Introduction
Anti-neutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV) is a life-threatening systemic
autoimmune condition of unknown cause that affects small- to
medium-sized blood vessels (1). AAV
is comprised of three different diseases entities: Eosinophilic
granulomatosis with polyangiitis, microscopic polyangiitis and
granulomatosis with polyangiitis (2).
Renal involvement is frequent in AAV. The characteristic clinical
and pathological features are rapidly progressive
glomerulonephritis and pauci-immune necrotizing crescentic
glomerulonephritis, respectively (3).
Renal impairment has been shown to be one of the negative
prognostic factors for mortality in AAV (4,5).
Multiple myeloma (MM) is a malignant proliferation
of monoclonal plasma cells in the blood. The most frequent
complications of MM are painful pathological fractures, anemia,
hypercalcemia, renal failure and recurrent bacterial infections
(6). Renal impairment is a common and
severe complication of MM, with an incidence rate as high as 50%
(7). The United States Renal Data
System showed that the 2-year all-cause mortality rate of patients
with end-stage renal disease (ESRD) due to MM was 58 vs. 31% in all
other patients (8). AAV and MM cause
acute kidney injury via different physiopathological mechanisms,
with the renal vasculature most impaired in AAV and tubular damage
mainly in MM (9). A previous study
reported a case in which immunoglobulin (Ig)A myeloma presented as
Henoch-Schönlein purpura with nephritis (10). However, to the best of our knowledge,
AAV coexisting with MM has not been previously reported. The
current study presents a rare case of AAV coexisting with MM.
Case report
A 64-year-old female presented to Xinqiao Hospital
(Chonqing, China) with fatigue and a poor appetite that had been
apparent for 6 weeks. The patient was admitted to a local hospital
1 month previously. At this time, laboratory tests revealed a serum
creatinine level of 1.33 mg/dl (reference range, 0.52–1.19 mg/dl),
a cytoplasmic (c)-ANCA titer of 1:10 (reference range, negative)
and a 24-h urinary protein excretion level of 1.27 g (reference
range, 0.00–0.12 g). The patient's creatinine level rapidly
increased to 9.05 mg/dl 2 weeks later and a diagnosis of acute
renal failure was formed. The patient was transferred to the
Institute of Nephrology of Chongqing and Kidney Center of the
People's Liberation Army, Xingqiao Hospital, for further
