Introduction
Male breast cancer is a rare disease accounting for
<1% of all diagnosed breast cases (1). The only ethnic group with a higher than
average incidence of male breast cancer is Jewish men, regardless
of their residence (2). A previous
study determined a strong family predisposition for the disease,
with an odds ratio of 3.3 (3). It is
recognized that breast cancer (BRCA) mutations may be
detected in a significant portion of male breast cancer cases
(primarily BRCA2) and the National Comprehensive Cancer
Network guidelines recommend genetic testing for such patients
(4). It has been suggested that male
breast cancer is associated with a poorer prognosis compared with
females, largely due to delays in diagnosis, lack of clinical care
pathways and associated comorbidities due to the advanced age of
patients (5). A recent comparative
analysis of menopausal status in female patients with breast cancer
concluded poorer outcomes for male patients with breast cancer
compared with postmenopausal females and similar outcomes to
premenopausal female patients with breast cancer (6). Recommendations for treatment in males
are extrapolated from extensive evidence in women. Due to lack of
breast tissue, simple mastectomy is commonly the preferred surgical
procedure. Sentinel lymph node biopsy is feasible and is being
performed more frequently in cases of male breast cancer. Given the
absence of terminal lobules in the male breast, the disease is
typically of a ductal type and hormone receptor-positive. Human
epidermal growth factor receptor 2 (HER2)-positivity is rare, with
a recent large multinational study reporting that this
characteristic was observed in <5% of cases (7). In the same study, a trend of improvement
in overall survival (OS) was also observed over time in men with
breast cancer (7).
The aim of the present study was to examine the
clinical and pathological characteristics, treatment patterns and
outcomes of a series of male patients with breast cancer, who were
consecutively treated at a single institution from January 1995 to
December 2015.
Patients and methods
Patients
The current study retrospectively reviewed medical
records of all male patients diagnosed with breast cancer over the
last 20 years from January 1995 to December 2015 at the Oncology
Department, Faculty Hospital Trenčín (Trenčín, Slovakia), which
serves a population of >600,000 inhabitants. All 21 cases
identified in this time period were included in the present study.
Individual patient records were reviewed and the relevant data was
obtained, including stage at presentation [based on the 7th edition
of the Tumor-Node-Metastasis (TNM) classification system] (8), histological classification and tumor
biomarkers, and details regarding treatment and follow-up. The
study was approved by the Ethics Committee of Faculty Hospital
Trenčín.
Statistical analysis
Statistical analyses were performed using MedCalc
15.2 software (www.medcalc.org). The patients'
characteristics were summarized using the median (range) for
continuous variables and frequency (percentage) for categorical
variables. Survival analysis was used only for patients treated
with curative intent. Kaplan-Meier estimates were performed to
calculate OS rate from the date of initial diagnosis to the date of
last follow-up or mortality, and disease-free survival (DFS) was
calculated from the date of initial diagnosis to the date of
progression or mortality.
Results
Patient clinicopathological
characteristics
All 21 male patients were diagnosed with invasive
breast cancer during the study period, which was <1% of the
number of female cases diagnosed at the same time. The median age
was 65.6 years (range, 51–86) and the majority of patients were
over 60 years at diagnosis (71%). As presented in Table I, the primary tumors in 8 patients
were staged as pT1, whilst 6 patients were staged as pT2 and 7 as
pT4. No patients presented with pT3 or Tis primary tumors. Axillary
lymph node involvement was present in 11 patients (52%) and 1
patient presented with metastatic disease (cytologically verified
malignant pleural effusion) at the time of diagnosis. According to
the TNM staging system (8), 6
patients were diagnosed with stage I disease, while 8 patients were
stage II, 6 patients stage III and 1 patient stage IV. With regards
to histological subtypes, invasive ductal cancer (invasive
carcinomas of no special type) was present in 19 patients and
invasive lobular cancer was present in 2 patients. Hormone receptor
status was examined in 18 patients, of which 15 (83%) were estrogen
receptor (ER)-positive and 13 (72%) were progesterone receptor
(PR)-positive. Only 1 out of 11 patients examined for HER2 status
was positive (9%). Only 2 patients were triple
(ER/PR/HER2)-negative. A total of 12 patients had grade 2 disease,
while 5 patients had grade 1 and 4 had grade 3. BRCA testing
was not performed on the majority of patients as reimbursement for
male breast cancer was not in place until recent years. All 6
patients analyzed had normal karyotypes without any BRCA
mutations.
 | Table I.Clinicopathological variables of male
patients with invasive breast cancer. |
Table I.
