Introduction
Inflammatory myofibroblastic tumor (IMT) was
formerly known as inflammatory pseudotumor, and is a lesion
characterized by myofibroblastic spindle cells accompanied by
inflammatory infiltrates. Although the lung is the most common site
of occurrence, IMT may arise in diverse extra-pulmonary locations.
The etiology of IMT is unclear; however, the anaplastic lymphoma
receptor tyrosine kinase gene may play a role in its pathogenesis
(1). The clinical presentation of IMT
is non-specific and may include fever, weight loss, malaise and
anemia. Retroperitoneal IMT usually grows slowly and may present as
a solid abdominal mass, accompanied by abdominal pain and weight
loss. Gastrointestinal and urinary symptoms may occur with an
enlarging expansile mass (2). There
are no specific radiographical features for IMT. For abdominal IMT,
a solid mass abutting and compressing various organs is the usual
presentation. Retroperitoneal IMT is rare, with only 12 cases
reported in English literature to date (2–10). The
present study describes a case of retroperitoneal IMT, which
recurred 30 months following initial surgery, and where
re-resection was successfully performed. Written informed consent
was obtained from the patient for the publication of the present
study.
Case report
A 74-year-old female initially presented with a
10-cm retroperitoneal mass, discovered incidentally during
cholecystectomy. The mass was resected via a midline
transperitoneal incision. Histopathological examination identified
the lesion as IMT. Patient history included a left pulmonary
lobectomy for bronchiectasis 20-years previously, and hypertension
for 40-years. Subsequently, the patient presented with a palpable
left lower-quadrant abdominal mass 30 months later. Hematological
and biochemical parameters, including carcinoembryonic antigen and
α-fetoprotein expression, were all within normal limits. Abdominal
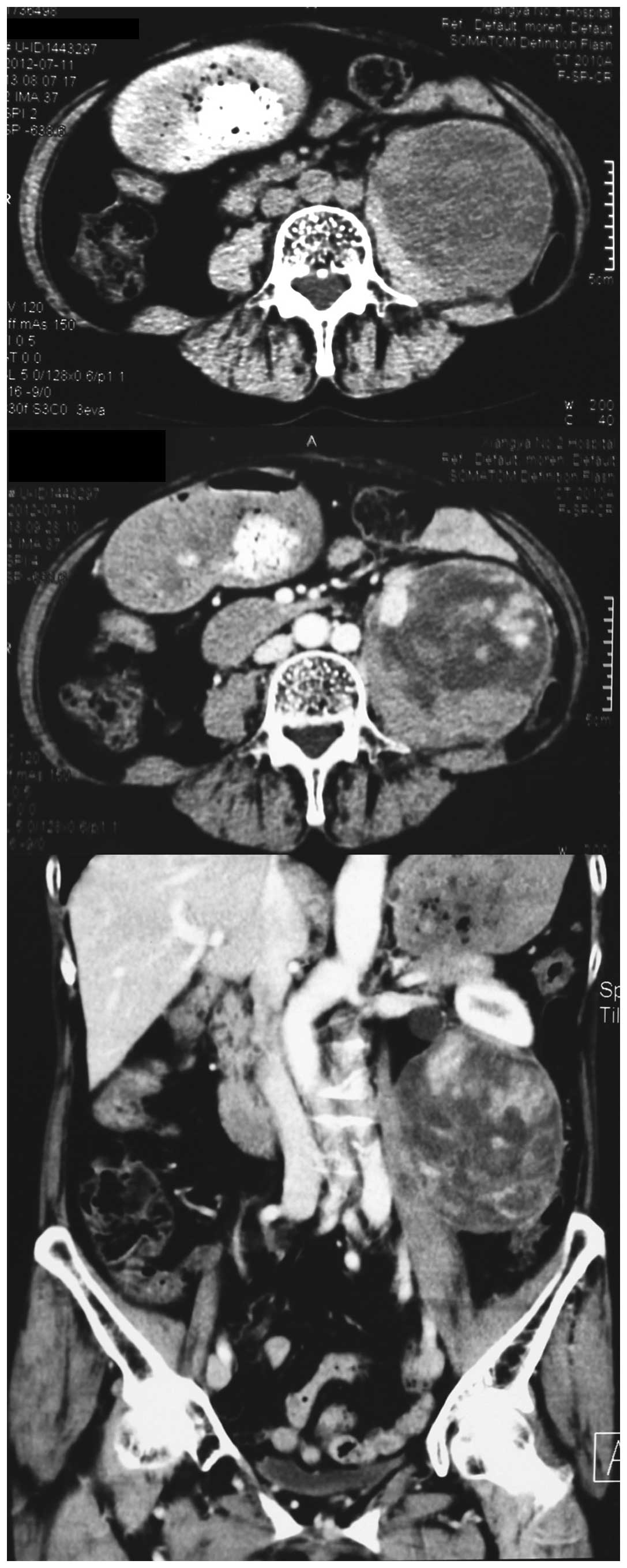
computerized tomography (CT) with contrast revealed an 8×7.5×8.5
cm, well-defined heterogeneous mass with uneven enhancement
(Fig. 1), associated with left
hydroureteronephrosis. There was no regional lymphadenopathy.
