Introduction
Cervical carcinoma is one of the most malignant
tumors in women (1). There are an
estimated 4.6 million new patients annually, with a mortality rate
of 20–35% (2). The occurrence and
development of cervical carcinoma have obvious stages, lasting 5–10
years, including cervical squamous epithelium, atypical hyperplasia
(mild, moderate and severe), cancer in situ, and early
invasive to invasive carcinoma (3).
Prior studies suggested that the persistent
infection rate of human papillomavirus type 16 (HPV16) ranges from
50 to 75%, which may be an important factor of neoplasia (4). Identifying a high-risk population in the
early stage and carrying out regular dynamic monitoring can promote
diagnosis, increase the chance of treatment and play an important
role in improving the prognosis. Although high-risk populations can
be identified by multifactorial analysis, including age and time of
gravidity or parity, risk levels and progression of the disease
remain to be determined (5). Recent
studies mainly focused on screening the specific oncogene and
oncoprotein expression in the different stages of cervical lesions.
In those studies, HPV16 E6 or E7 were used as markers with higher
sensitivity and specificity to diagnose cervical carcinoma
(6,7).
Nevertheless the boundary value of diagnosis and accuracy of
identifying high-risk populations of early stage cervical carcinoma
were not provided.
The aim of the present study was to examine the
value of E6 oncoprotein, in HPV16, in the diagnosis of early stage
cervical carcinoma and precancerous lesions. In addition, we
followed up the population at high risk and used the receiver
operating characteristic (ROC) curve to obtain the boundary value
and accuracy of diagnosis in order to provide valuable statistical
analyses for clinical studies. The results showed HPV16 E6
oncoprotein serves as an indicator with good sensitivity and
specificity in the diagnosis of early cervical carcinoma and
precancerous lesions. Thus, accuracy increased with the development
of the disease
Materials and methods
Subjects
From January, 2012 to June, 2013, 124 cases of
female patients diagnosed with persistent HPV16 infection were
selected. Inclusion criteria for the study were: i) age ≥18 and
<75 years; ii) patients were diagnosed for the first time and
did not receive treatments; and iii) clinical information of
patients were complete. Exclusion criteria for the study were: i)
pregnant women, lactating women and those during menstrual period;
ii) cases with other genito-urinary system diseases and those with
surgery and trauma history; and iii) cases with inaccurate
experimental images were also excluded. The average age of patients
was 46.7±6.9 years and the average duration of infection by HPV16
was 10.5±3.4 months. The average time of gravidity or parity was
1.2±0.5, and the average menopausal age was 48.9±3.3 years, and
usage rate of contraceptives was 45.8%.
This study was approved by the Ethics Committee of
the Bethune International Peace Hospital (Hebei, China). Informed
consent of patients and their relatives was also obtained.
Observation indicators and test
methods
A dynamic follow-up database was established up to
January 2016, and average follow-up time was 2.6±0.7 years. The
immunohistochemical Elivision method was used to detect the HPV16
E6 protein expression at different time points (first day, one year
after follow up and two years after follow up). Pathologic
diagnosis was used to analyze the results and clinical staging
criteria, revised by the International Federation of Gynecology and
Obstetrics (FIGO) in 2000, were used with regard to cervical
intraepithelial neoplasia (CIN) III, cancer in situ, early
invasive and invasive carcinoma as cervical carcinoma, and others
including precancerous lesions and normal tissues.
Main experimental reagents. Mouse anti-human HPV16
E6 monoclonal antibody (SC-460; dilution: 1:50) was purchased from
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and
immunohistochemical Elivision™ plus kit and DAB developer were
purchased from Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China. To prepare phosphate-buffered saline (PBS) buffer
solution 29 g of Na2HPO4·12H2O, 3
g NaH2PO4·2H2O and 85 g of NaCl
were mixed and distilled water up to 1,000 ml was added. The buffer
was used to prepare 0.1 M PBS buffer solution with pH 7.4 (the
solution was diluted 10 times using distilled water, to obtain 0.01
M PBS solution buffer with pH 7.4).
