Introduction
Malignant peripheral nerve sheath tumors (MPNSTs)
are highly malignant sarcomas derived from the neural crest, and
account for 5–10% of all soft tissue sarcomas (1). MPNSTs exhibit a high incidence of local
recurrence and distant metastases. Metastases are most frequently
identified in the lung, followed by the bone and pleura (2). Currently, there are no recommended
adjuvant treatments for MPNST as there is for other soft tissue
sarcomas. The European Society for Medical Oncology guidelines
state that surgery remains to be the optimal treatment, with the
aim of achieving clear surgical margins. Despite multimodal
treatment, the prognosis of MPNST is poor with 5-year survival
rates between 23–69% (3). Brain
metastasis from soft tissue sarcomas, including MPNST, is rare with
a reported prevalence of 1–6% of all soft tissue sarcomas (1,4,5). In particular, brain metastasis arising
from soft tissue sarcomas, in the absence of lung metastasis, is
considered a relatively rare event (1,4,5). Furthermore, to the best of our
knowledge, no cases of medullary metastasis without preceding lung
metastasis have been reported in the literature to date. The
current study presents the case of an 81-year-old woman with
medullary metastasis arising from a MPNST of the brachial plexus,
who experienced loss of medullary function.
Case report
In July 2012, an 81-year-old woman presented to
Shingu Municipal Medical Center (Shingu, Japan) with numbness in
the upper left arm. At this hospital, the patient was diagnosed
with unknown neuritis and thus received no treatment. A total of 6
months later in January 2013, the patient was referred to Mie
University Hospital (Tsu, Japan) due to paralysis of the left arm,
which was suspected to be a disorder of the brachial plexus.
Clinically, no neck or shoulder masses were evident during physical
examination. The patient was unable to contract any muscles in the
left upper extremity and experienced severe pain in the upper left
limb, which could not be relieved by major analgesics (including