Metastatic workup, including a chest CT scan and radionuclide bone
scan, was negative.
Following multidisciplinary consultation, laparotomy
was performed via a left subcostal incision. A 10-cm mass was
identified, which was compressing the kidney and proximal ureter
and adherent to the descending colon. The kidney and ureter were
successfully dissected free but partial left colectomy was required
to facilitate en bloc removal of the tumor mass. The postoperative
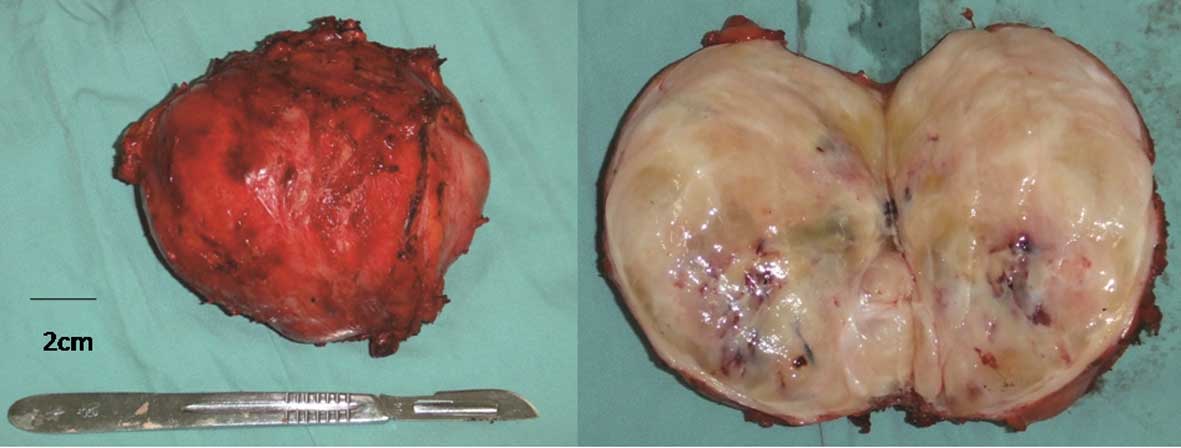
course was uneventful. Gross histopathological examination revealed
the tumor to be encapsulated, with a grey-whitish cut surface
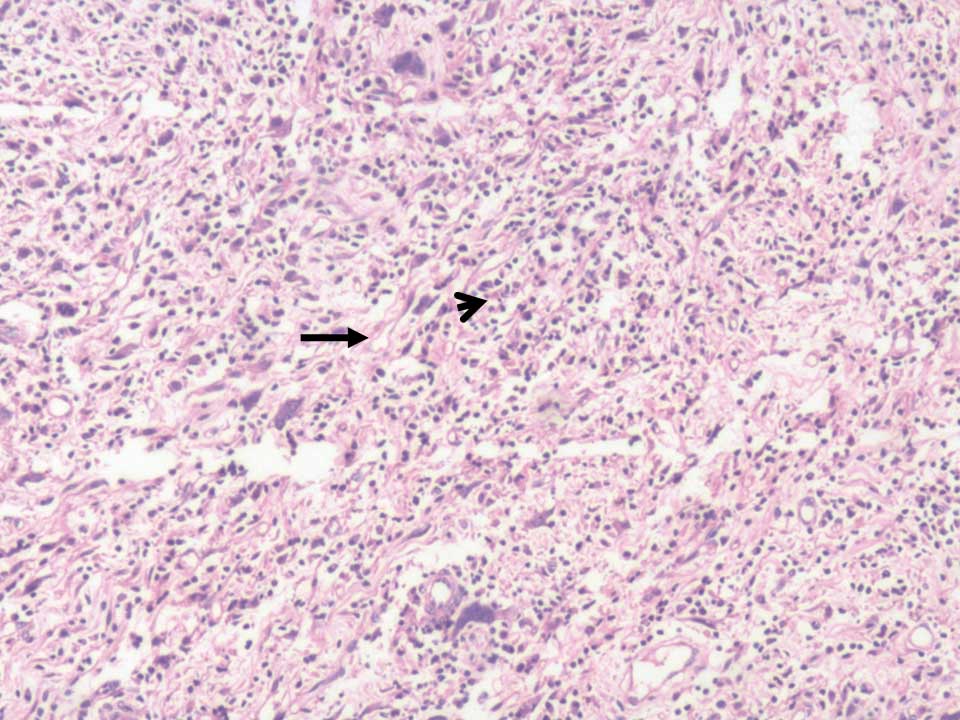
(Fig. 2). Microscopically, the tumor
demonstrated proliferation of spindle cells and infiltration of
lymphocytes, plasma cells and eosinophils (Fig. 3). Immunohistochemistry revealed
diffuse positivity for vimentin, CD68 and desmin; focal staining
for myogenin and Ki-67; and negativity for CD34, cytokeratin,
smooth muscle actin and S100.
The multidisciplinary treatment team did not
recommend postoperative adjuvant therapy. The patient has remained
free of local or distant recurrence 24 months subsequent to
re-resection, at the time of the present report.
Discussion
Inflammatory myofibroblastic tumor (IMT) is a
distinct lesion, composed of myofibroblastic spindle cells with
intermediate biological potential, accompanied by an inflammatory
infiltration of plasma cells, lymphocytes and eosinophils (5,6). The most
common sites of IMT are the lungs and soft-tissue viscera in
children and young adults. The etiology is unclear, although
trauma, surgery, inflammation and infection by Epstein-Barr virus
