (1.16±0.18 and
0.45±0.16; P<0.05; Fig. 4A and C).
Further investigation revealed that relative TRPM8 mRNA (2.89±0.21)
and protein (1.23±0.22) levels in the patients with clinical stage
IIB/III were also notably higher than in those with clinical stages
I+IIA (1.25±0.24 and 0.33±0.11; P<0.05; Fig. 4B and D). These data suggest that an
elevated TRPM8 level predicts stronger aggressiveness and a higher
clinical stage in OS patients.
Association between TRPM8 expression
and clinicopathological characteristics of OS patients
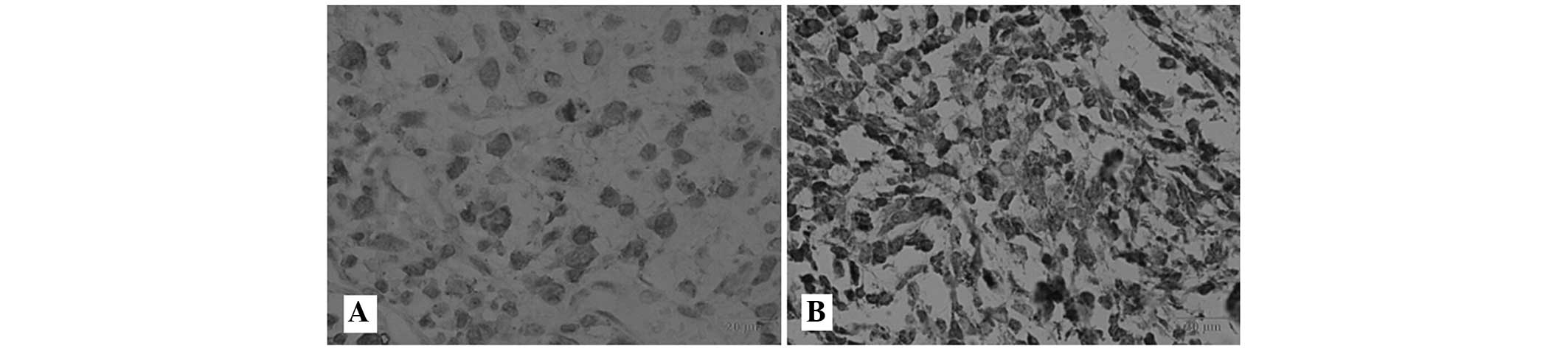
To further evaluate whether TRPM8 protein
upregulation was associated with the clinical characteristics of OS
patients, the expression of TRPM8 protein in 98 OS tissue samples
was examined by immunohistochemistry. The positive immunoreactivity
of TRPM8 was localized in the membrane and cytoplasm of the OS
cells. In accordance with the TRPM8 immunoreactive intensity, 60.2%
(59/98) patients were classified as having positive TRPM8, and
39.8% (39/98) were classified as having negative TRPM8 (Fig. 5). Table
I shows the associations between TRPM8 expression and
clinicopathological characteristics in OS patients. TRPM8 protein
expression was noted to be strongly associated with distant
metastasis and clinical stage (P=0.030 and P=0.007), but not
associated with age (P=0.481), gender (P=0.905), tumor size
(P=0.429), histological subtype (P=0.207) or anatomical location
(P=0.369), suggesting that the TRPM8 protein level may be closely
associated with the development and progression of OS.
