Introduction
The transdifferentiation of epithelial cells into
motile mesenchymal cells, a developmental program termed
epithelial-mesenchymal transition (EMT), has been demonstrated to
play a critical role in promoting tumor invasion and metastasis
(1,2).
The functional hallmark of the EMT program is to endow stationary
epithelial cells with migratory and invasive ability (2). Thus, the transition into a mesenchymal
phenotype is considered as a marker of metastatic potential in
numerous epithelium-derived carcinomas, including cervical cancer
(3,4).
The EMT program is triggered and controlled by
multiple signaling pathways that are activated in response to
extracellular stimuli (5,6). Among these, the transforming growth
factor (TGF)β signaling pathway has a predominant role in promoting
epithelial plasticity that may progress to EMT (6–8). However,
a large number of non-invasive tumor cells did not undergo
TGFβ-induced EMT in vitro (9),
suggesting that TGFβ may not be able to efficiently activate
signaling pathways in non-invasive tumor cells. It has been
previously observed that the effect of TGFβ1 may be enhanced by
other factors that could cooperate with TGFβ1 to induce sufficient
activation of multiple signaling pathways (10). While extracellular factors may
cooperate with TGFβ (7,8), it remains unclear whether the increased
expression of transcription factors in tumor cells could increase
the sensitivity of tumor cells to TGFβ stimulation and promote
TGFβ-induced EMT.
Sine oculis homeobox homolog 1 (SIX1) is a
transcription factor associated with development that is rarely
expressed in the majority of adult tissues (11,12).
However, overexpression of SIX1 has been observed in various
malignancies, and frequently correlates with poor clinical
prognosis (12–14). In cervical cancer, SIX1 is induced by
the E7 oncoprotein of human papillomavirus, and is closely
associated with tumorigenesis of cervical epithelium (15). Increased expression of SIX1 could
promote tumor growth by accelerating the cell cycle process
(15), and enhance the metastatic
potential of cervical cancer cells by upregulating the expression
of α5β1 integrin (16). Importantly,
the present authors recently reported that SIX1 could amplify TGFβ
signals, interacting with SMAD2/3 proteins to regulate gene
expression (17). Based on these
findings, the present study investigated whether SIX1 and TGFβ were
responsible for modulating the EMT program in cervical cancer. The
data obtained indicated that SIX1 could coordinate with TGFβ to
induce EMT, thus enhancing the metastatic capacity of cervical
cancer cells.
Materials and methods
Cells and transfection
The human cervical cancer cell line SiHa was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;
Hyclone; GE Healthcare Life Sciences, Beijing, China) supplemented
with 10% fetal bovine serum (Hyclone; GE Healthcare Life Sciences)
at 37°C and 5% CO2. Cells were transduced with
CMV-Fluc-IRES-RFP lentiviral particles (GeneChem, Co., Ltd.,
Shanghai China) expressing luciferase and red fluorescent protein
(RFP). Cells with stable transfection, which expressed RFP, were
isolated by fluorescence-activated cell sorting on a FACSCalibur
flow cytometer and analyzed with CellQuest Pro 6.0 software (BD
Biosciences, Franklin Lakes, NJ, USA).
SiHa cells were transfected with plasmid
pcDNA3.1-control (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), which harboured neomycin-resistance, or with pcDNA3.1-SIX1,
which was kindly donated by Dr Kong-Ming Wu (Department of Cancer
Biology, Thomas Jefferson University, Philadelphia, PA, USA), using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
The expression of TGFβ receptor 1 (TβR1) was knocked down using
puromycin-resistance small hairpin (sh)RNA lentiviral particles
(GeneChem, Co., Ltd.) targeting 5′-CTGTAATTCTGCTGTAATA-3′. As a
negative control (NC), shNC, a shRNA not targeting any known gene,
was used. Cells with stable transfection were selected with G418
and puromycin (Sigma-Aldrich, St. Louis, MO, USA). The transfected
cells were designated as SiHa-control, SiHa-SIX1, SiHa-SIX1-shNC
and SiHa-SIX1-shTβR1. To downregulate the expression of SMAD2 and
SMAD3, the corresponding small interfering (si)RNAs (Guangzhou
RiboBio Co., Ltd., Guangzhou, China) were transfected into tumor
cells using Lipofectamine 2000, according to the manufacturer's
protocol.
Bioinformatic analysis
The normalized RNA-sequencing data of cervical
cancer samples was publicly available from The Cancer Genome Atlas
(TCGA) database (https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp).
Differential gene expression based on SIX1 expression level was
determined as previously described (15) using Bioconductor edgeR software
package (18), freely available at
www.bioconductor.org. Differences were
considered to be statistically significant when false discovery
rate was <0.25.