Main instruments
The following instruments were purchased: Tissue
embedding machine (Bnu-III, domestic; Biosharp, Hefei, China),
tissue processor (Tissue-Tek; Sakura Finetechnical Co., Tokyo,
Japan), paraffin slicing machine (Leica-2025; Leica Microsystems,
Wetzlar, Germany), adjustable micropipettor (Gilson Inc., Villiers
le Bel, France), and light microscope (Olympus, Tokyo, Japan).
Main steps
i) Formaldehyde (10%) was used to fix tissue
samples. Samples were sliced into 0.5 cm sections and placed in AF
liquid for fixation (1 h). The samples were then transferred to 95%
alcohol (overnight), followed by dehydration (using absolute
alcohol and xylene) and then placed in impregnated wax boxes
(Biosharp). The samples were embedded and paraffin blocks were
constructed. ii) Elivision method was employed in
immunohistochemical staining. PBS was used as a negative control,
and a positive control image was purchased from Beijing Zhongshan
Jinqiao Biotechnology Co., Ltd. Sections (4 µm) were prepared and
then adhered to slide glass of polylysine attached membrane at 60°C
and left overnight. Xylene was used to dewax the sections
conventionally and graded ethanol to dehydrate, and running and
distilled water were used to rinse the samples three times in PBS
(3 times for 3 min each time). The samples were soaked in citrate
buffer solution (pH 6.0). High pressure repairing antigen was
employed, and the samples were cooled to 20̊C. One drop of 3%
H2O2 was added to each section, and the
samples were incubated at room temperature for 10 min to inhibit
endogenous oxidase activity. PBS solution was used to wash the
samples (3 times for 3 min each time). Subsequently, 50 µl primary
antibody was added to each section (working concentration of
anti-HPV16 E6 protein was 1:50), followed by incubatation at room
temperature for 10 min. PBS solution was used for washing (3 times
for 3 min each). Then one drop of reagent A (polymer enhancer) was
add to each section, and the samples were incubated at room
temperature for 20 min. After rinsing with PBS (3 times for 3 min
each time), one drop of reagent B (enzyme labeled anti-mouse
polymer) was added to each section. The sections were then
incubated at room temperature for 30 min.
After washing with PBS (3 times for 3 min each time)
one drop or 50 µl of freshly prepared DAB solution was added to
each section. The samples were washed under running water,
hematoxylin was added to restain the samples for 1 min and the
sections were washed under running water and PBS solution. Gradient
ethanol was used to dry and dehydrate the sections, which became
transparent in xylene and were fixed using neutral balata.
Interpretation of the results
Presence of brown particles in the cell, revealed
the expression of HPV16 E6 protein. At a magnification of ×400 five
horizons were randomly selected, 200 tumor cells were counted
(total of 1,000 tumor cells), and the proportion/horizon of
positive cells was counted.
Statistical methods
SPSS 19.0 software (IBM, Armonk, NY, USA) was used
to analyze data. Measurement data were presented as mean ± standard
deviation, and one-way ANOVA was used for comparisons among groups.
Enumeration data were used to indicate cases or (%), and the
χ2 test was used to make comparisons among groups. Area
under curve (AUC) of ROC was used to compare accuracy of diagnosis.
P<0.05 was used to indicate statistically significant
results.
Results
Comparisons of the positive expression
of HPV16R6 among groups
HPV16 E6 protein expression increased with the
development of the disease. Twenty-five positive cases (20.2%) were
identified at the inception of the study, 57 cases (46.0%) at 1
year after follow-up, and 70 cases (56.5%) at 2 years after
follow-up. Differences among the groups were of statistical
significance (P<0.05) (Table
I).
 | Table I.Comparisons of positive expression of
HPV16R6 among groups (proportioin/horizon). |
Table I.
Comparisons of positive expression of
HPV16R6 among groups (proportioin/horizon).