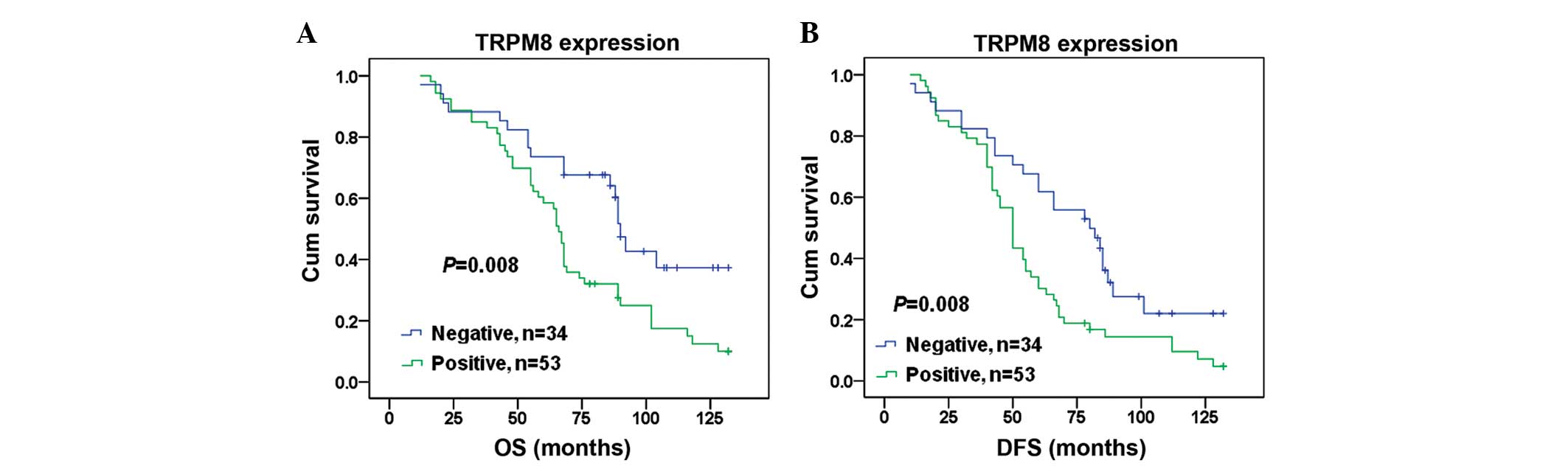
Prognostic significance of TRPM8
expression in OS
Finally, the prognostic value of TRPM8 expression in
OS was assessed. Kaplan-Meier analysis demonstrate a significantly
shorter median overall survival and disease-free survival time in
patients with positive TRPM8 OS compared with those with negative
TRPM8 OS (71 vs. 91 months, P=0.008 and 57 vs. 76 months, P=0.008,
respectively; Fig. 6). Univariate
analyses demonstrated that tumor size (P=0.036 and P=0.042),
clinical stage (P=0.005 and P=0.034), distant metastasis (P=0.001
and P=0.009), anatomic location (P=0.036 and P=0.003) and TRPM8
(P=0.011 and P=0.011) were significant predictors of overall
survival and disease-free survival in OS patients (Table II). The multivariate survival
analysis (Table II) further
confirmed that TRPM8 was an independent prognostic factor
(P=0.040), as were distant metastasis (P=0.024) and tumor size
(P=0.011) in overall survival, while distant metastasis (P=0.027),
tumor size (P=0.030) and anatomic location (P=0.028) were
independent prognostic factors in disease-free survival.
 | Table II.Cox regression analysis of
clinicopathological data associated with OS and DFS in
osteosarcoma. |
Table II.
Cox regression analysis of
clinicopathological data associated with OS and DFS in
osteosarcoma.
|
| OS | DFS |
|---|
|
|
|
|
|---|
|
| Univariate | Multivariate | Univariate | Multivariate |
|---|
|
|
|
|
|
|
|---|
| Factor | RR | Pa | RR | Pb | RR | Pa | RR | Pb |
|---|
| Age, years
(≥18/<18) | 1.309 | 0.289 | 1.230 | 0.434 | 1.263 | 0.322 | 1.164 | 0.536 |
| Gender
(male/female) | 0.904 | 0.694 | – | – | 1.109 | 0.666 | – | – |
| Tumor size, cm
(≥5/<5) | 1.711 | 0.036 | 2.018 | 0.011 | 1.617 | 0.042 | 1.723 | 0.030 |
| Histological
subtype (mixed/fibroblastic/chondroblastic/osteoblastic) | 0.978 | 0.827 | – | – | 1.017 | 0.855 | – | – |
| Clinical stage
(IIB/III/I+IIA) | 2.065 | 0.005 | 0.369 | 0.251 | 4.294 | 0.034 | 0.308 | 0.166 |
| Distant metastasis
(present/absent) | 2.364 | 0.001 | 7.196 | 0.024 | 1.885 | 0.009 | 6.579 | 0.027 |
| Anatomic location
(tibia/femur/elsewhere) | 1.709 | 0.036 | 1.408 | 0.288 | 2.017 | 0.003 | 1.752 | 0.028 |
| TRPM8
(positive/negative) | 2.045 | 0.011 | 1.855 | 0.040 | 1.910 | 0.011 | 1.700 | 0.051 |
Discussion
To the best of our knowledge, this is the first
study involving a large number of clinical samples that aimed to
assess the expression of TRPM8 in OS tissues and its association
with OS clinicopathological characteristics and to evaluate the
association between TRPM8 expression levels and the prognosis of OS
patients. The study firstly confirms that expression levels of
TRPM8 mRNA and protein are significantly higher in OS tissues
compared with matched normal bone tissues, which is consistent with
the findings of a previous study (18). The present study further indicated
that TRPM8 may play an essential role in the development and
progression of OS.