Assay of gene expression by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
treated with Ambion Turbo DNA-free DNase (Thermo Fisher Scientific,
Inc.; Austin, TX, USA), according to the manufacturer's
instructions. RNA (2 µg) was used for first-strand cDNA synthesis
with reverse transcriptase (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. cDNA was amplified by
qPCR in a CFX96 Touch Real-Time PCR Detection system with CFX
Manager 3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and eukaryotic
translation elongation factor 1 alpha 1 (EEF1A1) were used as
reference genes, since this was reported to be the most reliable
gene combination in cervical cancer (19). The primer sequences for RT-qPCR were
as follows: GAPDH, sense 5′-GACAGTCAGCCGCATCTTCT-3′ and antisense
5′-TTAAAAGCAGCCCTGGTGAC-3′; EEF1A1, sense 5′-TGCGGTGGGTGTCATCAAA-3′
and antisense 5′-AGAGTGGGGTGGCAGGTATTG-3′; SIX1, sense
5′-CACCAGTTCTCGCCTCACA-3′ and antisense 5′-CACCCGATATTTGCCCAC-3′;
E-cadherin, sense 5′-AGGCCAAGCAGCAGTACATT-3′ and antisense
5′-ATTCACATCCAGCACATCCA-3′; and N-cadherin, sense
5′-CCATCAAGCCTGTGGGAATC-3′ and antisense 5′-GCAGATCGGACCGGATACTG-3′
(Beijing Qing Ke New Industrial Biotechnology Co., Ltd., Beijing,
China). The reaction included 5 ng cDNA and 10 pM of each primer.
The thermal cycling conditions for qPCR were as follows:
Denaturation at 95°C for 10 min; 40 cycles of denaturation at 95°C
for 10 sec, annealing at 60°C for 30 sec and extension at 72°C for
30 sec. Gene expression was quantified using the comparative Cq
method (19). The expression levels
of each mRNA were normalized to those of GAPDH and EEF1A1 mRNAs,
and expressed as n-fold difference relative to the control.
Immunohistochemistry
Cervical squamous cell carcinoma samples were
acquired from the Clinical Database and Biobank of Patients with
Gynecologic Neoplasms (ClinicalTrials.gov Identifier: NCT01267851), with
informed consent obtained from all subjects participating in the
study. The samples were obtained by surgery from cancer patients
without preoperative treatment between February 2007 and August
2009 at Tongji Hospital of Tongji Medical College (Wuhan, China).
Tumor samples were processed into tissue microarrays (TMAs) at
Shanghai Outdo Biotech Co., Ltd. (Shanghai, China). TMAs and tissue
sections were subjected to immunohistochemical analysis using the
Vectastain ABC kit (ZSGB-BIO, Beijing, China), according to the
manufacturer's protocol. Rabbit polyclonal SIX1 antibody (1:200;
catalogue no. HPA001893; Sigma-Aldrich) and rabbit monoclonal
E-cadherin antibody (1:500; catalogue no. EP700Y; Abcam, Cambridge,
UK) were used as primary antibodies. Low or high protein levels of
SIX1 in tissue were evaluated as previously described (17). Since E-cadherin is expressed on the
cellular membrane of normal cervical epithelium (3), positive membrane expression of
E-cadherin was classified as normal, while significant reduction of
membrane expression of E-cadherin was classified as a
low-E-cadherin level.
Western blot assay
Western blot assay was performed as previously
described (20). Briefly, the cells
were harvested and lysed using ice-cold RIPA lysis buffer [50 mM
Tris-HCl (pH 7.4), 150 mM sodium chloride, 1% Nonidet P-40 and 0.5%
sodium deoxycholate; Google Wuhan Biological Technology Co., Ltd.,
Wuhan, China]. Following centrifugation at 10,000 × g for 15 min at
4°C, the proteins in the supernatants were quantified using
Bradford method, separated on 10% SDS-PAGE gel (Google Wuhan
Biological Technology Co., Ltd.) and electrotransferred from the
gel to a nitrocellulose membrane (Merck & Co., Inc., Whitehouse
Station, NJ, USA). After blocking with 5% skimmed milk (Google
Wuhan Biological Technology Co., Ltd.) in phosphate-buffered
saline, the membranes were immunoblotted with the primary
antibodies at 4°C overnight. The primary antibodies used were as
follows: Anti-human SIX1 (1:1,100; catalogue no. HPA001893;
Sigma-Aldrich), anti-E-cadherin (1:10,000; catalogue no. ab40772;
Abcam), anti-N-Cadherin (1:1,000; catalogue no. ab18203; Abcam),
anti-SMAD2 (1:1,000; catalogue no. 5339; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-SMAD3 (1:1,000; catalogue no. 9523;
Cell Signaling Technology, Inc.) and β-actin (1:500; catalogue no.
ab8229; Abcam). Signals were detected using a SuperSignal West Pico
Chemiluminescent Substrate kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) with a ChemiDoc XRS+ machine (Bio-Rad
Laboratories, Inc.). Protein levels of β-actin were employed as
loading controls. The relative protein expression levels were
analyzed using Image Lab software (version 4.1; Bio-Rad
Laboratories, Inc.).
Migration assay
Migration assay was performed using a Transwell
chamber (Corning Incorporated, Corning, NY, USA). Tumor cells
(1×104 cells) were placed in the upper chamber.
Following 8-h incubation at 37°C in a humidified incubator with 5%
CO2, the cells that passed through the membrane were
fixed with 4% paraformaldehyde and stained with 0.1% crystal violet
(both from Google Wuhan Biological Technology Co., Ltd.) prior to
be counted under a microscope (BX53; Olympus Corporation Tokyo,
Japan). Cells from three randomly selected fields in each membrane
were counted, and the average number of cells per field was
calculated.