or human herpes virus have been proposed as potential causative
factors (11). Furthermore,
chromosomal rearrangement involving the ALK gene results in
activation of a tyrosine kinase receptor and may induce abnormal
expression. Immunohistochemically, ~50% of IMTs are positive for
ALK (12), suggesting that the ALK
gene may have a role in the pathogenesis of IMT (1).
The clinical presentation of IMT is non-specific and
may include fever, weight loss, malaise, anemia, thrombocytosis,
polyclonal hyper-globulinemia and/or elevated erythrocyte
sedimentation rate (2). Frequently,
IMT induces anatomical site-specific presentations. Retroperitoneal
IMT typically grows slowly and may present as a solid abdominal
mass, accompanied by abdominal pain and weight loss.
Gastrointestinal and urinary symptoms, particularly bowel and
ureteral obstruction, respectively, may occur with an enlarging
expansile mass (2). The patient in
the present report was asymptomatic with the primary tumor, while
the recurrence presented with a palpable mass but an unremarkable
hematological and biochemical profile.
There are no radiographic features specific to IMT.
In abdominal IMT, a solid mass abutting and compressing various
organs is the typical presentation, frequently accompanied by
obstruction or invasion of the affected organ(s). CT and magnetic
resonance imaging may identify a homogeneous or heterogeneous,
hypodense or isodense lesion. Enhancement may be variable and may
reveal central necrosis or fibrosis (13).
The diagnosis of IMT mainly occurs via pathological
examination. Macroscopically, IMT may be firm, fleshy or
gelatinous, with a white or tan cut surface. The tumors vary in
size, reaching up to 20 cm in the greatest dimension.