Accumulating evidence has demonstrated that tumor
metastasis and clinical stage are often considered to be the most
significant prognostic indicators in various tumors (20–24). To
prove whether TRPM8 affects the occurrence of tumor metastasis and
the clinical stage, and thereby regulates the development and
progression of OS, the present study analyzed TRPM8 mRNA and
protein levels in OS tissues with various metastatic states and
clinical stages using RT-qPCR and western blot analysis. It was
observed that TRPM8 mRNA and protein levels were markedly higher in
OS tissues with metastasis and higher clinical stage (IIB/III) than
in those without metastasis and with a lower clinical stage
(I/IIA), indicating that TRPM8 may be a novel predictor for
metastasis and advanced status in patients with OS.
It is well documented that age, tumor size,
resectability of the primary tumor, metastatic status and clinical
stage are notable clinical characteristics in OS (25). Several studies have demonstrated that
TRPM8 overexpression was frequently associated with a number of
clinicopathological features, which also confirms the significance
of TRPM8 in the development and progression of numerous tumors. In
order to explore the possible roles of TRPM8 in OS, the association
between TRPM8 protein expression and various clinicopathological
features was investigated. In line with the expectations of the
present study, it was revealed that TRPM8 protein expression was
strongly associated with distant metastasis and clinical stage,
further suggesting that TRPM8 may play a major role in the
development and progression of OS.
Several studies have demonstrated that TRPM8
overexpression is unfavorable in the prognosis of patients with
various tumors (14–17), suggesting a connection between TRPM8
and prognosis of tumor patients, and thus highlighting TRPM8 as a
crucial prognosis factor for these tumors. Therefore, to seek new
molecular therapeutic targets for patients with OS, the association
between TRPM8 and the prognosis of OS patients was further
investigated. The present findings revealed that TRPM8 expression
was closely associated with disease-free and overall survival in OS
patients, with the disease-free and overall survival of patients
with positive TRPM8 expression being significantly shorter than in
those with negative TRPM8 expression, indicating that TRPM8 may be
a novel prognosis predictor and therapeutic target in patients with
OS.
Although the precise role of TRPM8 in OS remains
unclear, the present study hypothesizes that TRPM8 overexpression
may lead to the generation of more Ca2+ release channels
in OS cell membrane and cytoplasm, including endoplasmic reticulum
and mitochondria, in which TRPM8 is possibly located. This may
result in a greater influx of Ca2+ through the TRPM8
channel (26). The increasing
concentration of Ca2+ may enhance the participation of
mitochondria in numerous metabolic functions including cell growth,
death and apoptosis (12), and could
account for a significant positive association between the
expression of TRPM8 and distant metastasis and clinical stage in
OS, with poor survival rates observed in OS patients with high
TRPM8 expression.
There are several limitations to the present study,
as it was a single-center and retrospective study, using smaller
samples. Therefore, to validate the roles of TRPM8 in the
pathogenesis and oncogenesis of OS, it will be necessary to carry
out in vitro and xenograft functional research in the
future, in order to detect the biological alteration of OS cells by
the regulation of TRPM8 expression.
In conclusion, elevated TRPM8 expression may play a
pivotal role in the occurrence, development and progression of OS.
Detection of TRPM8 levels may serve as a clinical predictor in the
diagnosis or prediction of clinical outcome in OS patients. Data
from the present study may provide novel prospects for molecular
target therapy in OS patients, although details are still to be
clarified through ongoing investigation.
Acknowledgments
This study was supported by grants from the General
Science Research Program of the Education Department of Liaoning
Province in China (no. L2014428) and the Natural Science Foundation
of Liaoning Province (no. 2013225086).
References
|
1
|
Gill J, Ahluwalia MK, Geller D and Gorlick
R: New targets and approaches in osteosarcoma. Pharmacol Ther.
137:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: state of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Min D, Zhao H, Wang Z, Qi W, Zheng
S, Tang L, He A, Sun Y, Yao Y and Shen Z: The prognostic role of
Ezrin immunoexpression in osteosarcoma: a meta-analysis of
published data. PLoS One. 8:e645132013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Y, Wu Y, Gu S, Sun Z, Rui Y, Wang J,
Lu Y, Li H, Xu K and Sheng P: Prognostic role of CD44 expression in
osteosarcoma: evidence from six studies. Diagn Pathol. 9:1402014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukahara T, Kawaguchi S, Torigoe T,
Kimura S, Murase M, Ichimiya S, Wada T, Kaya M, Nagoya S, Ishii T,
et al: Prognostic impact and immunogenicity of a novel osteosarcoma
antigen, papillomavirus binding factor, in patients with
osteosarcoma. Cancer Sci. 99:368–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Legrand G, Humez S, Slomianny C, Dewailly
E, Vanden Abeele F, Mariot P, Wuytack F and Prevarskaya N: Ca2+
pools and cell growth. Evidence for sarcoendoplasmic Ca2+-ATPases
2B involvement in human prostate cancer cell growth control. J Biol
Chem. 276:47608–47614. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thebault S, Flourakis M, Vanoverberghe K,
et al: Differential role of transient receptor potential channels
in Ca2+ entry and proliferation of prostate cancer epithelial
cells. Cancer Res. 66:2038–2047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vanoverberghe K, Vanden Abeele F, Mariot
P, et al: Ca2+ homeostasis and apoptotic resistance of
neuroendocrine-differentiated prostate cancer cells. Cell Death
Differ. 11:321–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skryma R, Mariot P, Bourhis XL, et al:
Store depletion and store-operated Ca2+ current in human prostate
cancer LNCaP cells: involvement in apoptosis. J Physiol. 527:71–83.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaneko Y and Szallasi A: Transient
receptor potential (TRP) channels: a clinical perspective. Br J
Pharmacol. 171:2474–2507. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen J, Luan Y, Yu R, Zhang Z, Zhang J and
Wang W: Transient receptor potential (TRP) channels, promising
potential diagnostic and therapeutic tools for cancer. Biosci
Trends. 8:1–10. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knowlton WM and McKemy DD: TRPM8: From
cold to cancer, peppermint to pain. Curr Pharm Biotechnol.
12:68–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsavaler L, Shapero MH, Morkowski S and
Laus R: Trp-p8, a novel prostate-specific gene, is up-regulated in
prostate cancer and other malignancies and shares high homology
with transient receptor potential calcium channel proteins. Cancer
Res. 61:3760–3769. 2001.PubMed/NCBI
|
|
14
|
Kim SH, Nam JH, Park EJ, Kim BJ, Kim SJ,
So I and Jeon JH: Menthol regulates TRPM8-independent processes in
PC-3 prostate cancer cells. Biochim Biophys Acta. 1792:33–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chodon D, Guilbert A, Dhennin-Duthille I,
Gautier M, Telliez MS, Sevestre H and Ouadid-Ahidouch H: Estrogen
regulation of TRPM8 expression in breast cancer cells. BMC Cancer.
10:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yee NS, Zhou W and Lee M: Transient
receptor potential channel TRPM8 is over-expressed and required for
cellular proliferation in pancreatic adenocarcinoma. Cancer Lett.
297:49–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao N, Jiang LM, Ge B, Zhang TY, Zhao XK
and Zhou X: Over-expression of TRPM8 is associated with poor
prognosis in urothelial carcinoma of bladder. Tumour Biol.
35:11499–11504. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Yang Z, Meng Z, Cao H, Zhu G, Liu
T and Wang X: Knockdown of TRPM8 suppresses cancer malignancy and
enhances epirubicin-induced apoptosis in human osteosarcoma cells.
Int J Biol Sci. 10:90–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spiessl B, Scheibe O and Wagner G: Soft
tissue sarcomas. In: International Union Against Cancer (UICC)
TNM-Atlas. Illustrated Guide to the Classification of Malignant
Tumours. Springer-Verlag. (Berlin). 170–172
|
|
20
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Greene FL, Stewart AK and Norton HJ: A new
TNM staging strategy for node-positive (stage III) colon cancer: an
analysis of 50,042 patients. Ann Surg. 236:416–421, (Discussion
421). 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jatoi I, Hilsenbeck SG, Clark GM and
Osborne CK: Significance of axillary lymph node metastasis in
primary breast cancer. J Clin Oncol. 17:2334–2340. 1999.PubMed/NCBI
|
|
23
|
Madu CO and Lu Y: Novel diagnostic
biomarkers for prostate cancer. J Cancer. 1:150–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swanson RS, Compton CC, Stewart AK and
Bland KI: The prognosis of T3N0 colon cancer is dependent on the
number of lymph nodes examined. Ann Surg Oncol. 10:65–71. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rech A, Castro CG Jr, Mattei J, Gregianin
L, Di Leone L, David A, Rivero LF, Tarrago R, Abreu A and Brunetto
AL: Clinical features in osteosarcoma and prognostic implications.
J Pediatr (Rio J). 80:65–70. 2004.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Wang X, Yang Z, Wang B and Li S:
Menthol induces cell death via the TRPM8 channel in the human
bladder cancer cell line T24. Oncology. 77:335–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|