Introduction
Breast cancer is one of the most prevalent cancers
and is a major public health concern among women. The most recent
statistics for Japan document >76,000 cases per year (1), with a mortality rate of >13,000 per
year (2). Breast cancer care and
research has improved early detection and treatment. However, even
after apparently successful localized treatments, there are
long-term risks of recurrence and metastasis (3).
Breast cancer is currently classified into subtypes
based on immunohistochemical (IHC) classification (4,5). Subtypes
are defined by clinicopathological biomarkers, including estrogen
receptor (ER), progesterone receptors (PgR), human epidermal growth
factor receptor 2 (HER2) and Ki-67; these biomarkers can indicate
the optimum treatment and play a key role in breast cancer
treatment to improve prognosis (6,7). To
facilitate the treatment of cancer, it is important to exploit new
biomarkers that can improve the reliability of prognosis
prediction, and to develop targeted therapies for breast cancer
patients.
Recently, specific kinesin motor proteins have been
studied as key proteins that regulate mitotic events and potential
targets of therapy (8–11). KIF18A is a member of the kinesin
protein superfamily, which is associated with the molecular motor
proteins that use ATP hydrolysis to produce force and movement
along microtubules (12–17). The basic mechanism for these
activities is not well understood. However, recent studies have
demonstrated that KIF18A regulates chromosome congression (18) and suppresses kinetochore movements to
control mitotic chromosome alignment (19). Chromosome congression relies on the
presence of KIF18A, indicating that this motile microtubule
depolymerase has a key role in the dynamics of kinetochore
microtubules driving chromosome alignment in the pre-anaphase state
of the human cell cycle (18–20).
It has been reported that KIF18A is involved in
breast, colorectal and hepatocellular cancers, and
cholangiocarcinoma (21–24). However, there is essentially no
information regarding the clinical relevance of KIF18A in breast
cancer patients. Therefore, the present study investigated the
clinicopathological significance of KIF18A expression in human
breast cancer.
Materials and methods
Patients and specimen collection
Primary breast cancer and paired normal tissues were
obtained from the operative specimens of 144 patients, who
underwent radical surgery at the Medical Hospital of the Tokyo
Medical and Dental University (Tokyo, Japan) between January 2004
and December 2006. Normal tissue was collected from at least 1 cm
away from the primary breast cancer site and was
histopathologically identified as normal by a pathologist at the
Tokyo Medical and Dental University. Written informed consent was
obtained from all patients according to the guidelines approved by
the Institutional Research Board. Patients ranged in age from 26 to
91 years, with a mean age of 54 years. Patients were excluded if
they received anti-hormone therapy, chemotherapy or radiotherapy
prior to surgery. Patients with non-invasive breast cancer were
also not included in the study. All patients were closely followed
up after surgery at regular 3- to 6-month intervals, and the total
follow-up periods ranged from 3 months to 7.6 years, with a median
of 5.9 years. Following surgery, all patients were clearly
classified into a category of breast cancer based on the
clinicopathological criteria described by the Japanese Society for
Breast Cancer (25). All data,
including age, tumor size, lymphatic invasion, vascular invasion,
nuclear grade, lymph node metastasis, ER status, PgR status, HER2
score, recurrence, pathological histology and clinical stage, were
obtained from the clinical and pathological records. HER2 status
was scored using the HER2 expression criteria (26). For primary tumors with a HER2 score of
2+, the IHC results were additionally validated with fluorescence
in situ hybridization. All patients were treated with
anti-hormonal therapy, chemotherapy and/or radiotherapy subsequent
to surgery, according to breast cancer treatment guidelines in
Japan, which were based on St. Gallen International Breast Cancer
Conference and National Comprehensive Cancer Network
recommendations (27).