Animal studies
Female athymic nude (nu/nu) 4-week-old mice (n=40)
were purchased from Beijing HFK Bio-technology Co, Ltd. (Beijing,
China). The studies were approved by the Committee on the Ethics of
Animal Experiments of Tongji Medical College (Wuhan, China). Mice
were maintained in the accredited animal facility of Tongji Medical
College at 20–26°C in a 12-h light/dark cycle. A lymphatic
metastasis model of cervical cancer was used as previously
described (16). Briefly,
5×106 tumor cells in 50 µl serum-free DMEM (Boster
Biological Technology, Co., Ltd., Wuhan, China)/Matrigel (BD
Biosciences) at a 9:1 ratio were injected subcutaneously into the
claw pads of the mice. Tumor size (mm3) was measured and
calculated by the following formula: Volume = (width)2 ×
length / 2. When primary tumors reached ~150 mm3 in
size, metastases were tracked by optical imaging of luciferase
activity originating from tumor cells using the IVIS Spectrum
system (Caliper Life Sciences; PerkinElmer, Inc., Waltham, MA,
USA). Subsequently, when primary tumors reached ~250 mm3
in size, the mice were euthanized, and their popliteal and inguinal
lymph nodes were excised. Metastases of tumor cells in the lymph
nodes were confirmed by detection of tumor-expressed RFP under a
SZX16 dissecting microscope (Olympus Corporation). The incidence of
metastasis-positive mice in each group was calculated.
Statistical analysis
Each experiment was independently repeated ≥3 times.
SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The results were expressed as the mean ±
standard deviation, and interpreted by one-way analysis of variance
or χ2 test. P<0.05 was considered to indicate a
statistically significant difference.
Results
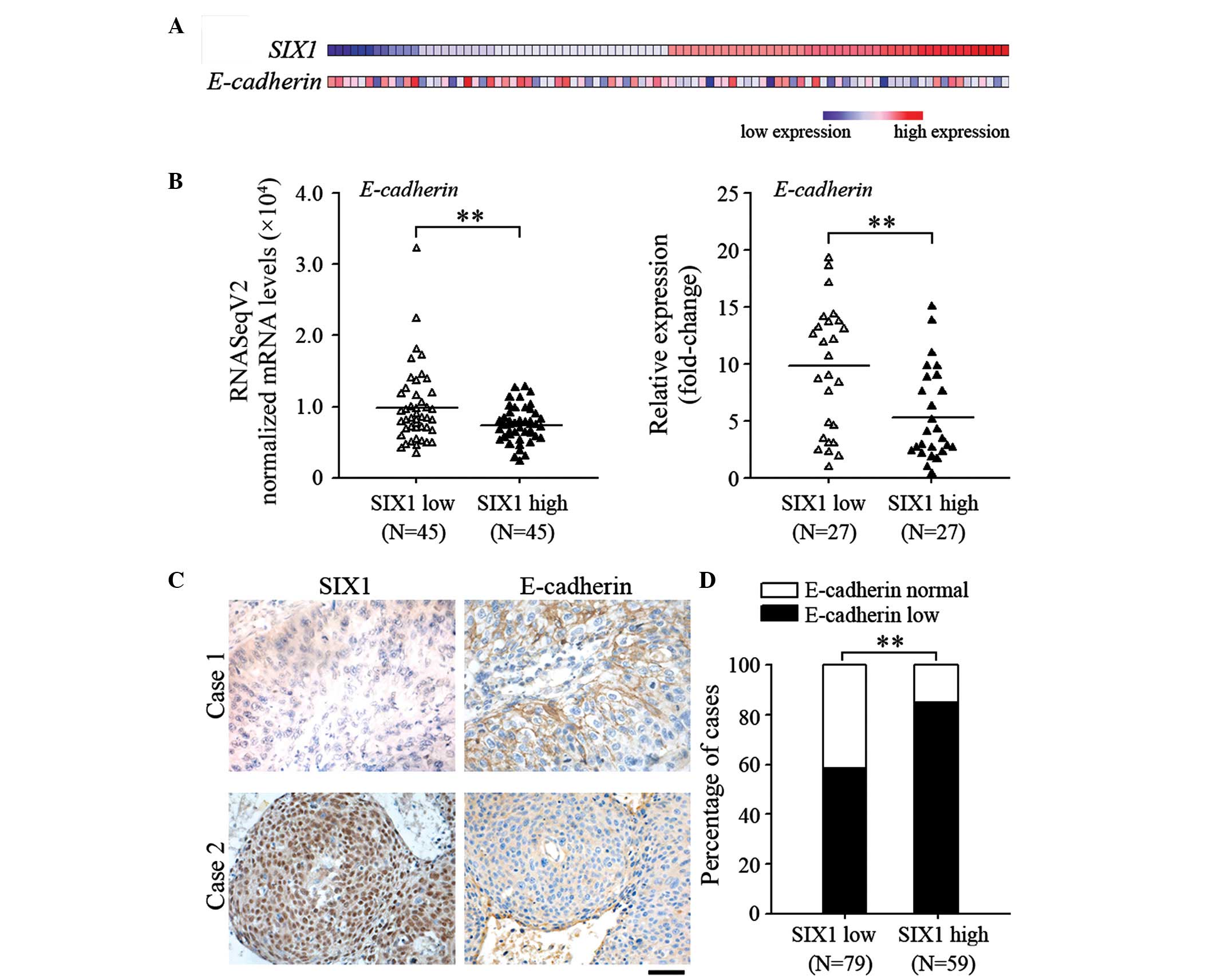
SIX1 expression is associated with EMT
of cervical cancer
To determine the effect of SIX1 on EMT of cervical
cancer, the effect of SIX1 on the expression of E-cadherin, which
is a common feature of EMT, was analyzed. The RNA-sequencing data
of cervical cancer specimens was obtained from the TCGA database.
