Introduction
Pancreatic cancer is one of the most lethal
neoplastic diseases with an overall 5-year survival rate of ~7% in
USA (1). It is the fourth leading
cause of cancer-related mortality in the USA, and its mortality
rate of ~7% has not decreased in Western countries over the past
few decades (1,2). Advanced stage pancreatic cancer with a
<5-year survival rate is more common in elderly individuals than
in younger patients (3,4). To better solve this health problem,
numerous studies are focusing on the molecular mechanism of
pancreatic cancer occurrence in order to develop novel treatment
strategies (5–8).
Non-coding RNAs (ncRNAs) were previously considered
to be transcriptional noise, however, they have more recently been
proven to have a pivotal role in cellular development and various
pathologies. Long ncRNAs (lncRNAs), which have a length of >200
nt, are a major group of ncRNAs that can be classified into five
categories: Sense, antisense, bidirectional, intronic and
intergenic (9). lncRNAs are involved
in almost every step of the life cycle of genes and regulate
diverse functions (10). Pseudogenes
have been emerging as a novel class of lncRNAs and have shown to be
important regulatory molecules involved in cancer (11). Welch et al identified 309
pseudogenes with significant differential expression in breast
cancer (12). Several transcribed
pseudogenes, such as phosphatase and tensin homolog pseudogene 1
(PTENP1), KRAS proto-oncogene, GTPase pseudogene 1
(KRASP1)and POU class 5 homeobox 1 (OCT4)-pg4, have
been known to promote tumor progression (13,14). In
addition, zinc finger family genes, such as zinc finger protein
(ZFP)185, have been revealed to have a strong inverse correlation
with prostate cancer progression (15). It has been reported that ZFP91
has a role in hematopoietic repopulating cells (16) and cell proliferation or anti-apoptosis
(17). Furthermore, ZFP91 mRNA
expression levels have been demonstrated to be upregulated in the
gastric tissue of patients with non-alcoholic steatohepatitis
(18). However, to the best of our
knowledge, no study to date has reported the role of ZFP91
pseudogenes (ZFP91-Ps) in pancreatic cancer and the cellular
function of ZFP91-P remains elusive.
The RNA interference (RNAi) technique, as a
loss-of-function assay, provides a novel approach for investigating
the molecular mechanism of cancer occurrence (19,20). In
the present study, ZFP91-P expression was specifically
knocked down in human pancreatic cancer cells by constructing
lentivirus-mediated short hairpin RNA (shRNA). Subsequently, cell
migration was evaluated using a scratch and Transwell migration
assay, cell proliferation was evaluated using an MTT assay, and the
molecular mechanism of ZFP91-P in pancreatic cancer
occurrence was determined.
Materials and methods
Cell culture
Human pancreatic cancer cell lines (BXPC-3 and
BXPC-3-H) were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The BXPC-3-H cell
line was generated in our laboratory by screening and verifying
high metastatic potential BXPC-3 cells by monoclonal and transwell
assays. The 293T human embryonic kidney cell line was purchased
from the Cell Bank of Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in Dulbecco's modified Eagle medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) with 10%
fetal bovine serum (FBS) and were maintained at 37°C in a 5%
CO2 humidified atmosphere.
Lentiviral vector construction
The shRNA sequence
(5′-GGGCTGCAGATCTAGTCTTCACTCGAGTGAAGACTAGATCTGCAGCCC-3′) was
designed to target human ZFP91-P gene (NR_024380; www.ncbi.nlm.nih.gov). The control shRNA sequence was:
5′-CTAGCCCGGCCAAGGAAGTGCAATTGCATACTCGAGTATGCAATTGCACTTCCTTGGTTTTTTGTTAAT-3′.
The shRNA sequences were designed by the present authors, and
synthesized by Genewiz, Inc. (Suzhou, China). The target and
control shRNAs were annealed, and ligated into the Nhe I/Pac I
(NEB, Ipswich, MA, USA)-linearized pFH-L vector (Shanghai Hollybio,
Shanghai, China), and termed pFH-L-shZFP91-P and pFH-L-shCon,
respectively.