Immunohistochemistry
IHC studies for KIF18A expression were performed on
formalin-fixed, paraffin-embedded breast cancer tissues. After
tissue sections (4 µm) were deparaffinized over 5 × 10 min
incubations in xylene, the sections were rehydrated, and antigen
retrieval was performed by incubation in antigen activation liquid
(pH 9.0) in a microwave processor at 98°C for 30 min. Endogenous
peroxidase activity was blocked using a solution of 3% hydrogen
peroxide in absolute methanol for 15 min. A polyclonal rabbit
anti-KIF18A antibody (catalog no., A301-080A; Bethyl Labotatories,
Montgomery, TX, USA) was applied at a dilution of 1:150 and
incubated overnight at 4°C. The Histofine Simple Stain MAX PO kit
(Nichirei Corporation, Tokyo, Japan) was used according to the
manufacturer's instructions to block non-specific binding and to
detect bound primary antibody. The color was developed by
diaminobenzidine (Nichirei Corporation) for 10 min at room
temperature. The sections were then counterstained with Mayer's
hematoxylin. Negative control staining was conducted by
substituting non-immune rabbit serum and phosphate-buffered saline
for primary antibodies. An expert pathologist selected and
evaluated five representative fields at 400x magnification from
each slide to produce digital photographs for measuring of the
staining intensity of KIF18A. KIF18A expression was quantified
using ImageJ software (Java 1.6.0_30 (32-bit); http://rsb.info.nih.gov/ij/index.html),
which calculates the staining intensity of an area by converting
RGB pixels to brightness values (11). The 5 most typically stained areas
within the tumor were selected for calculating the average
value.
Statistical analysis
Data from the IHC analysis was analyzed by JMP 10
software for Windows (version 10.0.1; SAS Institute, Cary, NC,
USA). Differences between groups were determined using the
χ2 test and analysis of variance. Disease-free survival
(DFS) rates were calculated actuarially according to the
Kaplan-Meier analysis and compared with the generalized log-rank
test. Variables with a value of P<0.05 in univariate analysis
were used in a subsequent multivariate analysis using nominal