The expression profiles of SIX1 and E-cadherin are shown in
Fig. 1A. The specimens were divided
into SIX1-low expression group and SIX1-high expression group.
Using Bioconductor edgeR software package, E-cadherin was
identified to be one of the significantly downregulated genes in
the SIX1-high expression group (false discovery rate=0.046;
Fig. 1B). These results were further
confirmed by quantifying the mRNA levels of SIX1 and E-cadherin in
the samples (P=0.003; Fig. 1C). A
significant decrease in the membrane expression levels of
E-cadherin was observed at the primary site of cervical cancer
samples that highly expressed SIX1 (P=0.001; Fig. 1D). These results suggest that
increased SIX1 expression is associated with mesenchymal phenotype
of cervical cancer.
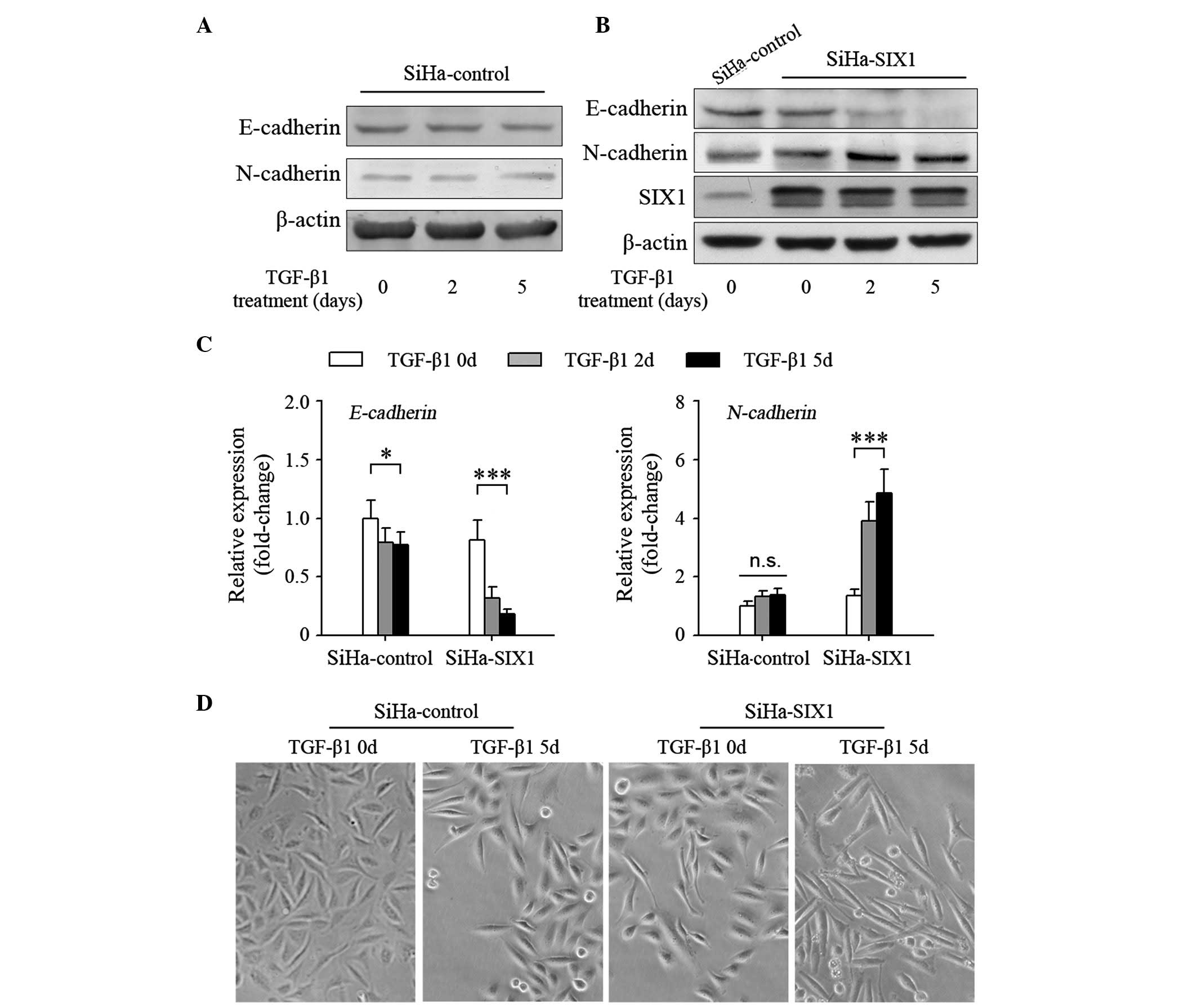
SIX1 enhances TGFβ-induced EMT in
cervical cancer cells
TGFβ is one of the most important inducers of EMT
(5,21). However, treatment with TGFβ1 only
suppressed slightly the expression of the epithelial marker
E-cadherin, and negligibly induced the expression of the
mesenchymal marker N-cadherin in SiHa cells, a cervical cancer cell
line with low expression levels of SIX1 (Fig. 2A) (17).