Lentiviral packaging and cell
infection
The reconstructed plasmids were transfected into
293T cells with pCMVΔR8.92 and pVSVG-I helper plasmids (Shanghai
Hollybio) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After transfection of 72 h at 37°C in a
humidified incubator containing 5% CO2, supernatants containing
Lv-shZFP91-P or Lv-shCon were harvested by purification and
precipitation. Then, BXPC-3-H cells (50,000 cells/well) were seeded
in 6-well plates and transduced with Lv-shZFP91-P or Lv-shCon at a
multiplicity of infection of 20. After 4 days infection, cells were
observed under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan). As the pFH-L lentiviral vector carries a green
fluorescence protein (GFP) reporter, the infection efficiency was
determined by counting the number of GFP-positive cells compared to
total cells.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and
synthesized into cDNA with random primers, according to the
manufacturer's protocol (Fermantas; Thermo Fisher Scientific,
Inc.). The reaction system of reverse transcription was as follows:
2 µg Total RNA, 1 µl oligo dT (0.5 µg/µl), 4 µl M-MLV buffer, 1.25
µl dNTPs, 0.5 µl RNasin, 0.75 µl M-MLV-RTase and nuclease-free
water. RT-qPCR were performed on a BioRad Real-Time PCR
platform using the following components: 10 µl 2X SYBR Premix
Ex-Taq, 0.8 µl primers, 5 µl cDNA and 4.2 µl ddH2O. The
primers used were as follows: Forward, 5′-ACCTGGGGAACAAAGGCTAC-3′
and reverse, 5′-TAGGACCGAGAGGCAAAGAC-3′ for ZFP91-P;
forward, 5′-ATTCCACTTTGCGTTCAAGG-3′ and reverse,
5′-CTTCAGAGAGAGGAAAGCCGA-3′ for vimentin; forward,
5′-AGCTACTGCCTCCGGTCTTC-3′ and reverse; 5′-GTGGTCAACAGCCAGCTCA-3′
for β-catenin; and forward, 5′-GTGGACATCCGCAAAGAC-3′ and reverse,
5′-AAAGGGTGTAACGCAACTA-3′ for β-actin. The reaction conditions were
an initial denaturation step at 95°C for 5 sec and 40 cycles of
denaturation at 95°C for 5 sec followed by elongation at 60°C for
20 sec. Absorbance values obtained at the end of every elongation
step were used to analyze fluorescence. Experiments were repeated
at least three times and the comparative quantification cycle
(2−ΔΔCq) method (21) was
used to analyze the relative mRNA expression levels of
ZFP91-P.
MTT assay
Infected BXPC-3-H (3,000 cells/well) were reseeded
in 96-well plates 4 days after lentivirus infection. The number of
viable cells was measured at daily intervals (days 1, 2, 3, 4, and
5). At each time point, 10 µl of 5 mg/ml MTT was added to the
cells. After incubation for 4 h, 150 µl acidic isopropanol (5%
isopropanol, 10% SDS and 0.01 mol/l HCl) was added to dissolve the
formazan crystals. The absorbance of each well was recorded at a
wavelength of 595 nm using microplate reader.
Scratch migration assay
The scratch assay is a convenient and inexpensive
method to analyze cell migration in vitro (22). Infected BXPC-3-H cells (1.0×104
cells/well) were seeded on 96-well plate at 37°C in a humidified
incubator containing 5% CO2 and, when cells were >90% confluent,
a scratch was made using the tip of 10 μl sterile pipette. The
initial scratch area and the degree of healing after scratching was
observed under a binocular light microscope (Olympus CH-2, Olympus
Corp., Tokyo, Japan)after 12 and 48 h.
Transwell migration assay
A Transwell® chamber (Corning, Inc, New
York, NY, USA) was used to determine the migration ability of
BXPC-3-H cells infected with target and control shRNA over 96 h.
shZFP91-P and shCon cells (3.0×104 cells/well)
were seeded into the upper chamber in 100 µl medium containing 0.1%
FBS. Subsequently, 1 ml medium, containing 10% FBS as a
chemoattractant, was added to the lower chamber. Then the migration
system was placed in an incubator for 6 h at 37°C in 5%
CO2. Finally, the migrated cells were fixed with 4%
paraformaldehyde and stained with crystal violet (0.5%). Cell
numbers were counted under a binocular light microscope in five
random fields (magnification, ×100) per filter and detected by the
spectrometric absorbance at 570 nm.
Western analysis
BXPC-3-H cells were collected 72 h post-infection
with recombinant lentiviruses, washed in ice-cold
phosphate-buffered saline and lysed in 2X SDS sample buffer. Total
protein (30 µg) was separated by electrophoresis using 10% SDS-PAGE
at 80 V for 30 min followed by 150 V for 1 h. Subsequently, the
separated protein samples were transferred onto a PVDF
transmembrane (Millipore, Bedford, MA, USA) under 300 mA and
blocked in 5% non-fat milk with Tris-buffered saline and 0.05%
Tween 20 (Sigma, St. Louis, MO, USA) for 2 h at room temperature.
The membrane was then incubated with following primary antibodies:
Rabbit anti-vimentin (1:1,000 dilution; cat no. #5741; Cell
Signaling Technology, Danvers, MA, USA); rabbit anti-β-catenin
(1:1,000 dilution; cat no. #8480; Cell Signaling Technology) and
rabbit anti-GAPDH (1:500,000 dilution; cat no. 10494-1-AP;
Proteintech Group, Inc., Chicago, IL, USA) at 4°C overnight,
followed by incubation with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat no. SC-2054; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 2 h at room
temperature. An ECL kit (Amersham; GE Healthcare Life Sciences) was
used to visualize the blot.
Statistical analysis
All statistical data are expressed as mean ±
standard error of three independent experiments. Student's
t-test was used to compare differences between groups.