logistic regression. P<0.05 was considered as to indicate
statistical significance.
Results
KIF18A expression in breast cancer
tissues
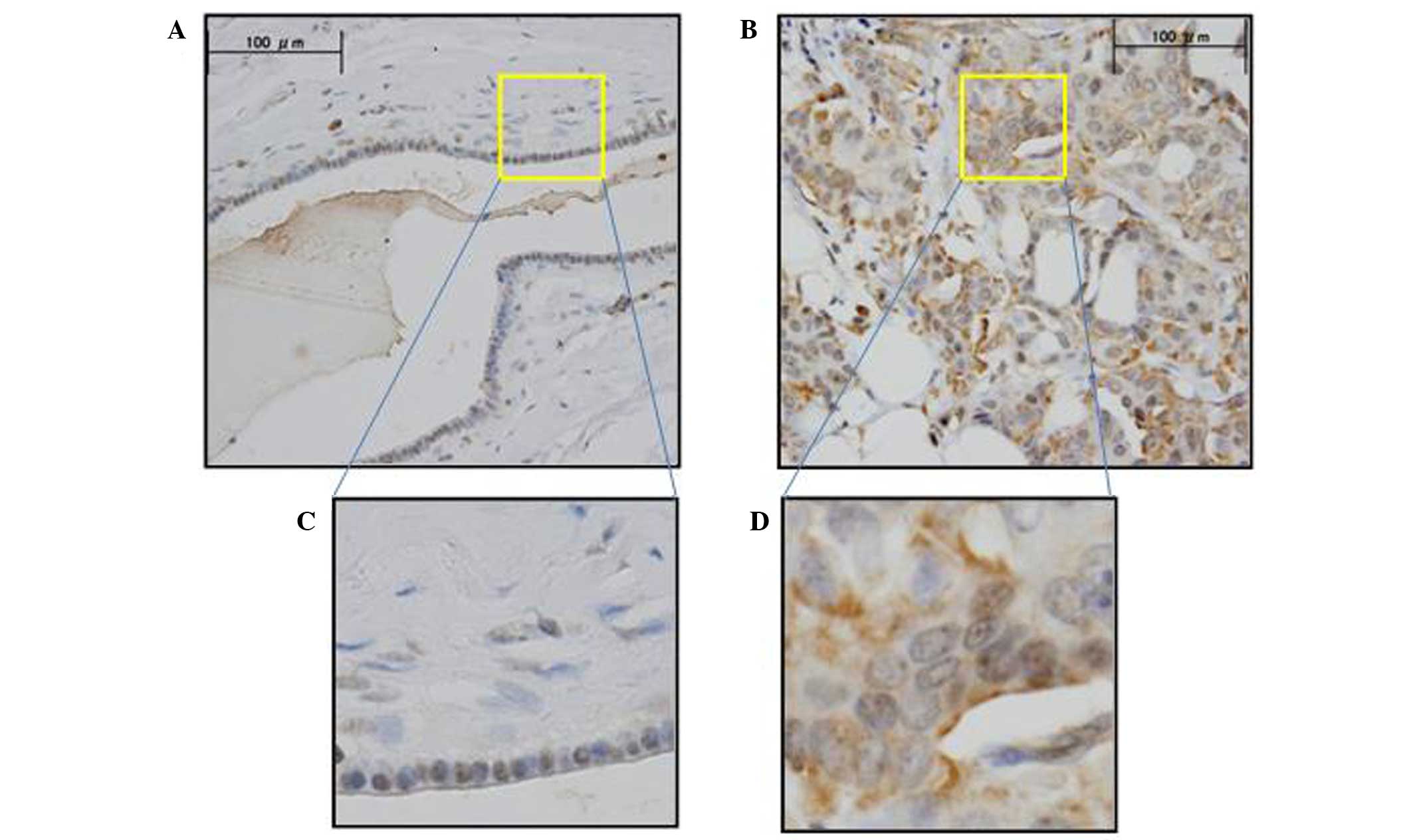
KIF18A expression was assessed by IHC analysis in
the primary breast cancer tissue and normal breast tissue samples.
IHC analysis with anti-KIF18A antibody verified that KIF18A was
highly expressed in cancer cells compared to normal cells (Fig. 1). The cancer and normal cell types
exhibited differential staining patterns: Positive IHC staining was
observed in the nucleus of normal and cancer cells; positive IHC
staining of the cytoplasm was observed predominantly in cancer
cells and slightly in normal cells. ImageJ software was used to
quantitatively evaluate the staining intensity of KIF18A in 144
breast cancer samples. Following assay optimization, a cutoff level
of expression was determined as 30.02 (the average expression level
of KIF18A in tumor): Breast cancer specimens <30.02 were
assigned to the low expression group (n=83; 57.6%), whereas those
with values ≥30.02 were assigned to the high expression group
(n=61; 42.4%).
Clinicopathological significance of
KIF18A expression in breast cancer tissue
The clinicopathological factors analyzed in relation
to KIF18A expression in breast cancer tissue are shown in Table I. The incidence of lymph node
metastasis was significantly higher (P=0.047) in the
high-expression group than in the low-expression group. Conversely,
no significant differences were observed with regard to age,
menopause status, tumor stage, lymphovascular invasion, nuclear
grade, hormone status, HER2 status or recurrence.
 | Table I.Clinicopathological significance of
the KIF18A expression ratio in breast cancer. |
Table I.
Clinicopathological significance of
the KIF18A expression ratio in breast cancer.
|
| KIF18A expression
ratio |
|
|---|
|
|
|
|
|---|
|
| Low | High |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factor | n | % | n | % | P-value |
|---|
| Age, years (mean ±
SD) | 54.6±12.9 |
| 54.0±13.5 |
| 0.814 |
| Menopause status |
|
|
|
| 0.876 |
| Pre | 37 | 44.6 | 28 | 45.9 |
|
| Post | 46 | 55.4 | 33 | 54.1 |
|
| Tumor stage |
|
|
|