These results suggested that cervical cancer cells may not be
sensitive to TGFβ1. Overexpression of SIX1 in SiHa cells affected
slightly the expression of E-cadherin and N-cadherin (Fig. 2B). Notably, TGFβ1 could effectively
induce SIX1-expressing tumor cells to undergo EMT (Fig. 2B). Similar results were obtained when
E-cadherin (SiHa-control 0 vs. 5 days, P=0.016; SiHa-SIX1 0 vs. 5
days, P<0.001) and N-cadherin (SiHa-control 0 vs. 5 days,
P=0.071; SiHa-SIX1 0 vs. 5 days, P<0.001) expression was
quantified using RT-qPCR (Fig. 2C).
Correspondingly, TGFβ1-treated SiHa-SIX1 cells became more fusiform
with decreased connection between the cells than TGFβ1-treated
SiHa-control cells (Fig. 2D). These
results demonstrate that SIX1 coordinates with TGFβ to induce EMT
in cervical cancer cells.
TGFβ signals are required for
SIX1-induced EMT
The present authors previously reported that SIX1
could interact with SMAD proteins, which are the signal transducers
of TGFβ signaling (17). To further
clarify the role of TGFβ signals in inducing EMT on SIX1-expressing
tumor cells, the components of the TGFβ signaling pathway were
inhibited in the present study. When the expression of SMAD2 or
SMAD3 was knocked down, the TGFβ1-induced EMT process in SiHa-SIX1
cells was abrogated (Fig. 3A). The
same results were obtained when E-cadherin (si-SMAD2, P<0.001;
si-SMAD3, P<0.001) and N-cadherin (si-SMAD2, P<0.001;
si-SMAD3, P<0.001) expression was quantified using RT-qPCR
(Fig. 3B). Albeit not significantly
induced EMT in vitro, overexpression of SIX1 alone resulted
in a decrease in E-cadherin expression in vivo (P=0.002;
Fig. 3C). However, silencing TβR1
completely abrogated the effect of SIX1 (P<0.001; Fig. 3C), further indicating that TGFβ
signals are required for SIX1 to induce EMT.
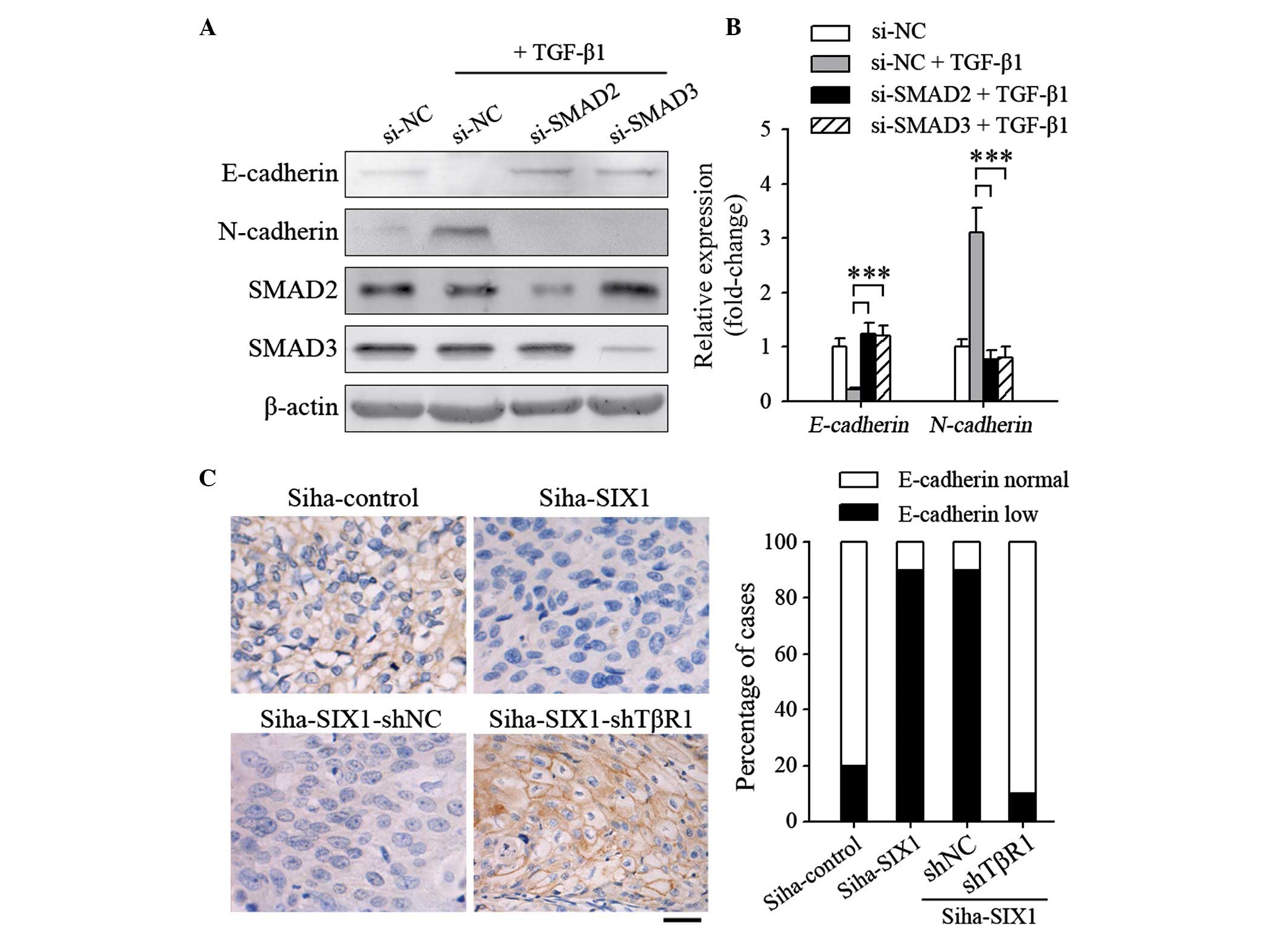 | Figure 3.TGFβ signals are necessary for SIX1 to
induce epithelial-mesenchymal transition in cervical cancer. (A and
B) At 24 h post-transfection with the indicated small interfering
RNAs, the cells were stimulated with TGFβ1 (1 ng/ml) for 5 days.