|
| At inception of the
study | 1 year after
follow-up | 2 years after
follow-up |
|---|
|
|
|
|
|
|---|
| Group | No. of cases |
| Positive
expression | No. of cases |
| Positive
expression | No. of cases |
| Positive
expression |
|---|
| Invasive
carcinoma | 3 |
|
72.5±13.2 | 10 |
|
76.4±14.3 | 16 |
|
79.3±15.2 |
| Early invasive
carcinoma | 9 |
|
48.6±10.4 | 22 |
|
52.3±12.2 | 24 |
|
54.6±13.2 |
| CIN III and cancer
in situ | 13 |
|
25.5±8.7 | 25 |
|
24.6±7.5 | 30 |
|
25.5±6.7 |
| CIN I and II | 59 |
|
13.6±4.3 | 40 |
|
17.7±4.6 | 36 |
|
18.2±4.3 |
| Normal cervix | 40 |
|
3.7±1.2 | 27 |
|
3.9±1.2 | 18 |
|
3.7±1.3 |
| F-test |
| 12.635 |
|
| 13.462 |
|
| 15.624 |
|
| P-value |
| <0.001 |
|
| <0.001 |
|
| <0.001 |
|
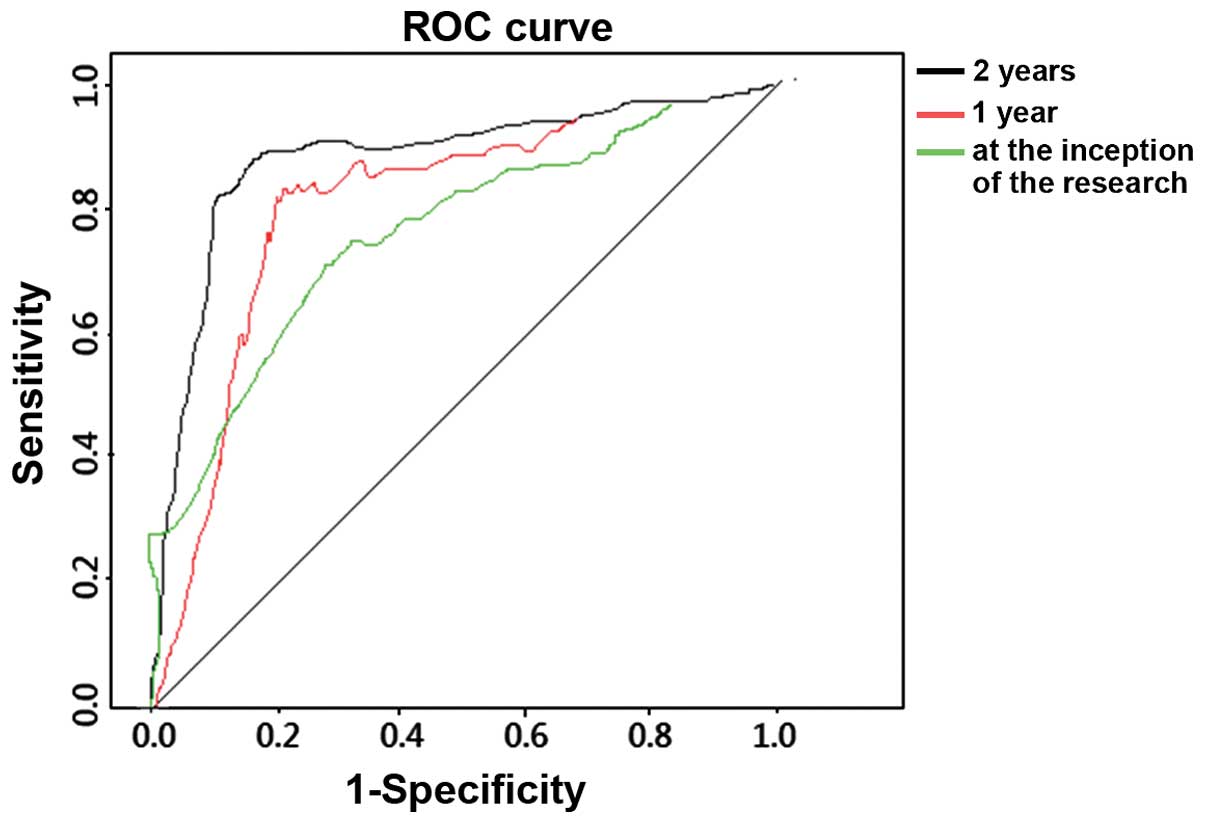
ROC analysis diagnosing HPV16 E6
The AUC of diagnosis at the inception of the study
was 0.635, and 95% CI was 0.375–0.821; sensitivity was 62.5%, while
specificity was 72.4%. The AUC of diagnosis 1 year after follow-up
was 0.719, and 95% CI was 0.462–0.873; sensitivity was 72.6%, while
specificity was 82.4%. The AUC of diagnosis 2 years after follow-up
was 0.821, and 95% CI was 0.488–0.893; sensitivity was 82.2%, while
specificity was 89.7%. Sensitivity, specificity and accuracy of
diagnosing HPV16 E6 increased over time. The differences were of
statistical significance (P<0.05) (Fig. 2).