Histologically, IMT is characterized by spindle cell proliferation
with a prominent inflammatory infiltrate. IMT lesions are also
positive for vimentin, smooth muscle actin, muscle-specific actin
and occasionally, desmin and cytokeratin (12).
IMT is a neoplasm with ‘intermediate biological
potential’ with a propensity for local recurrence, while rarely
metastasizing. In a series of 84 cases of extrapulmonary IMT,
recurrence was reported in 25% and distal metastasis in only 5% of
cases (12). Histopathological
features alone may not be sufficient for the prediction of
malignant transformation, as tumor size, cellularity, mitotic
activity and the presence of necrosis are not significantly
correlated with risk of metastasis. However, nuclear atypia and
ganglion-like cells may indicate more aggressive cellular behavior.
ALK-positive tumors have a notably low risk of metastasis, and ALK
reactivity has not been found to correlate with recurrence
(12). Compared with other
extra-pulmonary lesions, retroperitoneal IMTs tend to be more
aggressive. Of the 12 reported cases in the English literature,
summarized in Table I, 3 cases
developed metastases and one exhibited local recurrence, with
follow-up periods varying from 3 to 80 months (2–10).
 | Table I.Published cases of retroperitoneal
inflammatory myofibroblastic tumor. |
Table I.
Published cases of retroperitoneal
inflammatory myofibroblastic tumor.
| Reference | Age, years | Gender | Size, cm | Treatment | Outcome,
time-point |
|---|
| Coffin et al
(3) | 15 | M | NA | Diagnosed and treated
as immunoblastic lymphoma 2 months after biopsy | NED, 80 mo |
|
| 11 | F | 8 | Resection, local
recurrences, four re-excisions; regression with xanthogranulomatous
histology | NED, 22 mo |
| Przkora et al
(4) | 22 | M | NA | Ibuprofen 400 mg
bid | SD, 12 mo |
| Attili et al
(5) | 46 | F | 16×13.6×2.1 | Complete
resection | NED, 12 mo |
| Mali et al
(10) | 12 | M | 3.5 | Complete
resection | NED, 6 mo |
| Coffin et al
(6) | 14 | F | 22; liver
metastasis | NA | NA |
|
| 17 mo | F | 6; lung
metastasis | NA | AWD, 9 mo |
|
| 14 | NA | 19; bone
metastasis | NA | NA |
| Koirala et al
(7) | 52 | M | 12.5×10.5 | Complete
resection | NA |
| Ziadi et al
(8) | 41 | M | NA | Partial
resection | DOD, 3 mo |
| Chatzikokol et
al (2) | 68 | F | 3.3×4.5×4.5 | En bloc resection of
the upper section of the adrenal gland, tumor and spleen | NA |
| Tao et al
(9) | 14 | F | 3.3×4.5×4.5 | Resection failed;
postoperative chemotherapy and diclofenac sodium | NED, 36 mo |
The mainstay of management of IMT, as previously
reported, is by surgical excision for definitive or palliative
treatment (2–10). Incomplete tumor removal frequently
results in local recurrence (3),
which was likely the case in the present patient. The patient
underwent a second successful re-resection, with no recurrence at
30 months follow-up. However, this follow-up period is relatively
short and vigilant monitoring is required to facilitate rapid
detection of further recurrence.
Tao et al (9)
reported an unresectable retroperitoneal IMT, which was
successfully treated with methotrexate, cisplatin and diclofenac
sodium, facilitating maintenance of a clinical ‘free of disease’
status for 3 years. Przkora et al (4) also reported a case of unreseactable IMT,
treated with anti-inflammatory agent, ibuprofen, where the patient
was ‘stabilized’ for 12 months. The possible role of the ALK gene
in IMT pathogenesis highlights the potential for use of a
tyrosine-kinase inhibitor in a neoadjuvant or adjuvant capacity
(1). Furthermore, a study reported a
sustained partial response to the ALK-inhibitor, crizotinib, in a
patient with an ALK-translocated IMT (14). The role of radiotherapy in IMT is
unknown, although it may have potential benefits, particularly in
unresectable cases. To date, there is no established protocol for
the treatment of IMT, due to its rarity (9,15).