| 0.110 |
| T1 | 34 | 40.9 | 33 | 54.1 |
|
| T2-3 | 49 | 59.1 | 28 | 45.9 |
|
| Lymph node
metastasis |
|
|
|
| 0.047a |
|
Absent | 62 | 74.7 | 36 | 59.1 |
|
|
Present | 21 | 25.3 | 25 | 40.9 |
|
| Lymphatic
invasion |
|
|
|
| 0.649 |
|
Absent | 44 | 53.0 | 30 | 49.2 |
|
|
Present | 39 | 47.0 | 31 | 50.8 |
|
| Venous invasion |
|
|
|
| 0.929 |
|
Absent | 47 | 56.6 | 35 | 57.4 |
|
|
Present | 36 | 43.4 | 26 | 42.6 |
|
| Nuclear grade |
|
|
|
| 0.185 |
| Grade
1 | 47 | 56.6 | 40 | 65.6 |
|
| Grade
2 | 15 | 18.1 | 13 | 21.3 |
|
| Grade
3 | 21 | 25.3 | 8 | 13.1 |
|
| Nuclear atypia |
|
|
|
| 0.528 |
| Score
1 | 5 |
6.0 | 6 |
9.8 |
|
| Score
2 | 66 | 79.5 | 49 | 80.4 |
|
| Score
3 | 12 | 14.5 | 6 |
9.8 |
|
| Mitotic counts |
|
|
|
| 0.256 |
| Score
1 | 48 | 57.8 | 41 | 67.2 |
|
| Score
2 | 17 | 20.5 | 13 | 21.3 |
|
| Score
3 | 18 | 21.7 | 7 | 11.5 |
|
| Estrogen
receptor |
|
|
|
| 0.961 |
|
Absent | 12 | 14.5 | 9 | 14.8 |
|
|
Present | 71 | 85.5 | 52 | 85.2 |
|
| Progesterone
receptor |
|
|
|
| 0.629 |
|
Absent | 22 | 26.5 | 14 | 22.9 |
|
|
Present | 61 | 73.5 | 47 | 77.1 |
|
| HER2 score |
|
|
|
| 0.221 |
|
0–1 | 69 | 83.1 | 55 | 90.2 |
|
|
2–3 | 14 | 16.9 | 6 |
9.8 |
|
| Recurrence |
|
|
|
| 0.534 |
|
Absent | 69 | 83.1 | 53 | 16.9 |
|
|
Present | 14 | 86.9 | 8 | 13.1 |
|
DFS analysis
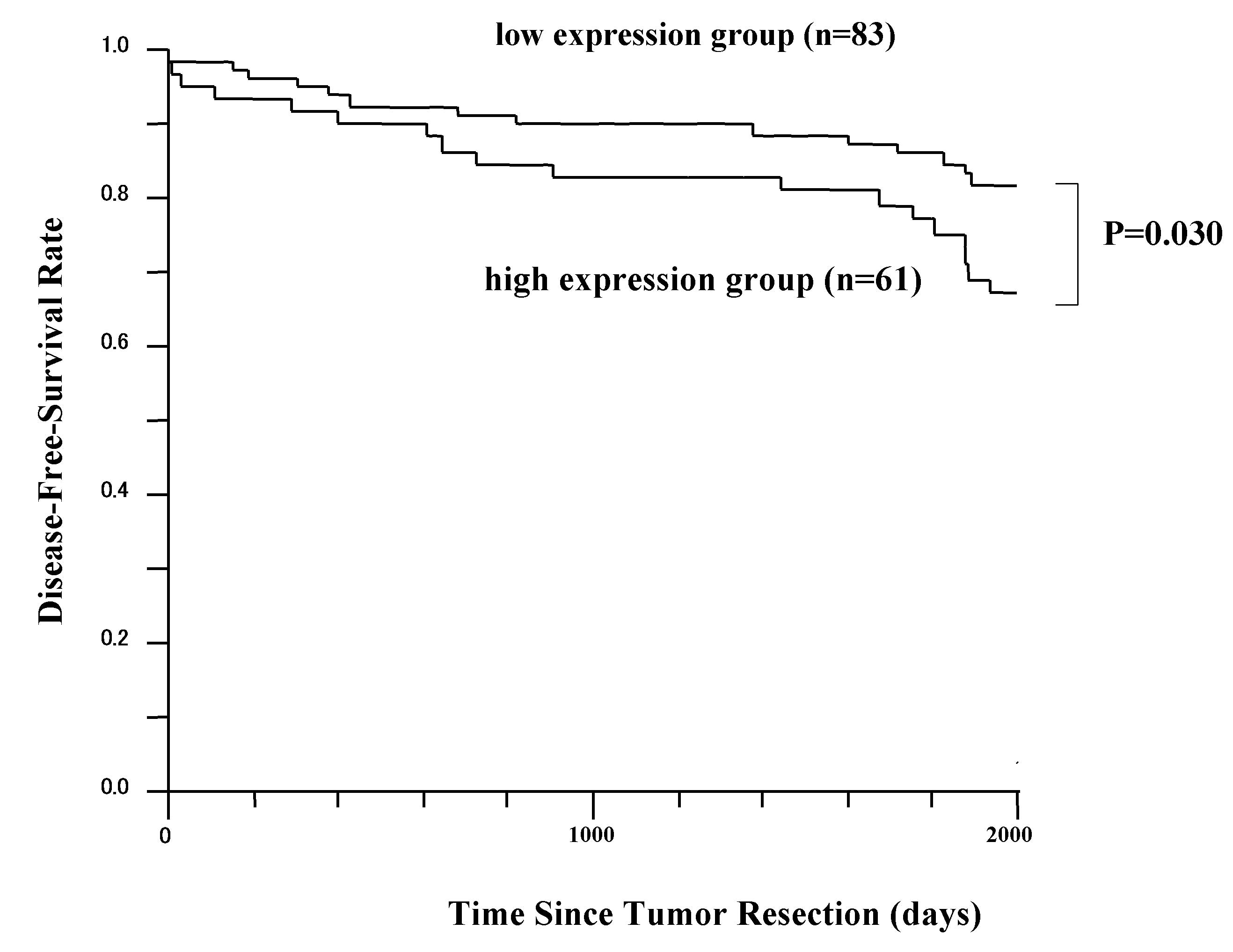
The 5-year DFS rates in patients with high KIF18A
expression and patients with low KIF18A expression are presented in
Fig. 2. The difference in DFS time
between these two groups was statistically significant (P=0.030;
log-rank test). However, the overall survival difference between
these two groups was not statistically significant (data not
shown). Patients received ≥1 postoperative therapy (anti-hormonal
treatment, chemotherapy or radiotherapy).
Univariate and multivariate
analysis
Univariate and multivariate logistic regression
analyses were performed for factors affecting lymph node metastasis
(Table II). Univariate analysis
revealed a significant associated between lymph node metastasis and
the following factors: Tumor size (P=0.009), lymphatic invasion
(P<0.001), venous invasion (P<0.001), recurrence (P=0.004)