Clinicopathological variables of male
patients with invasive breast cancer.
| Variable | n (%) |
|---|
| Tumor size |
| pT1 | 8 (38) |
| pT2 | 6 (29) |
| pT3 | 0(0) |
| pT4 | 7 (33) |
| Nodal status |
| N0 | 10 (48) |
| N+ | 11 (52) |
| Metastases |
| Yes | 1 (5) |
| No | 20 (95) |
| Grade |
| 1 | 5
(24) |
| 2 | 12 (57) |
| 3 | 4
(19) |
| Histology |
| Ductal
carcinoma in situ | 0 (0) |
| Invasive
ductal carcinoma | 19 (90) |
| Invasive
lobular carcinoma | 2
(10) |
| Estrogen
receptor |
|
Positive | 15 (83) |
|
Negative | 3
(17) |
| N/A | 3 (−) |
| Progesterone
receptor |
|
Positive | 13 (72) |
|
Negative | 5
(28) |
| N/A | 3 (−) |
| HER2 |
|
Positive | 1
(9) |
|
Negative | 10
(91) |
| N/A | 10 (−) |
Treatment regimens
As presented in Table
II, a total of 20 patients underwent primary surgical treatment
(simple mastectomy) for localized breast cancer. The axilla was
surgically staged in all 20 patients undergoing primary surgical
treatment; axillary lymph node dissection was performed in 19
patients and sentinel node biopsy was performed in 1 patient.
Adjuvant chemotherapy was administered to 9 patients. Indications
for adjuvant systemic treatment were extrapolated from breast
cancer in women. Anthracycline-based chemotherapy [adriamycin and
cyclophosphamide, or 5-fluorouracil (5-FU), adriamycin and
cyclophosphamide] was administered to 5 patients, sequential
chemotherapy based on anthracycline and taxane/trastuzumab was
administered to 1 patient and cyclophosphamide, methotrexate and
5-FU was given to 3 patients (who had been diagnosed >10 years
previously). Adjuvant radiotherapy was administered to 12 patients,
with a total dose of 50 Gy (25 fractions over 5 weeks) with/without
additional boosts to the tumor bed. Finally, 12 patients were
treated with 5-year-long adjuvant hormonal treatment, consisting of
9 patients who were administered tamoxifen alone and 3 patients who
underwent sequential hormonal therapy consisting of tamoxifen and
aromatase inhibitor. One HER2-positive patient was administered
adjuvant trastuzumab concurrently with adjuvant taxane treatment.
Adjuvant trastuzumab was continued for 1 year. Progression to stage
IV occurred in 3 patients, who were initially stage II or III,
following curative treatment. All of these patients were treated
with palliative chemotherapy (anthracycline-based, taxane-based or
capecitabine-based) and 2 also received additional hormonal therapy
(tamoxifen). Palliative radiotherapy was delivered to 1 patient for
skeletal metastases. One patient with initially metastatic disease
to the pleura was treated with palliative chemotherapy
(anthracycline-based) followed by tamoxifen, and upon progression
was treated with taxane and aromatase inhibitors, in addition to
luteinizing hormone-releasing hormone (LHRH) agonist.
 | Table II.Treatment details of male patients
with invasive breast cancer. |
Table II.
Treatment details of male patients
with invasive breast cancer.