P<0.05 was considered to indicate a statistically significant.
Comparisons were carried out by Student's t-test and one-way ANOVA
analysis using SPSS 22.0 statistical software (SPSS, Inc., Chicago,
IL, USA).
Results
Expression of ZFP91-P is downregulated
by Lv-shZFP91-P in pancreatic cancer cells
The expression of ZFP91-P was determined in
two pancreatic cancer cell lines, BXPC-3 and BXPC-3-H. As shown in
Fig. 1A, ZFP91-P mRNA levels
were significantly upregulated in BXPC-3-H cells compared with in
BXPC-3 cells (P<0.001). Thus, only BXPC-3-H cells were used in
subsequent experiments. To clarify the biological function of
ZFP91-P in pancreatic cancer cells, a lentivirus-mediated
RNAi system was used to suppress ZFP91-P expression in
BXPC-3-H cells. Fluorescence microscopy showed that >80% of
cells were GFP-positive in the Lv-shCon and Lv-shZFP91-P
groups 3 days after lentivirus infection (Fig. 1B). Subsequently, RT-qPCR was used to
analyze the knockdown efficiency of ZFP91-P. As shown in
Fig. 1C, expression of ZFP91 was
significantly inhibited by 52.5% in the Lv-shZFP91-P-s1 group and
81.4% in the Lv-shZFP91-P-s2 group compared with the Lv-shCon
group, respectively (P=0.0006; P=0.0002).
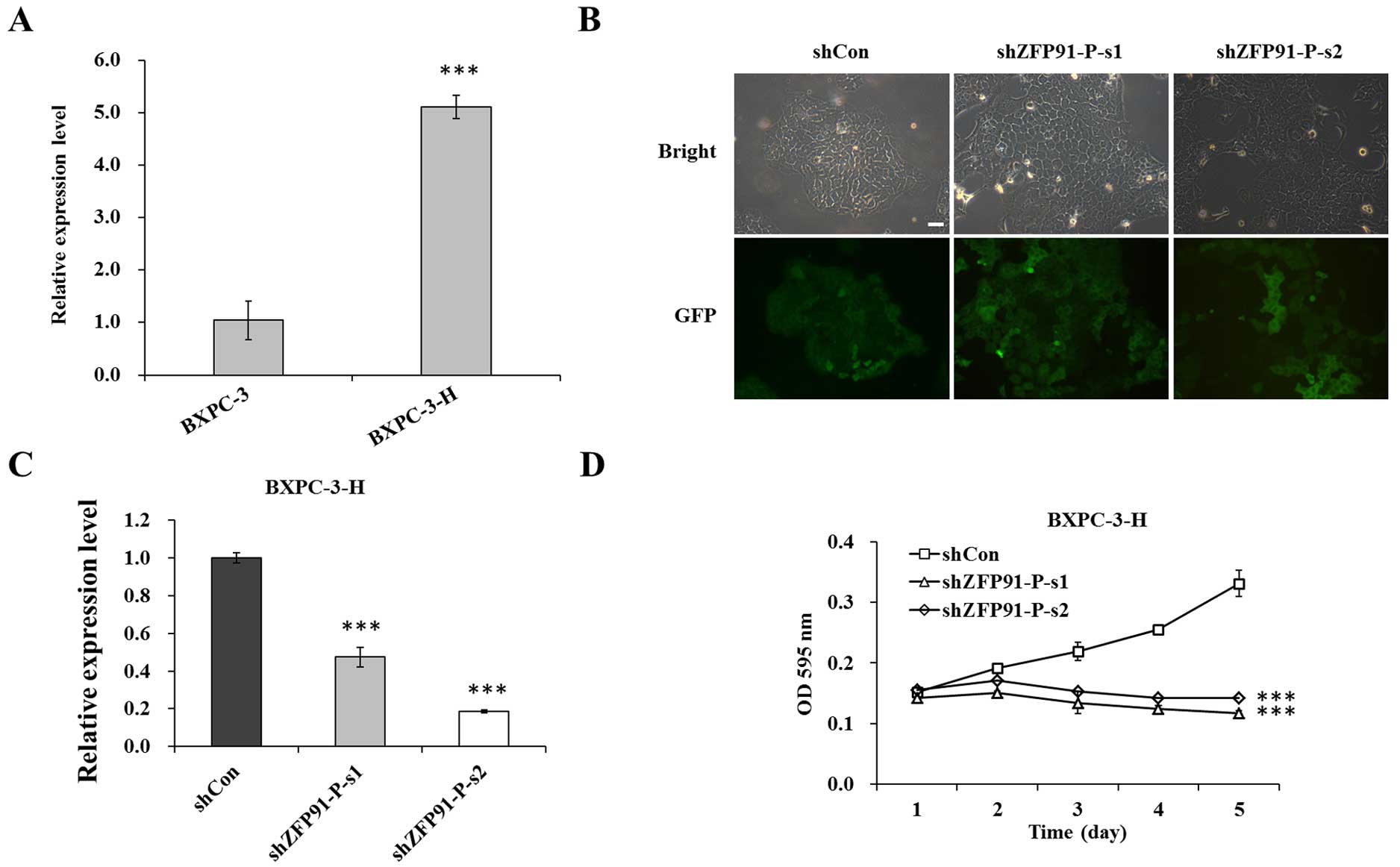 | Figure 1.Expression of ZFP91-P in
pancreatic cancer cells. (A) mRNA expression levels of
ZFP91-P in BXPC-3 and BXPC-3-H cells were determined by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). β-actin was used as an internal control gene.