and KIF18A expression (P=0.047). Multivariate analysis of these
parameters revealed that venous invasion (hazard ratio, 9.22; 95%
confidence interval, 3.90–23.66; P<0.001) and KIF18A expression
(hazard ratio, 3.20; 95% confidence interval, 1.34–6.09; P=0.010)
were independent predictive factors for lymph node metastasis.
 | Table II.Univariate and multivariate analyses
of clinicopathological factors affecting lymph node metastasis. |
Table II.
Univariate and multivariate analyses
of clinicopathological factors affecting lymph node metastasis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age
(<50/≥51) |
0.97 | 0.48–1.97 | 0.93 | – | – | – |
| T stage
(T1/T2-3) |
2.69 | 1.30–5.79 | <0.01 | 1.91 | 0.77–4.87 |
0.164 |
| LI
(absent/present) | 19.51 | 7.57–61.01 | <0.01 | – | – | – |
| VI
(absent/present)) |
9.97 | 4.48–23.92 | <0.01 | 9.22 | 3.90–23.66 | <0.001 |
| ER
(absent/present) |
1.60 | 0.58–5.16 | 0.39 | – | – | – |
| PgR
(absent/present) |
0.92 | 0.41–2.10 | 0.84 | – | – | – |
| HER2
(absent/present) |
1.51 | 0.55–3.95 | 0.41 | – | – | – |
| Recurrence |
3.90 | 1.54–10.27 | <0.01 | 2.54 | 0.81–8.33 | 0.113 |
| KIF18A
(low/high) |
2.05 | 1.01–4.21 | 0.047 | 3.20 | 1.34–8.09 | 0.010 |
Discussion
In recent years, cancer therapy research has focused
on proteins involved in the regulatory events of mitosis (8–11).
Infiltrating growth of cancer cells, which is associated with
abnormal, uncontrolled proliferation, requires the biological
activation of numerous proteins that serve central roles. Mitotic
inhibitor drugs, which include taxanes and vinca alkaloids, act to
target microtubules and have yielded various levels of success in
the treatment of various types of carcinomas (28). Several next-generation mitotic drug
targets have been developed, and small molecule inhibitors that
have been identified are already under investigation in clinical
trials (11).
KIF18A is a member of the kinesin 8 family and has
been demonstrated to play important roles in chromosome alignment
during mitosis (18–20). Through several in vitro assays,
it has been revealed that upregulation of KIF18A may affect the
biological characteristics of cancer cells (21–24).