The expression levels of E-cadherin and N-cadherin were detected by
(A) western blotting or (B) reverse transcription-quantitative
polymerase chain reaction. (C) SiHa cells were injected
subcutaneously into the claw pads of mice to form primary tumors,
and the protein expression levels of E-cadherin in the tumors were
analyzed by immunohistochemical analysis. Left panel,
representative images of SIX1 and E-cadherin staining of primary
tumors (magnification, ×400; scale bar, 50 µm). Right panel,
percentage of cases (n=10/group) with different intensity of
E-cadherin staining. ***P<0.001. TGF; transforming growth
factor; SIX1, sine oculis homeobox homolog 1; NC, negative control;
sh, small hairpin; si, small interfering; TβR1, TGFβ receptor
1. |
SIX1 coordinates with TGFβ to enhance
the metastatic capacity of cervical cancer cells
EMT is associated with the metastatic capacity of
tumor cells (5,6). Based on the above results, the effect of
SIX1 and TGFβ1 on the metastatic phenotype of cervical cancer cells
was further investigated. The migration of SiHa cells was increased
following TGFβ1 stimulation. TGFβ1 was more efficient than the
control in promoting the migratory capacity of tumor cells if the
cells expressed high levels of SIX1 (SiHa-control, P=0.003;
SiHa-SIX1, P<0.001; Fig. 4A). The
metastatic capacity of tumor cells was further tested using a
previously described lymphatic metastasis model (Fig. 4B). The results indicated that
SIX1-mediated promotion of lymph node metastasis was completely
abolished by silencing TβR1 (SiHa-SIX1 shNC vs. SiHa-SIX1 shTβR1,
P=2.61E-4; Fig. 4C). These results
demonstrate that SIX1 and TGFβ signals coordinate to enhance the
metastatic capacity of cervical cancer cells.
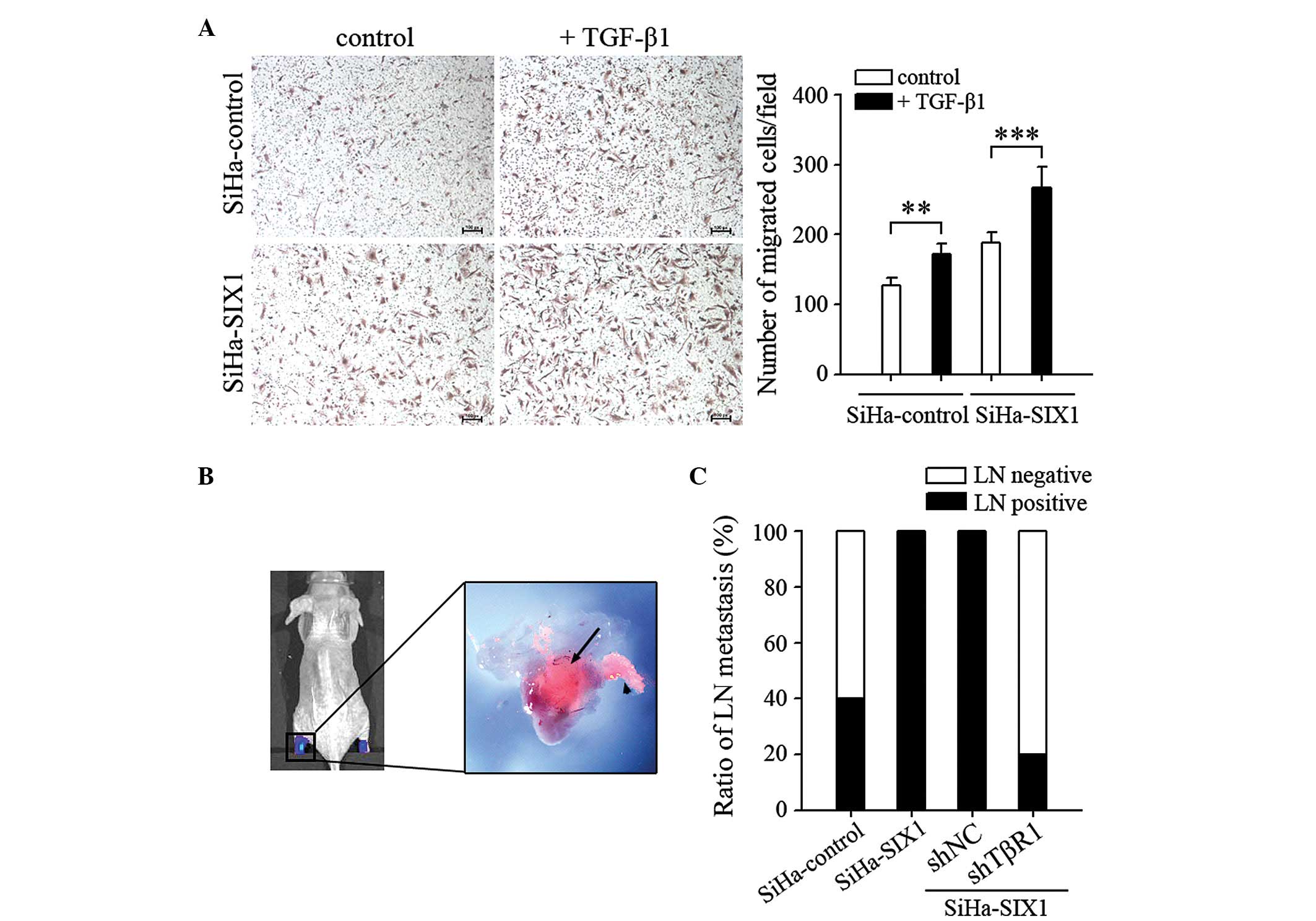 | Figure 4.SIX1 coordinates with TGFβ signals to
enhance the metastatic capacity of cervical cancer cells. (A)
Migration assay. SiHa cells, untransfected or transfected with
SIX1-expressing vector, were untreated or treated with TGFβ1 (1
ng/ml) for 5 days, and their migration ability was assessed (scale
bar, 100 µm). (B) In vivo bioluminescence and fluorescence
representative images of lymphatic metastasis in mice. Lymph nodes
in the popliteal and inguinal regions of the mice were detected by
bioluminescence and fluorescence signals, corresponding to
luciferase activity and red fluorescent protein expression,
respectively. The black arrow indicates tumor cells inside the
lymph node, while the arrowhead indicates a tumor-invaded lymphatic
vessel. (C) The ratios of lymph node metastasis were calculated
(n=10/group). **P<0.01; ***P<0.001. SIX1, sine oculis
homeobox homolog 1; TGF; transforming growth factor; LN, lymph
node; TβR1, TGFβ receptor 1; NC, negative control; sh, small
hairpin. |
Discussion
An increasing number of studies have provided strong
evidence for the critical role of the EMT program in tumor
progression and metastasis (5,6). Although
TGFβ is regarded as one of the most important inducers of EMT,
several non-invasive tumor cells were not able to undergo
TGFβ-induced EMT in vitro (9).
The present data suggest that the expression levels of the
transcription factor SIX1 could determine the sensitivity of tumor