| Treatment | n (%) |
|---|
| Surgery (n=21) |
|
Mastectomy | 20
(95) |
| Wide
local excision | 0
(0) |
| No breast
surgery | 1
(5) |
| Axillary
lymph node dissection | 19
(90) |
| Sentinel
lymph node biopsy | 1
(5) |
| No
surgery in axilla | 1
(5) |
| Adjuvant radiotherapy
(n=20) |
| Yes | 12 (60) |
| No | 8
(40) |
| Adjuvant chemotherapy
(n=20) |
| Yes | 9
(45) |
|
Anthracyclin-based | 5
(56) |
|
Antracyclin
followed by taxane/trastuzumab | 1
(11) |
|
CMF | 3
(33) |
| No | 3
(55) |
| Adjuvant hormonal
therapy (n=14) |
| Yes | 12 (86) |
|
Tamoxifen | 9
(75) |
|
Aromatase
inhibitors | 0 (0) |
|
Tamoxifen followed
by aromatase inhibitors | 3
(25) |
| No | 2
(14) |
Patient outcomes
A total of 4 patients succumbed during the median
follow-up period of 4.5 years. Two patients succumbed due to
progression of the disease and the cause of mortality regarding the
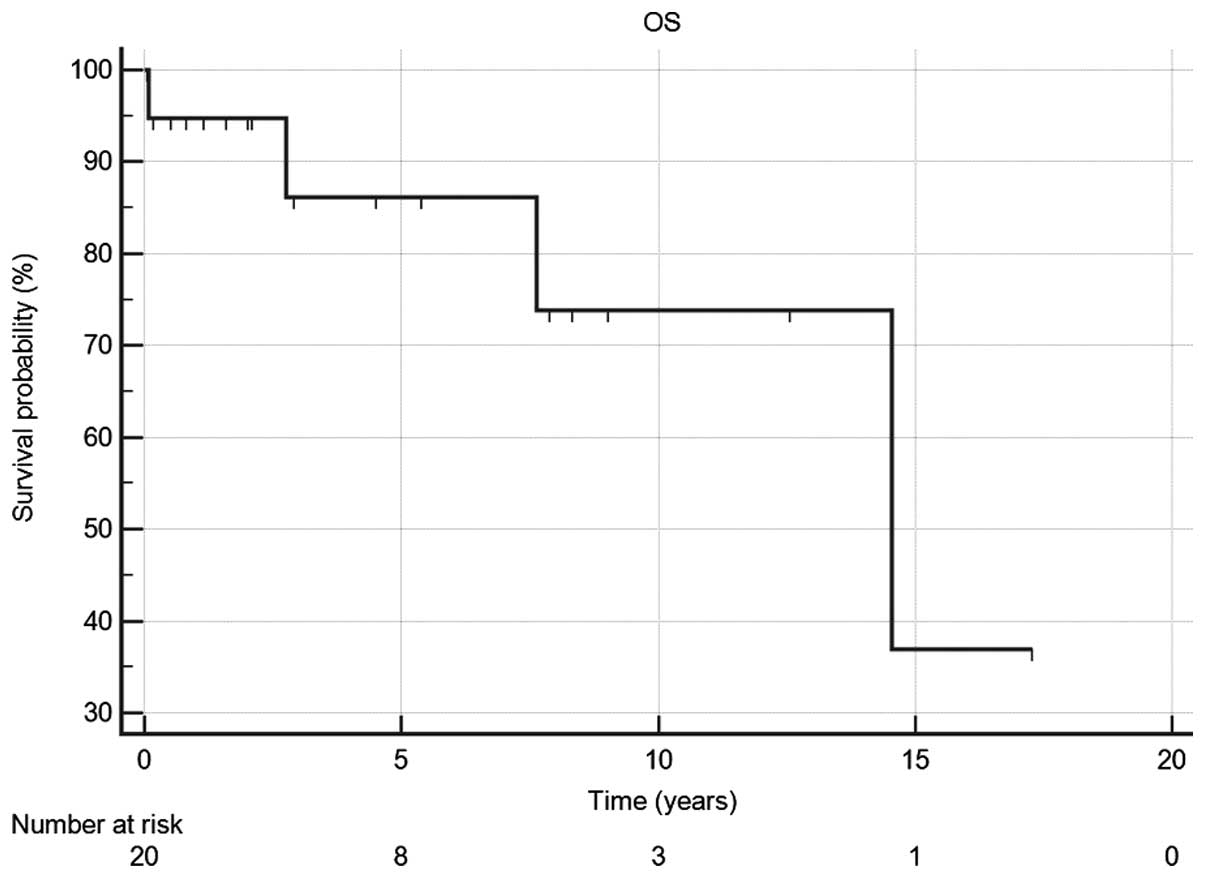
other two patients is unknown. The 5- and 10-year OS rates were 87
and 74%, respectively (Fig. 1). The
estimated median DFS for the same population was 9.5 years (95% CI,
6.2–14.6) and 5-year DFS rate was 86%.