Retroperitoneal IMT is a rare entity with unclear
etiology and non-specific clinical symptoms. Precise diagnosis
mainly occurs via histopathological analysis. Complete surgical
excision, where feasible, is the recommended treatment. In
addition, complete surgical re-resection is also recommended for
the treatment of recurrent lesions.
References
|
1
|
Cessna MH, Zhou H, Sanger WG, Perkins SL,
Tripp S, Pickering D, Daines C and Coffin CM: Expression of ALK1
and p80 in inflammatory myofibroblastic tumor and its mesenchymal
mimics: A study of 135 cases. Mod Pathol. 15:931–938. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chatzikokolis S, Troupis TG, Michalinos A,
Bafaloukas N, Filippidis T and Gennimatas V: Retroperitoneal
inflammatory myofibroblastic tumor. Am Surg. 78:E190–E191.
2012.PubMed/NCBI
|
|
3
|
Coffin CM, Watterson J, Priest JR and
Dehner LP: Extrapulmonary inflammatory myofibroblastic tumor
(inflammatory pseudotumor). A clinicopathologic and
immunohistochemical study of 84 cases. Am J Surg Pathol.
19:859–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Przkora R, Bolder U, Schwarz S, Jauch KW,
Spes J, Andreesen R and Mackensen A: Regression of nonresectable
inflammatory myofibroblastic tumours after treatment with
nonsteroidal anti-inflammatory drugs. Eur J Clin Invest.
34:320–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Attili SV, Chandra CR, Hemant DK, Bapsy
PP, RamaRao C and Anupama G: Retroperitoneal inflammatory
myofibroblastic tumor. World J Surg Oncol. 3:662005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coffin CM, Hornick JL and Fletcher CD:
Inflammatory myofibroblastic tumor: Comparison of
clinicopathologic, histologic and immunohistochemical features
including ALK expression in atypical and aggressive cases. Am J
Surg Pathol. 31:509–520. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koirala R, Shakya VC, Agrawal CS, Khaniya
S, Pandey SR, Adhikary S and Pathania OP: Retroperitoneal
inflammatory myofibroblastic tumor. Am J Surg. 199:e17–e19. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ziadi S, Trimeche M, Mestiri S, Boujelbene
N, Mokni M, Sriha B and Korbi S: Retroperitoneal myofibroblastic
inflammatory tumor. Tunis Med. 89:400–401. 2011.PubMed/NCBI
|
|
9
|
Tao YL, Wang ZJ, Han JG and Wei P:
Inflammatory myofibroblastic tumor successfully treated with
chemotherapy and nonsteroidals: A case report. World J
Gastroenterol. 18:7100–7103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mali VP, Tan HC, Loh D and Prabhakaran K:
Inflammatory tumour of the retroperitoneum - a case report. Ann
Acad Med Singapore. 34:632–635. 2005.PubMed/NCBI
|
|
11
|
Gómez-Román JJ, Sánchez-Velasco P,
Ocejo-Vinyals G, Hernández-Nieto E, Leyva-Cobián F and Val-Bernal
JF: Human herpesvirus-8 genes are expressed in pulmonary
inflammatory myofibroblastic tumor (inflammatory pseudotumor). Am J
Surg Pathol. 25:624–629. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gleason BC and Hornick JL: Inflammatory
myofibroblastic tumours: Where are we now? J Clin Pathol.
61:428–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aptel S, Gervaise A, Fairise A, Henrot P,
Leroux A, Guillemin F, Laurent V and Régent D: Abdominal
inflammatory myofibroblastic tumour. Diagn Interv Imaging.
93:410–412. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Butrynski JE, D'Adamo DR, Hornick JL, Dal
Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ,
Ramaiya N, et al: Crizotinib in ALK-rearranged inflammatory
myofibroblastic tumor. N Engl J Med. 363:1727–1733. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chavez C and Hoffman MA: Complete
remission of ALK-negative plasma cell granuloma (inflammatory
myofibroblastic tumor) of the lung induced by celecoxib: A case
report and review of the literature. Oncol Lett. 5:1672–1676.
2013.PubMed/NCBI
|