Discussion
The HPV16 E6 gene is located within
nucleotides 83–559 on the HPV16 viral genome, and is composed of
477 nucleotides. E6 protein is a small and basic protein with two
zinc finger motifs (8). The presence
of E6 has been shown to be closely associated with malignant
transformation, transcriptional activation, and interactions
between cells (8). HPV16E6 is a major
protein in the virus life cycle, and continuous expression of the
E6 protein is key to the cause of immortalization, malignant
transformation and malignant phenotype maintenance of host cells
and the progression of disease after HPV16 DNA integrates into the
host cell genome (9).
Previous findings showed that E6 protein can trigger
the degradation of tumor-inhibiting factors such as P53 and pRB. It
mediates cell apoptosis by forming complexes with related proteins
of ubiquitin ligase E6 (10) and the
activating transcription of the human telomerase catalytic subunit
gene. E6 protein decreases the stability of hosts' chromosomes and
promotes immortalization of host cells (11). Expression of E6 protein inhibits the
promoter activity of E-cadherin in epithelium and restrains
cytokines mediated by the transcript of E-cadherin to adhere to
epidermal antigen-presenting cells, both of which promote the
occurrence of immune escape of virus (12). Interacting with Death
domain-associated protein (Daxx), E6 protein can inhibit promoter
activity of Daxx, decrease Daxx protein expression and prevent
apoptosis (13). E6 protein may also
interact with host cell transcription factors such as cytokines of
activator protein-1, TNF-α and IL-1β. E6 plays an important role in
the development of cervical carcinoma (14). The HPV16 E6 gene locus mutation
is associated with persistent viral infection, high-grade cervical
lesions and squamous carcinoma of the cervix (15). Upregulation of E6 stimulates cell
multiplication and inhibits cell differentiation at the same time
(16). Generally speaking, it can be
said that E6 protein plays an important role in the carcinogenesis
of HPV16.
The findings suggest that the diagnostic rate of
cervical carcinoma increased with time. We detected 25 positive
cases (20.2%) at the inception of the study, 57 cases (46.0%) at 1
year after follow-up, and 70 cases (56.5%) at 2 years after
follow-up. These results suggested that persistent HPV16 infection
is an important factor in the occurrence and development of
cervical carcinoma. The positive expression of HPV16 E6 increased
as the disease developed, suggesting that HPV16 E6 was highly
expressed in cervical carcinoma and its presence was positively
correlated with the tumor stage. Sensitivity, specificity and
accuracy of diagnosis using E6 improved with time and the
sensitivity and specificity at 1 and 2 years after follow-up were
up to 75%, and the accuracy was 70%. Sensitivity, specificity and
accuracy results suggested that the E6 oncoprotein can be used as
an indicator with acceptable sensitivity and specificity to
diagnose early cervical carcinoma and precancerous lesions. The
accuracy increased as the disease developed.
In summary, efforts should be focused on the
high-risk populations for persistent infection and offer dynamic
monitoring analyses for this group. HPV16 E6 protein had an
important value of identifying early cervical carcinoma, and it
provided accurate quantitative test data at the same time to
distinguish lesions of different stages, both of which provided new
methods for clinical practices. For future studies, we suggest
using larger samples and randomized clinical control studies.
References
|
1
|
Mocarska A, Staroslawska E,
Zelazowska-Cieślińska I, Łosicki M, Stasiewicz D, Kieszko D and
Burdan F: Epidemiology and risk factors of the cervical squamous
cell carcinoma. Pol Merkur Lekarski. 33:101–106. 2012.(In Polish).