cells to TGFβ stimulation.
In the present study, the expression of SIX1 was
negatively correlated with that of the epithelial marker E-cadherin
in cervical cancer. Consistently, increased SIX1 expression in
tumor cells could sufficiently reduce E-cadherin expression and
promote lymph node metastasis in vivo. However, increased
SIX1 expression in SiHa cells could not significantly induce EMT
in vitro, suggesting that SIX1 could not efficiently induce
EMT by itself. The present data demonstrated that the main
contribution of SIX1 to EMT was to increase the sensitivity of
tumor cells to TGFβ stimulation. Increased expression of SIX1 could
promote EMT of cervical cancer cells in a TGFβ-dependent manner.
Based on the overexpression of SIX1, TGFβ induced more remarkable
changes in the transition of phenotype than the SiHa-control group.
Therefore, SIX1 and TGFβ coordinated to promote cell motility and
tumor metastasis of cervical cancer.
Previous studies have described the roles of
TGFβ-activated SMADs in EMT. Increased expression of SMAD2 or SMAD3
was reported to induce EMT, whereas expression of dominant negative
versions of SMAD2 or SMAD3 blocked TGFβ-induced EMT (7,22).
Additionally, TGFβ activates SMAD and non-SMAD signals, including
Rho-like guanosine triphosphatases,
phosphatidylinositol-4,5-bisphosphate 3-kinase and
mitogen-activated protein kinase signaling pathways, which also
contribute to EMT (6,23,24). In
the present study, TGFβ could efficiently induce EMT in
vitro and lymph node metastasis in vivo if tumor cells
expressed high levels of SIX1. However, knocking down the
expression of SMAD2 or SMAD3 could completely block the EMT process
induced by SIX1 and TGFβ. In a previous study, the present authors
demonstrated that SIX1 was involved in the SMAD2/3 protein complex
and could enhance TGFβ-SMAD signaling (17). Therefore, enhancing TGFβ-SMAD
signaling is an important mechanism by which SIX1 promotes EMT and
lymphatic metastasis in cervical carcinoma.
The signal transduction of TGFβ forms a complex
network, involving the activation of SMAD and non-SMAD signals, and
the crosstalk among various signal transduction pathways such as
wingless-related integration site and Notch signaling pathways
provides context-dependent effects during EMT (25,26).
Therefore, context-specific outcomes may be generated according to
the availability of repressors/activators, distinct intensity or
duration of SMAD/non-SMAD signaling activity or changes in the
levels of interacting protein partners (7). The present data revealed that TGFβ1
stimulation was not able to induce EMT in SiHa cells, which are
cervical cancer cells with low expression of SIX1 (17). By contrast, when SiHa-SIX1 cells were
stimulated with TGFβ1, significant changes were observed in the
expression of EMT markers, cell morphology and metastatic capacity
of the cells. In addition, higher expression levels of E-cadherin
in SIX1-low clinical samples and in vivo experiments also
suggested that environmental factors such as TGFβ were ineffective
in inducing EMT in a SIX1-low context. Therefore, the coordination
of SIX1 and TGFβ may be critical for inducing EMT in cervical
carcinoma.