Discussion
Male patients with breast cancer tend to present
later in life than females and the incidence of male breast cancer,
although much lower than in females, is rising (1). Incidence of male breast cancer in
Slovakia is ~0.8% of all female breast cancer cases, which is
similar to that of Western Europe (9). Furthermore, 83% of the patients in the
current study were hormone receptor-positive, while only 9% of
patients were HER2-positive, which is in line with a recent
retrospective study by Cardoso et al (7). Given this fact, the luminal A molecular
subtype was predominantly observed in 58% of patients in the same
study (7). Additional comparative
biomarker studies of matched cohorts confirm a higher predominance
of ER-positivity and luminal A subtypes in men (10). This knowledge is important for
practicing oncologists, as all newly diagnosed males with breast
cancer with negative hormonal status should undergo a thorough
pathological review so that the opportunity for hormonal treatment
is not missed. The present study observed only 2 cases of lobular
carcinoma, which is a similar number reported by a previous
population-based study (11).
Surgical treatment of male breast cancer usually includes
mastectomy, as cosmetic outcomes are typically less important than
in women. Experience with sentinel lymph node biopsy in men is
gaining wider acceptance among breast surgeons and is a recommended
method of axillary staging of early cancer in women. The rarity of
male breast cancer precludes prospective studies that may
potentially guide the development of optimal treatment regimens.
The decisions made regarding treatment in the present study were
based on general guidelines for breast cancer in women, as
recommended by the European Society for Medical Oncology (12). Limitations of the current study
include the small number of patients diagnosed and treated over
several years for a relatively rare disease. The majority of
patients with positive axillary lymph nodes and/or pT4 primary
tumor stage received adjuvant chemotherapy and chest-wall
radiotherapy. All but 2 patients with ER-positive tumors received
hormone treatment, initially with tamoxifen. One patient without
hormonal treatment arrived from another institution and the other
is currently finishing adjuvant chemotherapy. Tamoxifen remains the
standard hormonal treatment in adjuvant and metastatic settings for
male breast cancer (13). Hormonal
treatment is preferred in palliative setting as outcomes are
comparable with chemotherapy with less toxicity in women (14). The present study incorporated hormonal
manipulation in the treatment strategies for all eligible
palliative male breast cancer patients and there was no requirement
for rapid tumor response.
Incorporation of aromatase inhibitors, a standard
treatment approach in women, remains a contentious issue in male
breast cancer. Aromatase, an estrogen synthetase, is a key enzyme
in estrogen synthesis, which is expressed not only in the gonads
and peripheral tissues (including fat and bone), but also
intratumorally (15). In men, 60% of
circulating estradiol (E2) is produced by the testes either
directly or by conversion from testicular androgens (16). The remaining portion is derived from
the conversion of adrenal androgens (16). Thus, the production of testicular and
adrenal androgens, together with activity of aromatase enzyme,
determine plasma estrogen concentration in men. LHRH agonists (also
known as gonadotropin-releasing hormone agonists) are used in
premenopausal women with breast cancer to reduce the production of
ovarian estrogen and in castrate-sensitive prostate cancer in
males. LHRH administration yields reduction of testosterone and
estrogen produced by the testes (17). Combination with aromatase inhibitors
may further block estrogen production via aromatization.
Prospective and retrospective measurements of plasma E2,
follicle-stimulating hormone (FSH) and testosterone in men treated
with only aromatase inhibitors have been reported (18,19), with
results demonstrating that E2 levels decreased with treatment, but
testosterone and FSH levels increased. These measurements confirmed
a positive feedback loop between increasing FSH and testosterone.
As testosterone is a substrate for aromatase, further E2
suppression may be achieved with LHRH analogs. A recent pooled
analysis of 105 male breast cancer cases demonstrated the
effectiveness of the aforementioned strategy (a 3-fold increase in
clinical benefit rate) (20), and in
a smaller analysis of 60 patients, a survival benefit was observed
following use of the combination strategy (21). However, a phase II trial investigating
this further was closed prematurely due to poor accrual (22). As this preclinical data suggests that
complete hormonal suppression with aromatase inhibitors in men is
not possible, they are (as monotherapy) not considered standard
treatment in male breast cancer. All in all, tamoxifen continues to
be administered as a standard adjuvant treatment in cases of male
breast cancer, but dual treatment with LHRH agonists (or
orchiectomy) and aromatase inhibitors is gaining ground in
metastatic male breast cancer (23).