***P<0.001 vs. BXPC-3. (B) Fluorescence photomicrographs of
BXPC-3-H cells infected by lentivirus. Multiplicity of infection,
20. Magnification, ×100. Scale bar, 10 µm. (C) RT-qPCR analysis of
ZFP91-P mRNA expression in BXPC-3-H cells with three
treatments. β-actin was used as an internal control gene.
***P<0.001 vs. shCon. (D) BXPC-3-H cell proliferation after
ZFP91-P silencing, as determined by an MTT assay. Cells with
three treatments including shCon, shZFP91-P-s1, and shZFP91-P-s2
groups. shCon, BXPC-3-H cells infected with control shRNA;
shZFP91-P-s1, BXPC-3-H cells infected with ZFP91-P shRNA 1;
shZFP91-P-s2, BXPC-3-H cells infected with ZFP91-P shRNA2.
***P<0.001 vs. shCon. Data are presented as mean ± standard
error of the mean. sh, shRNA; Con, control; ZFP91-P, zinc finger
protein 91 pseudogene; GFP, green fluorescent protein; OD, optical
density. |
Knockdown of ZFP91-P significantly
inhibits BXPC-3-H cell proliferation
To evaluate the effect of shZFP91-P on
pancreatic cancer cell proliferation, an MTT assay was conducted in
BXPC-3-H cells. The results showed that the growth curves of the