evaluation. Upon the current admission, a physical examination
revealed mild elevated blood pressure (149/77 mmHg) and a normal
temperature of 36.5°C. Laboratory tests showed that in a complete
blood count, the white blood cell count was 2,997
cells/mm3, the hemoglobin level was 4.3 g/dl and the
platelet count was 149,000/mm3. No microscopic hematuria
was observed. Further results were as follows: 24-h urinary protein
excretion, 3.5 g; blood urea nitrogen, 99.9 mg/dl; serum
creatinine, 10.31 mg/dl; serum albumin, 2.05 g/dl; globulin, 3.96
g/dl; IgG, 2.48 g/dl; IgA, 219.00 mg/dl; IgM, 106.00 mg/dl; and
complement 3, 0.58 g/l. The patient's c-ANCA titer was 1:10, the
result for myeloperoxidase was positive and the antinuclear
antibody titer was 1:100. The patient exhibited an elevated
erythrocyte sedimentation rate of 78.0 m/h, a C-reactive protein
level of 49 mg/dl, and a negative result for anti-glomerular
basement membrane and hepatitis B virus surface antigen. The
patient was diagnosed with rapid progressive glomerulopathy and
started hemodialysis immediately. Meanwhile, abdominal
ultrasonography revealed that the kidneys were increased in size. A
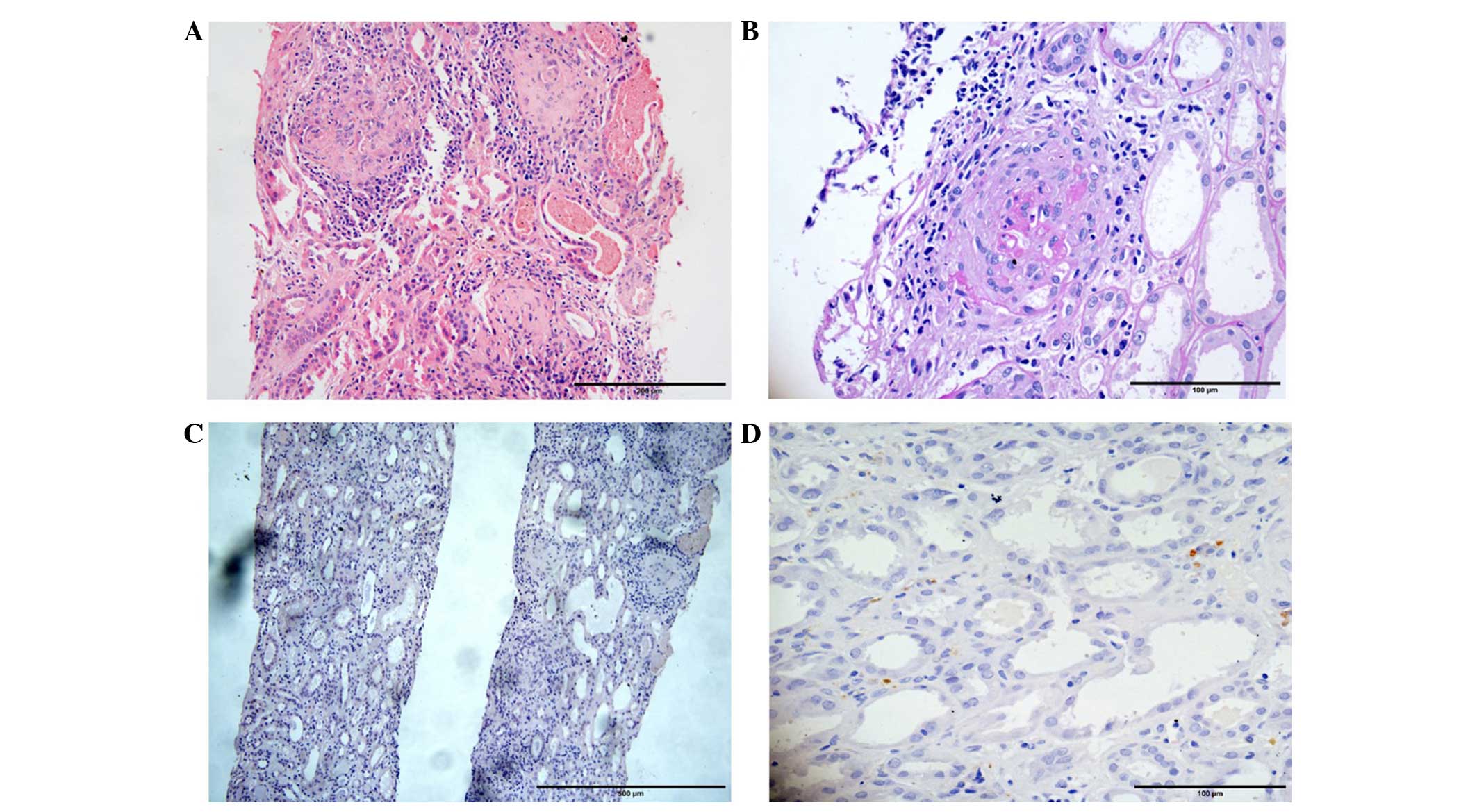
renal biopsy was undertaken carefully and 10 glomeruli were
obtained; 5 glomeruli exhibited glomerulosclerosis and 1 glomerulus
exhibited segmental glomerulosclerosis. A renal biopsy was
carefully undertaken, and 10 glomeruli were obtained, of which, 4
glomeruli exhibited glomerulosclerosis and 1 glomerulus exhibited
segmental glomerulosclerosis. Among the 10 glomeruli, 1 glomerulus
was a cellular crescent, 3 glomeruli were fibrocellular crescents
and 2 glomeruli were fibrous crescents. Prominent capillary loops
and interstitial small artery fibrinoid necrosis were formed.
Lymphocytic infiltration and fibrous tissue proliferation were
observed in certain interstitial regions (Fig. 1). Renal biopsy specimens were
processed for light, immunofluorescence and electron microscopy.
For light microscopy, tissue specimens were fixed with 10% neutral
formalin and embedded in paraffin, and 2-µm-thick serial sections
were stained for hematoxylin and eosin (H&E), periodic
acid-Schiff, periodic acid-methenamine silver (Jones) and Masson's
trichrome stains (Dako, Glostrup, Denmark). For immunofluorescence,
4–5-µm-thick serial cryostat sections were stained with
fluoresceinated antisera specific for IgG (cat. no. F020202-2;
1:20; Dako), IgA (cat. no. F020402-2; 1:50; Dako), IgM (cat. no.