tramadol hydrochloride 112.5 mg/day and acetaminophen 975 mg/day).
The patient was administered oxycodone (10 mg/day) and pregabalin
(125 mg/day), which successfully alleviated the pain. A
fluorodeoxyglucose positron emission tomography-computed tomography
(CT) scan (Discovery PET/CT 690; GE Healthcare Bio-Sciences,
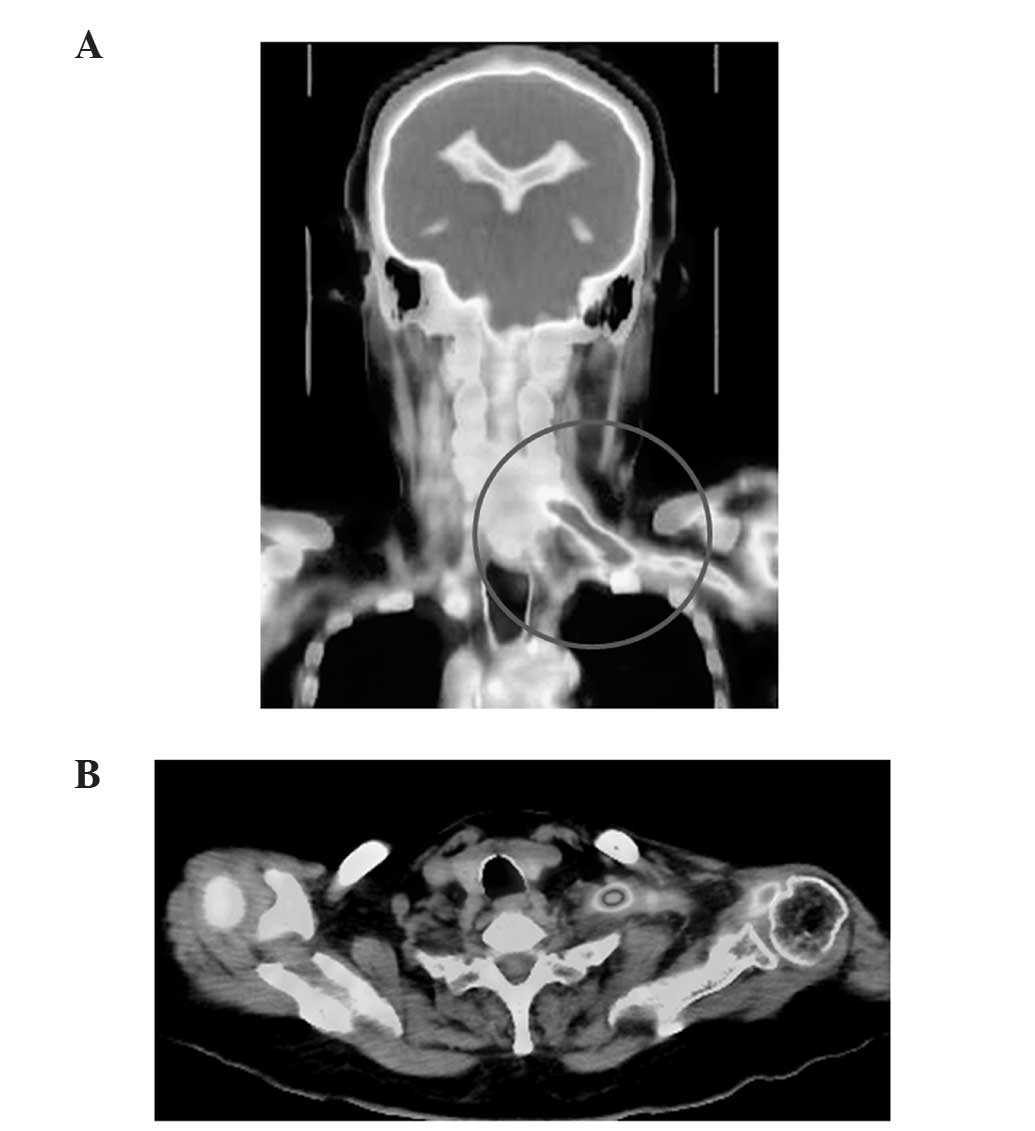
Pittsburgh, PA, USA) revealed increased tracer uptake in the left
brachial plexus (Fig. 1A) arising
from the cervical nerve root (Fig.
1B), which indicated the presence of a tumor. Magnetic
resonance imaging (MRI; Ingenia 1.5T; Philips Healthcare, DA Best,
The Netherlands) identified a soft tissue mass along the nerve at
the left brachial plexus, which exhibited low signal intensity on
T1-weighted images and high signal intensity on T2-weighted
images.
A CT-guided needle biopsy was subsequently
performed. Tissues were cut into 3.0 µm sections and stained with
hematoxylin and eosin. The histological findings demonstrated
interweaving of atypical spindle cells with a high mitotic rate,
consistent with a high-grade sarcoma. Tissues were incubated with
the following antibodies: Monoclonal mouse anti-human integrase
interactor 1 (INI-1; dilution, 1:2,400; #612110; BD Biosciences,
Franklin Lakes, NJ, USA), polyclonal rabbit anti-human S100A (ready
for use/dilution, 1:1; #760-2523; Ventana Medical Systems, Inc.,
Tucson, AZ, USA), monoclonal mouse anti-human cytokeratin
(dilution, 1:800; #M3515; Dako, Glostrup, Denmark), monoclonal
mouse anti-human cluster of differentiation (CD)34 (dilution,
1:400; #413361; Nichirei Corporation, Tokyo, Japan), monoclonal
mouse anti-human p16 (dilution, 1:800; #551153; BD Pharmingen, San
Diego, CA, USA), monoclonal mouse anti-human α-smooth muscle actin
(α-SMA; dilution, 1:800; #M0851; Dako) and monoclonal mouse
anti-human epithelial membrane antigen (EMA; ready for
use/dilution, 1:1; #M1504; Dako). Antibodies for cytokeratin,
INI-1, EMA and p16 were incubated at 100°C for 30 min, whereas
antibodies for CD34, α-SMA and S100A required no pretreatment.