Lv-shZFP91-P group were significantly lower than those of
the Lv-shCon group (P<0.001; Fig. 1D), suggesting that knockdown of
ZFP91-P could significantly inhibit pancreatic cancer cell
proliferation.
Depletion of ZFP91-P significantly
inhibits the migratory ability of metastatic pancreatic cancer
cells
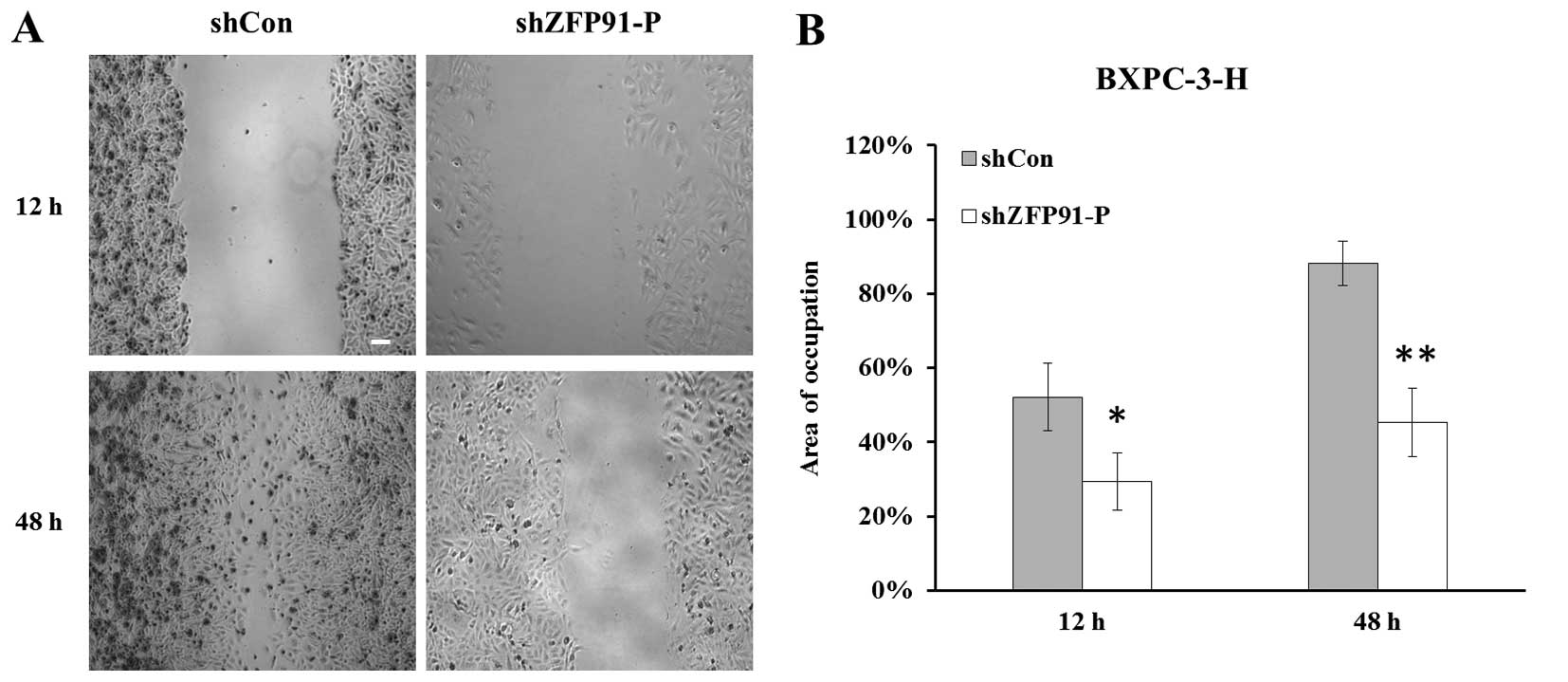
A scratch migration assay was performed to evaluate
the migration ability of BXPC-3-H cells in vitro. At the
selected time points of 12 and 48 h, cell migration distance was
observed under a microscope (magnification, ×100) (Fig. 2A). Cell migration ability was
significantly inhibited in the Lv-shZFP91-P group compared
with the Lv-shCon group, with control BXPC-3-H cells showing a
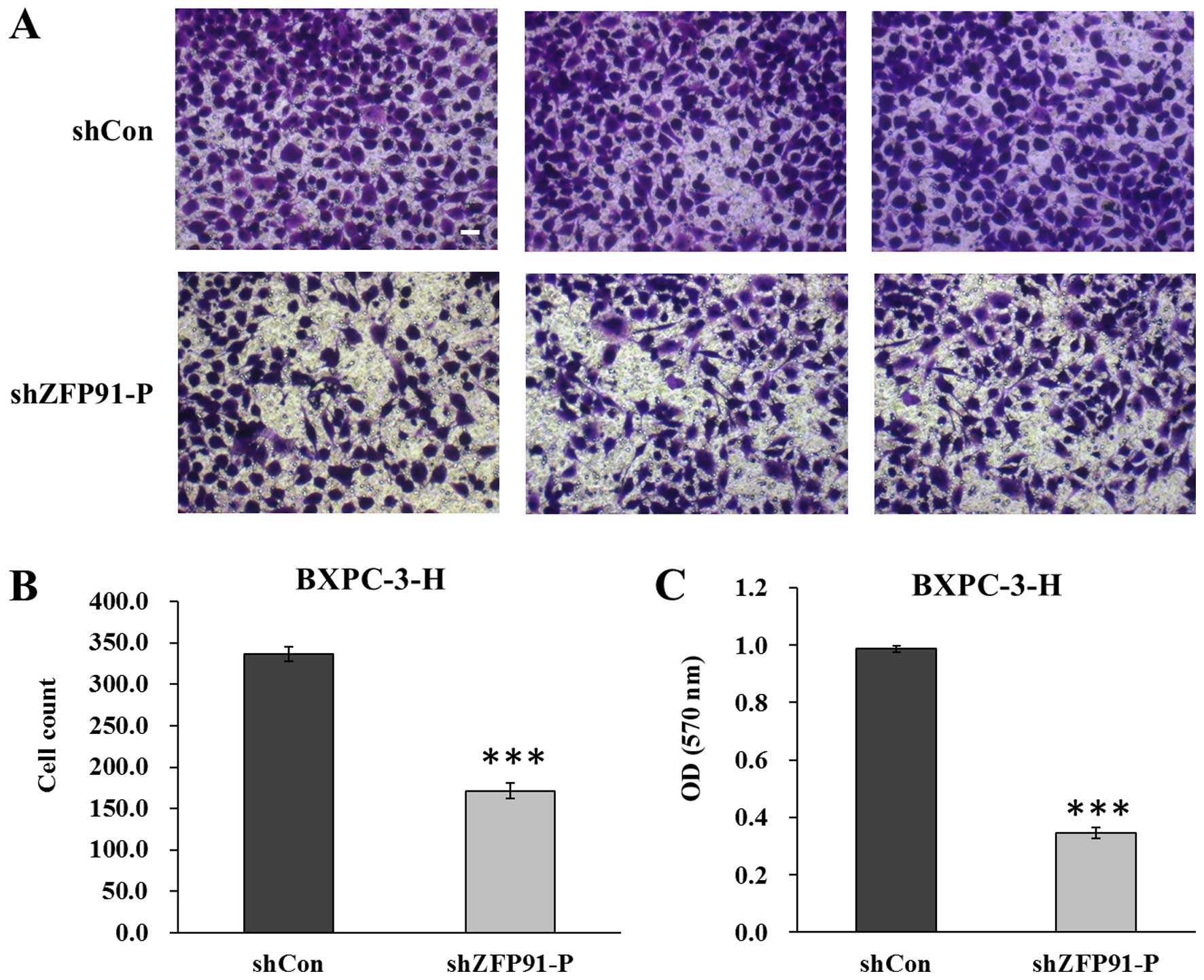
better ability to heal the scratch (P<0.05) (Fig. 2B). Subsequently, a Transwell assay was
used to determine the effect of ZFP91-P knockdown in
regulating pancreatic cancer cell migration (Fig. 3A). As indicated in Fig. 3B, significantly fewer cells in the
Lv-shZFP91-P group migrated to the lower surface of the
membrane, compared with cells in the Lv-shCon group (P<0.001).