In the current study, the expression of KIF18A in
breast cancer tissues and the association between KIF18A expression
and clinicopathological factors in breast cancer were explored
using IHC analysis. The results revealed that KIF18A protein
expression was significantly higher in breast cancer tissues than
in normal breast tissues (21). This
suggests that breast cancer cells may take advantage of KIF18A
overexpression to control mitotic chromosome alignment and increase
their rate of repetitive cell division.
The present study also revealed that KIF18A
overexpression in breast cancer was associated with lymph node
metastasis and poor prognosis. The group with high KIF18A
expression had a poorer prognosis compared with that of the
low-expression group in terms of DFS. KIF18A overexpression was
prevalent in breast cancer cells and was also associated with
prognostic factors and shorter survival time; these results may
suggest that the overexpression of this mitotic protein is
associated with aggressive primary tumors. To the best of our
knowledge, this is the first study to demonstrate the clinical
relevance of KIF18A in invasive breast cancer and its relation to
disease outcome.
The axillary lymph node status is the most
consistent prognostic factor used in adjuvant therapy
decision-making (29). Currently, the
sentinel node biopsy is a common surgical procedure to determine
the stage of the cancer and select an appropriate treatment plan
(30,31). In multivariate analysis, KIF18A
overexpression in breast cancer was determined to be an independent
and significant predictive factor for lymph node metastasis. Based
on these findings, in cases where low KIF18A expression is
identified prior to breast surgery, it may be possible to avoid
performing the sentinel node biopsy in selected patients with
clinically and radiologically normal axilla.
In summary, this is the first report of
clinicopathological analysis of KIF18A in breast cancer patients.
KIF18A expression was correlated with lymph node metastasis and was
an independent predictive factor for the lymph node metastasis and
DFS. Kaplan-Meier analyses revealed that the DFS rate was
significantly lower in the high KIF18A expression group. These
findings suggest that KIF18A may be a useful predictive biomarker
of lymph node metastasis, which could aid in the development of
optimum adjuvant treatments.
Acknowledgements
The authors would like to thank Ms. Yoko Takagi from
the Department of Surgical Oncology, Graduate School of Medicine
and Dental Science, Tokyo Medical and Dental University (Tokyo,
Japan) for providing technical support.
References
|
1
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H: Japan Cancer Surveillance Research
Group: Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the monitoring
of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katanoda K, Hori M, Matsuda T, Shibata A,
Nishino Y, Hattori M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report on the trends in cancer incidence and mortality in
Japan. Jpn J Clin Oncol. 45:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peto R, Boreham J, Clarke M, Davies C and
Beral V: UK and USA breast cancer deaths down 25% in year 2000 at
ages 20–69 years. Lancet. 355:18222000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harbeck N, Thomssen C and Gnant M: St.
Gallen 2013: Brief preliminary summary of the consensus discussion.
Breast Care (Basel). 8:102–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St. Gallen international expert consensus on the primary
therapy of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Falck AK, Bendahl PO, Chebil G, Olsson H,
Fernö M and Rydén L: Biomarker expression and St Gallen molecular
subtype classification in primary tumours, synchronous lymph node
metastases and asynchronous relapses in primary breast cancer
patients with 10 years' follow-up. Breast Cancer Res Treat.
140:93–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcus AI, Peters U, Thomas SL, Garrett S,
Zelnak A, Kapoor TM and Giannakakou P: Mitotic kinesin inhibitors
induce mitotic arrest and cell death in taxol-resistant and
-sensitive cancer cells. J Biol Chem. 280:11569–11577. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miglarese MR and Carlson RO: Development
of new cancer therapeutic agents targeting mitosis. Expert Opin
Investig Drugs. 15:1411–1425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huszar D, Theoclitou ME, Skolnik J and
Herbst R: Kinesin motor proteins as targets for cancer therapy.
Cancer Metastasis Rev. 28:197–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaestner P and Bastians H: Mitotic drug
targets. J Cell Biochem. 111:258–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sharp DJ, Rogers GC and Scholey JM:
Microtubule motors in mitosis. Nature. 407:41–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miki H, Setou M, Kaneshiro K and Hirokawa
N: All kinesin superfamily protein, KIF, genes in mouse and human.