Immunohistochemical analysis demonstrated that the tumor was
positive for INI-1, and negative for S100A, cytokeratin, CD34, p16,
α-SMA and EMA. As wide resection of the tumor was considered to be
difficult due to the tumor location, the patient underwent carbon
ion radiotherapy (70.4 Gy equivalence for a total of 16 fixed
fractions for 3 weeks) at the Research Center Hospital for Charged
Particle Therapy, National Institute of Radiological Sciences
(Chiba, Japan). No severe acute toxicity associated with the carbon
ion radiotherapy was observed.
A total of four months subsequent to administration
of carbon ion radiotherapy (September 2013), the patient developed
a high-grade fever (40°C) and was admitted to Shingu Municipal
Medical Center with suspected pyelonephritis. Laboratory data
revealed an increased level of C-reactive protein (8.05 mg/dl;
normal range, <0.3 mg/dl) and a normal white blood cell count
(7,400/µl; normal range, 4,000–8,000/µl). A blood culture was
negative for infection. Although intensive antibiotic therapy was
administered (cefotiam 2 g/day for 12 days, followed by doripenem 1
g/day for 1 week), the high grade fever continued and the patient's
laboratory data did not improve. CT scans of the head, chest,
abdomen and pelvis revealed no source of infection. However,
recurrence of the MPNST at the shoulder, an area not previously
treated with carbon ion radiotherapy, was identified. No distant
metastases were observed. MRI revealed tumor recurrence in the
deltoid muscle. Therefore, the high-grade fever was suspected to be
a neoplastic fever. Following 24 h treatment with naproxen (300
mg/day for 3 weeks), the fever was alleviated. The patient was
subsequently referred to Mie University Hospital in October 2013 to
undergo wide excision of the recurrent tumor. The histopathological
findings revealed interweaving atypical cells with a high mitotic
rate, necrosis and degeneration, consistent with a high-grade
MPNST. A total of 3 weeks subsequent to surgery, the patient
suddenly developed a stagger and dysarthria. Treatment with all
drugs, including oxycodone and pregabalin, was discontinued due to
suspected opioid overdosing; however, the symptoms did not improve.
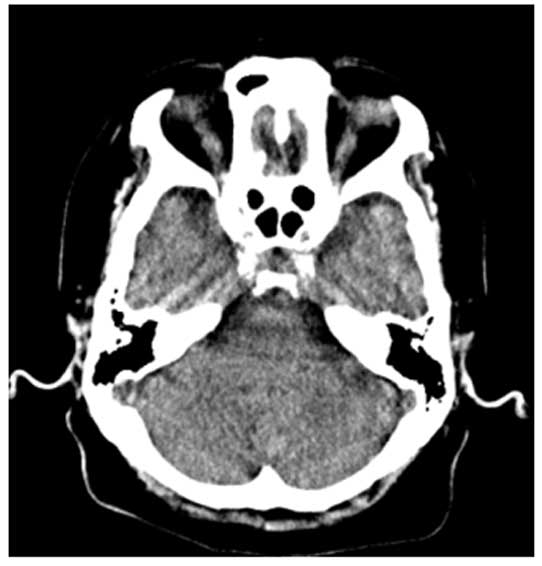
Plain CT scanning (Aquilion ONE; Toshiba Corporation, Tokyo, Japan)
performed in November 2013 revealed no significant changes in the
brain and chest (Fig. 2). Dysphagia
emerged and left upper limb pain was alleviated the following day.