F020302-2; 1:50; Dako), C3 (cat. no. F020102-2; 1:50; Dako), C4
(cat. no. F016902-2; 1:50; Dako), κ (cat. no. F019802-2; 1:50;
Dako) and λ (cat. no. F019902-2; 1:50; Dako) light chains.
Immunofixation electrophoresis was performed by Helena Laboratories
REP Rapid Electrophoresis Analyzer (Helena Biosciences, Münster,
Germany). Immunofluorescence analysis noted only IgG deposition in
the glomeruli, while Congo red staining and light chain staining
were negative (Fig. 1). Moreover,
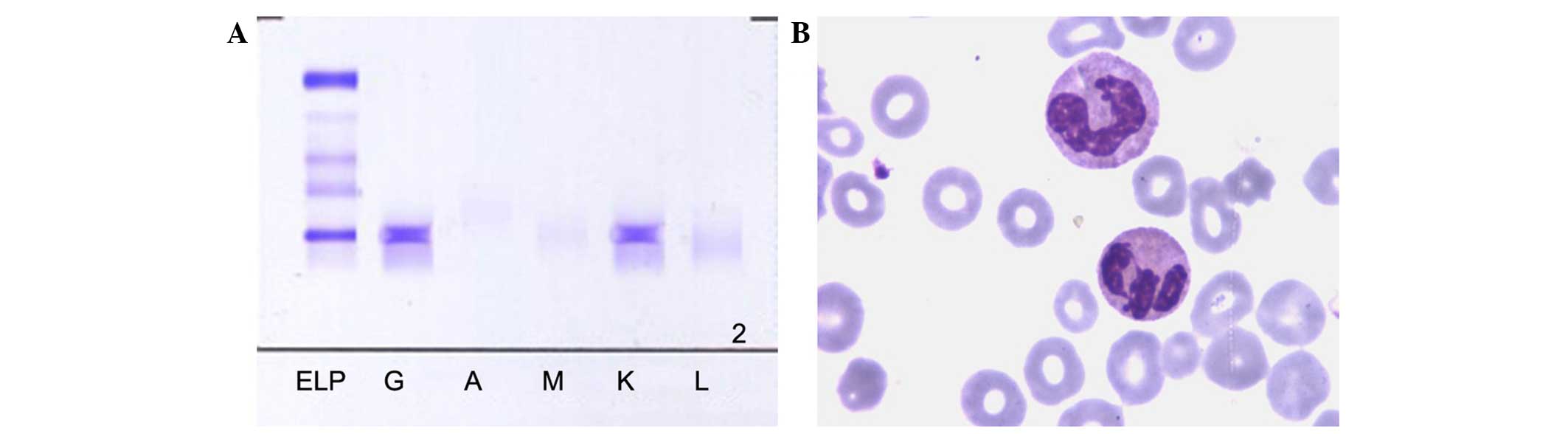
monoclonal protein was found by serum protein electrophoresis, and
confirmed by serum immunofixation electrophoresis, type IgG κ
(Fig. 2). Furthermore, bone marrow
aspiration was also performed and showed significantly active
proliferation and plasmocytes accounting for 15% of the total cells
(Fig. 2). As a result, MM was
diagnosed. Overall, the patient was finally diagnosed with AAV
coexisting with MM, based on the ANCA titer, crescent
glomerulonephritis and rapid progression of renal function
observed. Considering the irreversible loss to renal function and
the patient's weak physical condition, regular hemodialysis was
administered three times a week and arteriovenous fistula surgery
was performed during the hospital stay. Other treatments included
medication to control hypertension and supportive therapies. The
patient was discharged The patient was discharged after 3 weeks in
hospital and is followed up as an outpatient regularly every 3
months.
Discussion
To the best of our knowledge, the present study
reports the first case of AAV coexisting with MM. The diagnosis of
AAV was based on the serum ANCA titers and renal biopsy revealing
crescent glomerulonephritis, while MM was confirmed by the serum
immunofixation electrophoresis results combined with bone marrow
aspiration. Although renal biopsy Congo red stain for amyloidosis
was negative and immunochemistry for light chain was also negative,
no abnormal plasma cells infiltration was found by H&E staining
upon light microscopy in the renal tissue. Myeloma cast nephropathy
cannot be diagnosed and excluded, as the spectrum of renal lesions
in MM is heterogeneous, and it is undetectable in the early stage.