Proc Natl Acad Sci USA. 98:7004–7011. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kamal A and Goldstein LS: Principles of
cargo attachment to cytoplasmic motor proteins. Curr Opin Cell
Biol. 14:63–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karcher RL, Deacon SW and Gelfand VI:
Motor-cargo interactions: The key to transport specificity. Trends
in Cell Biol. 12:21–27. 2002. View Article : Google Scholar
|
|
16
|
Vale RD: The molecular motor toolbox for
intracellular transport. Cell. 112:467–480. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kline-Smith SL and Walczak CE: Mitotic
spindle assembly and chromosome segregation: Refocusing on
microtubule dynamics. Mol Cell. 15:317–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mayr MI, Hümmer S, Bormann J, Grüner T,
Adio S, Woehlke G and Mayer TU: The human kinesin Kif18A is a
motile microtubule depolymerase essential for chromosome
congression. Curr Biol. 17:488–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stumpff J, von Dassow G, Wagenbach M,
Asbury C and Wordeman L: The kinesin-8 motor Kif18A suppresses
kinetochore movements to control mitotic chromosome alignment. Dev
Cell. 14:252–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gardner MK, Odde DJ and Bloom K: Kinesin-8
molecular motors: Putting the brakes on chromosome oscillations.
Trends Cell Biol. 18:307–310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Zhu C, Chen H, Li L, Guo L, Jiang
W and Lu SH: Kif18A is involved in human breast carcinogenesis.
Carcinogenesis. 31:1676–1684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagahara M, Nishida N, Iwatsuki M,
Ishimaru S, Mimori K, Tanaka F, Nakagawa T, Sato T, Sugihara K,
Hoon DS and Mori M: Kinesin 18A expression: Clinical relevance to
colorectal cancer progression. Int J Cancer. 129:2543–2552. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rucksaken R, Khoontawad J, Roytrakul S,
Pinlaor P, Hiraku Y, Wongkham C, Pairojkul C, Boonmars T and
Pinlaor S: Proteomic analysis to identify plasma orosomucoid 2 and
kinesin 18A as potential biomarkers of cholangiocarcinoma. Cancer
Biomark. 12:81–95. 2012.PubMed/NCBI
|
|
24
|
Liao W, Huang G, Liao Y, Yang J, Chen Q,
Xiao S, Jin J, He S and Wang C: High KIF18A expression correlates
with unfavorable prognosis in primary hepatocellular carcinoma.
Oncotarget. 5:10271–10279. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukai H, Aihara T, Yamamoto Y, Takahashi
M, Toyama T, Sagara Y, Yamaguchi H, Akabane H, Tsurutani J, Hara F,
et al: The Japanese Breast Cancer Society Clinical Practice
Guideline for systemic treatment of breast cancer. Breast Cancer.
22:5–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology; College of
American Pathologists: American Society of Clinical
Oncology/College of American Pathologists guideline recommendations
for human epidermal growth factor receptor 2 testing in breast
cancer. J Clin Oncol. 25:118–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komoike Y, Inokuchi M, Itoh T, Kitamura K,
Kutomi G, Sakai T, Jinno H, Wada N, Ohsumi S and Mukai H: Japanese
Breast Cancer Society: Japan Breast Cancer Society clinical
practice guideline for surgical treatment of breast cancer. Breast
Cancer. 22:37–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gradishar WJ, Anderson BO, Blair SL,
Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A, Giordano SH,
Goldstein LJ, et al: National Comprehensive Cancer Network Breast
Cancer Panel: Breast cancer version 3.2014. J Natl Compr Canc Netw.
12:542–590. 2014.PubMed/NCBI
|
|
29
|
Atalay C: New concepts in axillary
management of breast cancer. World J Clin Oncol. 5:895–900. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Giuliano AE, Dale PS, Turner RR, Morton
DL, Evans SW and Krasne DL: Improved axillary staging of breast
cancer with sentinel lymphadenectomy. Ann Surg. 222:394–399;
discussion 399–401. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ollila DW, Brennan MB and Giuliano AE: The
role of intraoperative lymphatic mapping and sentinel
lymphadenectomy in the management of patients with breast cancer.
Adv Surg. 32:349–364. 1999.PubMed/NCBI
|