In summary, the present study revealed the important
role of the coordination of SIX1 and TGFβ in inducing EMT in
cervical cancer. SIX1 induced EMT of cervical cancer cells in a
TGFβ-dependent manner, and increased SIX1 expression significantly
enhanced the TGFβ-induced transition of mesenchymal phenotype,
indicating that SIX1 and TGFβ coordinate to promote the EMT program
and the metastatic capacity of cervical cancer cells. In
conclusion, the present results demonstrate that SIX1 plays a
crucial role in the progression and metastasis of cervical cancer,
and suggest that targeting SIX1 and/or TGFβ signaling may be a
valuable strategy in cancer therapy.
Acknowledgements
The present authors would like to thank Dr Qi-Lin Ao
and Dr Shuang Guo (Department of Pathology, Union Hospital, Tongji
Medical College, Huazhong University of Science and Technology,
Wuhan, China) for reviewing the histology data. The present study
was supported by the National Science Foundation of China (Beijing,
China; grant nos. 81072135 and 81372801).
References
|
1
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Wang W, Wang W, Yang R, Wang T, Su
T, Weng D, Tao T, Li W, Ma D and Wang S: Correlation of TWIST2
up-regulation and epithelial-mesenchymal transition during
tumorigenesis and progression of cervical carcinoma. Gynecol Oncol.
124:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou XM, Zhang H and Han X: Role of
epithelial to mesenchymal transition proteins in gynecological
cancers: Pathological and therapeutic perspectives. Tumour Biol.
35:9523–9530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu J, Lamouille S and Derynck R:
TGF-beta-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brown KA, Aakre ME, Gorska AE, Price JO,
Eltom SE, Pietenpol JA and Moses HL: Induction by transforming
growth factor-beta1 of epithelial to mesenchymal transition is a
rare event in vitro. Breast Cancer Res. 6:R215–R231. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng XX, Liu M, Yan W, Zhou ZZ, Xia YJ, Tu
W, Li PY and Tian DA: β3 integrin promotes
TGF-β1/H2O2/HOCl-mediated induction of
metastatic phenotype of hepatocellular carcinoma cells by enhancing
TGF-β1 signaling. PLoS One. 8:e798572013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ford HL, Landesman-Bollag E, Dacwag CS,
Stukenberg PT, Pardee AB and Seldin DC: Cell cycle-regulated
phosphorylation of the human SIX1 homeodomain protein. J Biol Chem.
275:22245–22254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Micalizzi DS, Christensen KL, Jedlicka P,
Coletta RD, Barón AE, Harrell JC, Horwitz KB, Billheimer D,
Heichman KA, Welm AL, et al: The Six1 homeoprotein induces human
mammary carcinoma cells to undergo epithelial-mesenchymal
transition and metastasis in mice through increasing TGF-beta
signaling. J Clin Invest. 119:2678–2690. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ng KT, Man K, Sun CK, Lee TK, Poon RT, Lo
CM and Fan ST: Clinicopathological significance of homeoprotein
Six1 in hepatocellular carcinoma. Br J Cancer. 95:1050–1055. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Behbakht K, Qamar L, Aldridge CS, Coletta
RD, Davidson SA, Thorburn A and Ford HL: Six1 overexpression in
ovarian carcinoma causes resistance to TRAIL-mediated apoptosis and
is associated with poor survival. Cancer Res. 67:3036–3042. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu D, Zhang XX, Xi BX, Wan DY, Li L, Zhou
J, Wang W, Ma D, Wang H and Gao QL: Sine oculis homeobox homolog 1
promotes DNA replication and cell proliferation in cervical cancer.
Int J Oncol. 45:1232–1240. 2014.PubMed/NCBI
|
|
16
|
Liu D, Zhang XX, Wan DY, Xi BX, Ma D, Wang
H and Gao QL: Sine oculis homeobox homolo 1 promotes α5β1-mediated
invasive migration and metastasis of cervical cancer cells. Biochem
Biophys Res Commun. 446:549–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu D, Li L, Zhang XX, Wan DY, Xi BX, Hu
Z, Ding WC, Zhu D, Wang XL, Wang W, et al: SIX1 promotes tumor
lymphangiogenesis by coordinating TGFβ signals that increase
expression of VEGF-C. Cancer Res. 74:5597–5607. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen Y, Li Y, Ye F, Wang F, Lu W and Xie
X: Identification of suitable reference genes for measurement of
gene expression in human cervical tissues. Anal Biochem.
405:224–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang C, Zhou L, Li S, Wei J, Wang W, Zhou
T, Liao S, Weng D, Deng D, Weng Y, et al: C4orf7 contributes to
ovarian cancer metastasis by promoting cancer cell migration and
invasion. Oncol Rep. 24:933–939. 2010.PubMed/NCBI
|
|
21
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-beta and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamouille S and Derynck R: Emergence of
the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin
axis in transforming growth factor-β-induced epithelial-mesenchymal
transition. Cells Tissues Organs. 193:8–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Timmerman LA, Grego-Bessa J, Raya A,
Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick
F, Izpisúa-Belmonte JC and de la Pompa JL: Notch promotes
epithelial-mesenchymal transition during cardiac development and
oncogenic transformation. Genes Dev. 18:99–115. 2004. View Article : Google Scholar : PubMed/NCBI
|