In addition, the crystal violet staining intensity was
significantly lower in the Lv-shZFP91-P group than in the
Lv-shCon group (P<0.001) (Fig.
3C). These results suggest that ZFP91-P may have a key
role in pancreatic cancer metastasis.
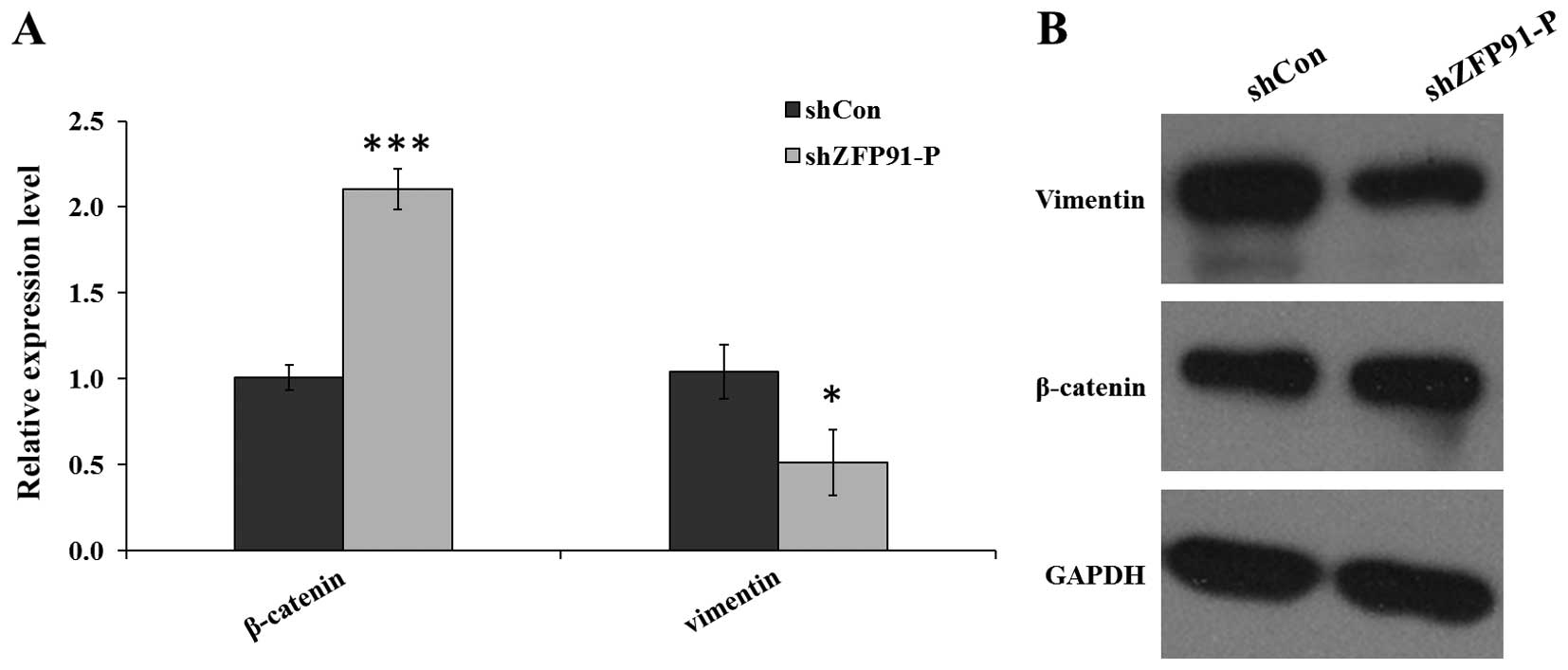
Downregulation of ZFP91-P elevates the
expression of β-catenin and inhibits the expression of vimentin via
epithelial-mesenchymal transition (EMT) signaling
To evaluate the regulatory role of ZFP91-P in
EMT signaling in human pancreatic cancer, the effect of
ZFP91-P silencing on the expression of β-catenin and
vimentin, which are critical for tumor invasion and metastasis, was
examined. RT-qPCR results showed that the mRNA expression levels of
β-catenin were significantly elevated (P<0.001) while vimentin
was significantly reduced (P<0.05) in the Lv-shZFP91-P
group compared with the Lv-shCon group in BXPC-3-H cells (Fig. 4A). Analysis of protein expression
levels revealed the same pattern (Fig.
4B).