PubMed/NCBI
|
|
2
|
Yung KW, Yung TT, Chung CY, Tong GT, Liu
Y, Henderson J, Welbeck D and Oseni S: Principles of cancer
staging. Asian Pac J Surg Oncol. 1:1–16. 2015.
|
|
3
|
Chiappetta C, Lendaro E, Cacciotti J,
Zaralli R, Puggioni C, Migliore G, Petrozza V, Rocca CD and Di
Cristofano C: The 16, 18, and 45 HPV infection in high grade
squamous cervical lesions in primary hr-HPV test screening program.
Eur J Gynaecol Oncol. 36:722–725. 2015.PubMed/NCBI
|
|
4
|
Smith JS, Lindsay L, Hoots B, Keys J,
Franceschi S, Winer R and Clifford GM: Human papillomavirus type
distribution in invasive cervical cancer and high-grade cervical
lesions: a meta-analysis update. Int J Cancer. 121:621–632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mzarico E, Gómez-Roig MD, Guirado L,
Lorente N and Gonzalez-Bosquet E: Relationship between smoking, HPV
infection, and risk of Cervical cancer. Eur J Gynaecol Oncol.
36:677–680. 2015.PubMed/NCBI
|
|
6
|
Zacapala-Gómez AE, Del Moral-Hernández O,
Villegas-Sepúlveda N, Hidalgo-Miranda A, Romero-Córdoba SL,
Beltrán-Anaya FO, Leyva-Vázquez MA, Alarcón-Romero LC and
Illades-Aguiar B: Changes in global gene expression profiles
induced by HPV 16 E6 oncoprotein variants in cervical carcinoma
C33-A cells. Virology. 488:187–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poljak M, Kocjan BJ, Oštrbenk A and Seme
K: Commercially available molecular tests for human
papillomaviruses (HPV): 2015 update. J Clin Virol. 76(Suppl 1):
S3–S13. 2015.PubMed/NCBI
|
|
8
|
Zanier K, ould M'hamed ould Sidi A,
Boulade-Ladame C, Rybin V, Chappelle A, Atkinson A, Kieffer B and
Travé G: Solution structure analysis of the HPV16 E6 oncoprotein
reveals a self-association mechanism required for E6-mediated
degradation of p53. Structure. 20:604–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang G, Sun L, Li Z, Si L, Song T, Huang
C and Zhang W: HPV-16E6 can induce multiple site phosphorylation of
p53. Oncol Rep. 21:371–377. 2009.PubMed/NCBI
|
|
10
|
Bernard X, Robinson P, Nominé Y, Masson M,
Charbonnier S, Ramirez-Ramos JR, Deryckere F, Travé G and
Orfanoudakis G: Proteasomal degradation of p53 by human
papillomavirus E6 oncoprotein relies on the structural integrity of
p53 core domain. PLoS One. 6:e259812011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee CJ, Suh EJ, Kang HT, Im JS, Um SJ,
Park JS and Hwang ES: Induction of senescence-like state and
suppression of telomerase activity through inhibition of HPV E6/E7
gene expression in cells immortalized by HPV16 DNA. Exp Cell Res.
277:173–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
D'Costa ZJ, Jolly C, Androphy EJ, Mercer
A, Matthews CM and Hibma MH: Transcriptional repression of
E-cadherin by human papillomavirus type 16 E6. PLoS One.
7:e489542012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dionne KR, Zhuang Y, Leser JS, Tyler KL
and Clarke P: Daxx upregulation within the cytoplasm of
reovirus-infected cells is mediated by interferon and contributes
to apoptosis. J Virol. 87:3447–3460. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
White EA, Kramer RE, Tan MJ, Hayes SD,
Harper JW and Howley PM: Comprehensive analysis of host cellular
interactions with human papillomavirus E6 proteins identifies new
E6 binding partners and reflects viral diversity. J Virol.
86:13174–13186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Niebler M, Qian X, Höfler D, Kogosov V,
Kaewprag J, Kaufmann AM, Ly R, Böhmer G, Zawatzky R, Rösl F, et al:
Post-translational control of IL-1β via the human papillomavirus
type 16 E6 oncoprotein: a novel mechanism of innate immune escape
mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog.
9:e10035362013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCloskey R, Menges C, Friedman A, Patel D
and McCance DJ: Human papillomavirus type 16 E6/E7 upregulation of
nucleophosmin is important for proliferation and inhibition of
differentiation. J Virol. 84:5131–5139. 2010. View Article : Google Scholar : PubMed/NCBI
|