Adjuvant chemotherapy is recommended for high-risk female patients
with breast cancer, and by extrapolation, its effectiveness is
assumed to apply to the treatment of males with the disease. In the
present study, out of 14 patients with stage II or III disease, 5
patients did not receive adjuvant chemotherapy due to advanced age,
patient or physician choice, and comorbidities. The 5- (87%) and
10-year (74%) OS rates and the 5-year DFS rate estimated in the
current study are consistent with previously published studies
(13,24–26).
Due to the rarity of male breast cancer and largely
anecdotal evidence from case reports, the International Male Breast
Cancer Program was created under auspices of the European
Organization for Research and Treatment of Cancer and the
Translational Breast Cancer Research Consortium. In this program, a
prospective cohort study analyzing a collection of DNA and tumor
samples is planned. This program aims to advance the treatment of
male breast cancer in a similar manner to how therapies have
progressed for women with the disease. Male breast cancer is a rare
disease and there are no prospective studies to inform current
practice. As the disease has numerous similarities to breast cancer
in women, treatment strategies are extrapolated from female breast
cancer. Over the last 20 years, significant progress has been made
in physician detection, investigation and treatment of male breast
cancer. The encouraging results of the present study may give
patients and professionals confidence in future treatment of the
disease, which was once considered to have a grave prognosis.
In conclusion, the present study analyzed patients
with breast cancer, who had been diagnosed over the last 20 years
at the Oncology Department, Faculty Hospital Trenčín. In line with
the published literature, the majority of patients presented with
invasive ductal carcinoma with positive hormone receptors. HER2
positivity was rare. In order to improve knowledge regarding the
treatment of patients with this rare cancer, the cases reviewed in
the present study should be entered into a prospectively maintained
international database. In addition, clinical trials designed for
this patient population are required. Alternatively, patients with
male breast cancer may benefit from being enrolled into selected
clinical trials originally designed for women, where extensive
networks of clinical research units already successfully
collaborate.
Glossary
Abbreviations
Abbreviations:
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
E2
|
estradiol
|
|
LHRH
|
luteinizing hormone-releasing hormone
agonist
|
|
FSH
|
follicle-stimulating hormone
|
References
|
1
|
Anderson WF, Jatoi I, Tse J and Rosenberg
PS: Male breast cancer: A population-based comparison with female
breast cancer. J Clin Oncol. 28:232–239. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mabuchi K, Bross DS and Kessler II: Risk
factors for male breast cancer. J Natl Cancer Inst. 74:371–375.
1985.PubMed/NCBI
|
|
3
|
Ewertz M, Holmberg L, Tretli S, Pedersen
BV and Kristensen A: Risk factors for male breast cancer-a
case-control study from Scandinavia. Acta Oncol. 40:467–471. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
NCCN: Genetic/Familial High-Risk
Assessment: Breast and Ovarian Cancer. 2015.http://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdfAccessed.
January 1–2016
|
|
5
|
Müller AC, Gani C, Rehm HM, Eckert F,
Bamberg M, Hehr T and Weinmann M: Are there biologic differences
between male and female breast cancer explaining inferior outcome
of men despite equal stage and treatment?! Strahlenther Onkol.
188:782–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu XF, Yang HJ, Yu Y, Zou DH and Miao LL:
A prognostic analysis of male breast cancer (MBC) compared with
post-menopausal female breast cancer (FBC). PLoS One.
10:e01366702015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cardoso F, Bartlett J and Slaets L:
Abstract S6-05: Characterization of male breast cancer: First
results of the EORTC10085/TBCRC/BIG/NABCG International Male BC
Program. Cancer Res. 75:S6–05. 2015. View Article : Google Scholar
|
|
8
|
Sobin LH, Gospodarowicz MK and Wittekind
C: The TNM Classification of Malignant Tumours (7th).
Wiley-Blackwell. Geneva: 2009.
|
|
9
|
Diba CS: Cancer incidence in the Slovak
Republic. National Health Information Center. Bratislava:
2009.http://www.nczisk.sk/Documents/publikacie/analyticke/incidencia_zhubnych_nadorov_2009.pdfAccessed.