Discussion
Pancreatic cancer is one of the most fatal
neoplastic diseases, as it is typically diagnosed at an advanced
stage. The majority of cases of pancreatic cancer are inoperable
and metastasized, therefore, it is difficult to overcome this
health problem. Thus far, numerous gene abnormalities have been
found to be involved in pancreatic cancer (23–25). Gene
therapy is designed to deliver a therapeutic gene into a target
site to regulate expression of the specific gene (26–28).
It has been reported that ZFP91 may have an
important role in cell proliferation (17) and may be involved in prostate cancer
(29). Several transcribed
pseudogenes, such as PTENP1, KRASP1 and OCT4-pg4,
have been known to promote tumor progression (13,14). These
findings prompted the present study to investigate the
ZFP91-P gene as a target site in pancreatic cancer
therapy.
In the present study, the association between
ZFP91-P and characteristics of pancreatic cancer were
initially examined. ZFP91-P showed high expression in
BXPC-3-H and BXPC-3 pancreatic cancer cells. Subsequently, the
expression of ZFP91-P was knocked down in BXPC-3-H cells
using a lentivirus-based shRNA system. The cell proliferation and
migration ability were impaired in the absence of ZFP91-P,
suggesting that ZFP91-P may promote the metastatic and
motility properties of pancreatic cancer cells.
EMT is a biological process that can enhance cell
migratory capacity and invasiveness. It is clear that EMT occurs in
three distinct biological settings, including organ development
(30), tumor growth and cancer
progression (31). Furthermore, it
has been reported that EMT acts as a major driver of tumor invasion
(32), with a crucial role in the
aggressiveness and invasion of pancreatic cancer (33). The expression of mesenchymal makers,
such as ZEB1, vimentin, Slug and Snail, are positive for majority
of cases of pancreatic cancer (34).
To further clarify these results, the present study identified the
signaling elements targeted by ZFP91-P for the promotion of
EMT in pancreatic cancer. ZFP91-P silencing appeared to
reverse EMT, as shown by increased expression of β-catenin and
decreased expression of vimentin. Vimentin, a major component of
the intermediate filament family, has been demonstrated to markedly
reverse EMT in pancreatic cancer cells, acting as a mesenchymal
marker (35). In addition, its
overexpression may result in accelerated cell growth, invasion and
poor prognosis in numerous types of cancer (36). The current results indicate that
ZFP91-P silencing can inhibit cell proliferation and
migration by reversing the EMT pathway. β-catenin not only has a
critical role in cell-cell adhesion by interacting with cadherins
at the plasma membrane, but is also involved in a signaling cascade
at the center of the Wnt signaling pathway. Several previous
studies have demonstrated that β-catenin is essential for normal
pancreatic organogenesis (37,38).
However, stable expression of β-catenin within the pancreatic
epithelium can give rise to the formation of tumors (39,40). By
contrast, concurrent activation of β-catenin and Kras prevents the
formation of pancreatic intraepithelial neoplasias (40). In addition, increased β-catenin
activity by overexpression of OCT-4 can inhibit cell
differentiation (40). Based on these
points, we propose that β-catenin may be concurrently activated
with certain genes during ZFP91-P silencing in pancreatic
cancer cells. However, further studies are required to investigated
the molecular mechanism of β-catenin in pancreatic cancer.
In conclusion, the current study indicates that
ZFP91-P is an important regulator of pancreatic cancer cell
migration and proliferation, and ZFP91-P silencing may
suppress the migration of pancreatic cancer cells by reversing EMT.
Therefore, ZFP91-P may be a useful target for gene therapy
in pancreatic cancer. However, the biological behavior of
ZFP91-P in pancreatic cancer needs to be thoroughly explored
in future studies.
Acknowledgements
The present study was funded by the Shanghai
Municipal Public Health Bureau (Shanghai, China), grant no.
20114178.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma J, Siegel R and Jemal A: Pancreatic
cancer death rates by race among US men and women, 1970–2009. J
Natl Cancer Inst. 105:1694–1700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rozenblum E, Schutte M and Goggins M:
Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res.
57:1731–1734. 1997.PubMed/NCBI
|
|
6
|
Luo J, Guo P and Matsuda K: Pancreatic
cancer cell-derived vascular endothelial growth factor is
biologically active in vitro and enhances tumorigenicity in vivo.
Int J Cancer. 92:361–369. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie K: Interleukin-8 and human cancer
biology. Cytokine Growth Factor Rev. 12:375–391. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamanaka Y, Friess H and Buchler M:
Overexpression of acidic and basic fibroblast growth factors in
human pancreatic cancer correlates with advanced tumor stage.
Cancer Res. 53:5289–5296. 1993.PubMed/NCBI
|
|
9
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grandér D and Johnsson P:
Pseudogene-expressed RNAs: Emerging roles in gene regulation and
disease. Curr Top Microbiol Immunol. 2015.
|
|
12
|
Welch JD, Baran-Gale J, Perou CM,
Sethupathy P and Prins JF: Pseudogenes transcribed in breast
invasive carcinoma show subtype-specific expression and ceRNA
potential. BMC Genomics. 16:1–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Poliseno L, Salmena L, Zhang J, Carver B,
Haveman WJ and Pandolfi PP: A coding-independent function of gene
and pseudogene mRNAs regulates tumour biology. Nature.