Esnault et al (9) found that
patients with monoclonal (MIg) may be positive for ANCA. While AAV
is rare in hematological malignancies, Cil et al (11) detected ANCA positivity in 8 out of 60
patients with Hodgkin's lymphoma, while ANCA was not detected in
non-Hodgkin's lymphoma patients (0/119). In addition, Nocente et
al (12) reported a case of
Sweet's syndrome associated with MIg of IgG-λ type and perinuclear
(p)-ANCA positivity, and concluded that the MIg, possibly directed
to neutrophils such as an antibody, may cause their fragmentation
and the release of antigens responsible for the appearance of
p-ANCA. Conte et al (13)
reported a case of Henoch-Schönlein purpura in a patient with MM.
Henoch-Schönlein purpura is also recognized as a type of
vasculitis, but without ANCA positivity. These cases lead us to
believe that there is an association between the two diseases,
although there is no previous direct evidence showing that AAV is
associated with MM, although the two entities are each associated
with the immune system. Thus, whether AAV and MM have an intrinsic
association or whether their joint occurrence is purely coincidence
requires further study.
In conclusion, AAV coexisting with MM is a rare
disease and a pathological biopsy analysis is required for the
diagnosis. The two disease entities are common in elderly patients
and can cause acute kidney injury, progressing to ESRD, which
requires kidney replacement. It appears that elderly patients
should be screened for AAV and MM on the occurrence of unexplained
renal failure. Meanwhile, the treatments for AAV coexisting with MM
are worthy of clinical attention and further study.
References
|
1
|
Wilde B, van Paassen P, Witzke O and
Tervaert JW: New pathophysiological insights and treatment of
ANCA-associated vasculitis. Kidney Int. 79:599–612. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen
EC, et al: 2012 revised International chapel hill consensus
conference nomenclature of vasculitides. Arthritis Rheum. 65:1–11.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Savage CO: Pathogenesis of anti-neutrophil
cytoplasmic autoantibody (ANCA)-associated vasculitis. Clin Exp
Immunol. 164(Suppl 1): S23–S26. 2011. View Article : Google Scholar
|
|
4
|
Luqmani RA, Bacon PA, Beaman M, Scott DG,
Emery P, Lee SJ, Howie AJ, Richards N, Michael J and Adu D:
Classical versus non-renal Wegener's granulomatosis. Q J Med.
87:161–167. 1994.PubMed/NCBI
|
|
5
|
Weidner S, Geuss S, Hafezi-Rachti S, Wonka
A and Rupprecht HD: ANCA-associated vasculitis with renal
involvement: An outcome analysis. Nephrol Dial Transplant.
19:1403–1411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alexanian R and Dimopoulos M: The
treatment of multiple myeloma. N Engl J Med. 330:484–489. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knudsen LM, Hippe E, Hjorth M, Holmberg E
and Westin J: Renal function in newly diagnosed multiple myeloma-a
demographic study of 1353 patients. The Nordic Myeloma Study Group.
Eur J Haematol. 53:207–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abbott KC and Agodoa LY: Multiple myeloma
and light chain-associated nephropathy at end-stage renal disease
in the United States: Patient characteristics and survival. Clin
Nephrol. 56:207–210. 2001.PubMed/NCBI
|
|
9
|
Esnault VL, Jayne DR, Keogan MT, Brownlee
AA, Testa A, Lecarrer D, Brown DL and Lockwood CM: Anti-neutrophil
cytoplasm antibodies in patients with monoclonal gammopathies. J
Clin Lab Immunol. 32:153–159. 1990.PubMed/NCBI
|
|
10
|
Zickerman AM, Allen AC, Talwar V, Olczak
SA, Brownlee A, Holland M, Furness PN, Brunskill NJ and Feehally J:
IgA myeloma presenting as Henoch-Schönlein purpura with nephritis.
Am J Kidney Dis. 36:E192000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cil T, Altintas A, Isikdogan A and Batun
S: Prevalence of antineutrophil cytoplasmic antibody positivity in
patients with Hodgkin's and non-Hodgkin lymphoma: A single center
experience. Int J Hematol. 90:52–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nocente R, Cammarota G, Silveri Gentiloni
N, Rotoli M, Zannoni GF, Bertazzoni G, Ceccanti M and Gasbarrini G:
A case of Sweet's syndrome associated with monoclonal
immunoglobulin of IgG-lambda type and p-ANCA positivity. Panminerva
Med. 44:149–150. 2002.PubMed/NCBI
|
|
13
|
Conte G, Conte FJ, Ojeda JM, Araos D,
Poniachik J, Murray G and Flores C: Severe Henoch-Schönlein purpura
in a patient with multiple myeloma. Rev Med Chil. 128:1255–1260.
2000.(In Spanish). PubMed/NCBI
|