January 2–2016
|
|
10
|
Shaaban AM, Ball GR, Brannan RA, Cserni G,
Di Benedetto A, Dent J, Fulford L, Honarpisheh H, Jordan L, Jones
JL, et al: A comparative biomarker study of 514 matched cases of
male and female breast cancer reveals gender-specific biological
differences. Breast Cancer Res Treat. 133:949–958. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leone JP, Leone J, Zwenger AO, Iturbe J,
Vallejo CT and Leone BA: Prognostic significance of tumor subtypes
in male breast cancer: A population-based study. Breast Cancer Res
Treat. 152:601–609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Senkus E, Kyriakides S, Ohno S,
Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S and Cardoso
F: ESMO Guidelines Committee: Primary breast cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 26(Suppl 5): v8–v30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bradley KL, Tyldesley S, Speers CH, Woods
R and Villa D: Contemporary systemic therapy for male breast
cancer. Clin Breast Cancer. 14:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Partridge AH, Rumble RB, Carey LA, Come
SE, Davidson NE, Di Leo A, Gralow J, Hortobagyi GN, Moy B, Yee D,
et al: Chemotherapy and targeted therapy for women with human
epidermal growth factor receptor 2-negative (or unknown) advanced
breast cancer: American Society of Clinical Oncology Clinical
Practice Guideline. J Clin Oncol. 32:3307–3329. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasano H and Harada N: Intratumoral
aromatase in human breast, endometrial and ovarian malignancies.
Endocr Rev. 19:593–607. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Ronde W and de Jong FH: Aromatase
inhibitors in men: Effects and therapeutic options. Reprod Biol
Endocrinol. 9:932011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nordman IC and Dalley DN: Breast cancer in
men: Should aromatase inhibitors become first-line hormonal
treatment? Breast J. 14:562–569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doyen J, Italiano A, Largillier R, Ferrero
JM, Fontana X and Thyss A: Aromatase inhibition in male breast
cancer patients: Biological and clinical implications. Ann Oncol.
21:1243–1245. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bighin C, Lunardi G, Del Mastro L, Marroni
P, Taveggia P, Levaggi A, Giraudi S and Pronzato P: Estrone
sulphate, FSH and testosterone levels in two male breast cancer
patients treated with aromatase inhibitors. Oncologist.
15:1270–1272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zagouri F, Sergentanis TN, Azim HA Jr,
Chrysikos D, Dimopoulos MA and Psaltopoulou T: Aromatase inhibitors
in male breast cancer: A pooled analysis. Breast Cancer Res Treat.
151:141–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clinical trials: S0511, goserelin and
anastrozole in treating men with recurrent or metastatic breast
cancer. https://clinicaltrials.gov/ct2/show/NCT00217659Accessed.
January 25–2016
|
|
22
|
Di Lauro L, Pizzuti L, Barba M, Sergi D,
Sperduti I, Mottolese M, Amoreo CA, Belli F, Vici P, Speirs V, et
al: Role of gonadotropin-releasing hormone analogues in metastatic
male breast cancer: Results from a pooled analysis. J Hematol
Oncol. 8:532015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Korde LA, Zujewski JA, Kamin L, Giordano
S, Domchek S, Anderson WF, Bartlett JM, Gelmon K, Nahleh Z, Bergh
J, et al: Multidisciplinary meeting on male breast cancer: Summary
and research recommendations. J Clin Oncol. 28:2114–2122. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Ieso PB, Potter AE, Le H, Luke C and
Gowda RV: Male breast cancer: A 30-year experience in south
Australia. Asia Pac J Clin Oncol. 8:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iorfida M, Bagnardi V, Rotmensz N, Munzone
E, Bonanni B, Viale G, Pruneri G, Mazza M, Cardillo A, Veronesi P,
et al: Outcome of male breast cancer: A matched single-institution
series. Clin Breast Cancer. 14:371–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masci G, Caruso M, Caruso F, Salvini P,
Carnaghi C, Giordano L, Miserocchi V, Losurdo A, Zuradelli M,
Torrisi R, et al: Clinicopathological and immunohistochemical
characteristics in male breast cancer: A retrospective case series.
Oncologist. 20:586–592. 2015. View Article : Google Scholar : PubMed/NCBI
|