465:1033–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayashi H, Arao T, Togashi Y, Kato H,
Fujita Y, De Velasco MA, Kimura H, Matsumoto K, Tanaka K, Okamoto
I, et al: The OCT4 pseudogene POU5F1B is amplified and promotes an
aggressive phenotype in gastric cancer. Oncogene. 34:199–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanaja DK, Cheville JC, Iturria SJ and
Young CY: Transcriptional silencing of zinc finger protein 185
identified by expression profiling is associated with prostate
cancer progression. Cancer Res. 63:3877–3882. 2003.PubMed/NCBI
|
|
16
|
Kiem HP, Ironside C, Beard BC and
Trobridge GD: A retroviral vector common integration site between
leupaxin and zinc finger protein 91 (ZFP91) observed in baboon
hematopoietic repopulating cells. Exp Hematol. 38:819–822. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Unoki M, Okutsu J and Nakamura Y:
Identification of a novel human gene, ZFP91, involved in acute
myelogenous leukemia. Int J Oncol. 22:1217–1223. 2003.PubMed/NCBI
|
|
18
|
Al Dulaimi D: Recent advances in gastric
cancer. Gastroenterol Hepatol Bed Bench. 7:238–240. 2014.PubMed/NCBI
|
|
19
|
Kim DH, Behlke MA, Rose SD, Chang MS, Choi
S and Rossi JJ: Synthetic dsRNA Dicer substrates enhance RNAi
potency and efficacy. Nat Biotechnol. 23:222–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo W, Zhang Y, Chen T, Wang Y, Xue J,
Zhang Y, Xiao W, Mo X and Lu Y: Efficacy of RNAi targeting of
pyruvate kinase M2 combined with cisplatin in a lung cancer model.
J Cancer Res Clin Oncol. 137:65–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shain AH, Giacomini CP, Matsukuma K,
Karikari CA, Bashyam MD, Hidalgo M, Maitra A and Pollack JR:
Convergent structural alterations define SWItch/Sucrose
NonFermentable (SWI/SNF) chromatin remodeler as a central tumor
suppressive complex in pancreatic cancer. Proc Natl Acad Sci USA.
109:E252–E259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones S, Zhang X, Parsons DW, Lin JC,
Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, et
al: Core signaling pathways in human pancreatic cancers revealed by
global genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Efthimiou E, Crnogorac-Jurcevic T, Lemoine
NR and Brentnall TA: Inherited predisposition to pancreatic cancer.
Gut. 48:143–147. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang H: RNAi-mediated knockdown of target
genes: A promising strategy for pancreatic cancer research. Cancer
Gene Ther. 14:677–685. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gartel AL and Kandel ES: RNA interference
in cancer. Biomol Eng. 23:17–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gleich LL: Gene therapy for head and neck
cancer. Laryngoscope. 110:708–726. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paschke L, Rucinski M, Ziolkowska A,
Zemleduch T, Malendowicz W, Kwias Z and Malendowicz LK: ZFP91-a
newly described gene potentially involved in prostate pathology.
Pathol Oncol Res. 20:453–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeisberg M, Hanai J, Sugimoto H, Mammoto
T, Charytan D, Strutz F and Kalluri R: BMP-7 counteracts
TGF-beta1-induced epithelial-to-mesenchymal transition and reverses
chronic renal injury. Nat Med. 9:964–968. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YL, Dong FL, Yang J, Li Z, Zhi QM,
Zhao X, Yang Y, Li DC, Shen XC and Zhou J: Suppression of the
epidermal growth factor-like domain 7 and inhibition of migration
and epithelial-mesenchymal transition in human pancreatic cancer
PANC-1 cells. Asian Pac J Cancer Prev. 16:4065–4069. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murtaugh LC, Law AC, Dor Y and Melton DA:
Beta-catenin is essential for pancreatic acinar but not islet
development. Development. 132:4663–4674. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wells JM, Esni F, Boivin GP, Aronow BJ,
Stuart W, Combs C, Sklenka A, Leach SD and Lowy AM:
Wnt/beta-catenin signaling is required for development of the
exocrine pancreas. BMC Dev Biol. 7:42007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hochedlinger K, Yamada Y, Beard C and
Jaenisch R: Ectopic expression of Oct-4 blocks progenitor-cell
differentiation and causes dysplasia in epithelial tissues. Cell.
121:465–477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heiser PW, Cano DA, Landsman L, Kim GE,
Kench JG, Klimstra DS, Taketo MM, Biankin AV and Hebrok M:
Stabilization of beta-catenin induces pancreas tumor formation.
Gastroenterology. 135:1288–1300. 2008. View Article : Google Scholar : PubMed/NCBI
|