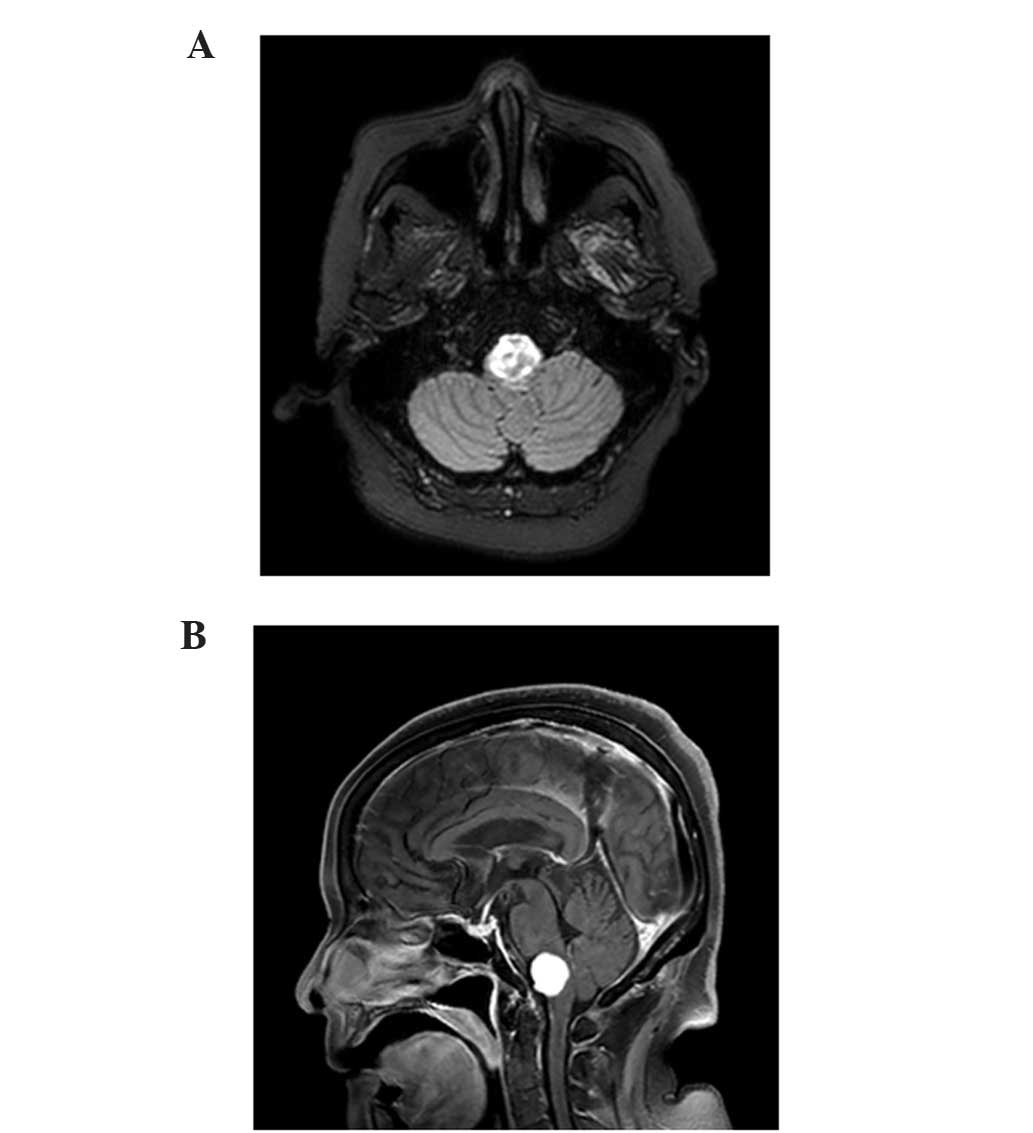
MRI revealed metastasis in the medulla (Fig. 3A and B). A total of 5 days subsequent
to the onset of dysarthria, the patient succumbed due to
respiratory failure.
Discussion
To the best of our knowledge, no previous cases of
medullary metastasis arising from a MPNST, in the absence of lung
metastasis, have been reported in the literature. Although brain
metastases are estimated to develop in 15–40% of cancer patients,
they are considered a rare event in soft tissue sarcoma patients,
with a reported prevalence of 1–6% (4). Furthermore, brain metastasis occurring
in the absence of lung metastasis is considered a relatively rare
event (4–6). Notably, Ogose et al (6) reported that alveolar soft part sarcoma,
extraskeletal Ewing's sarcoma and rhabdomyosarcoma exhibit
relatively high incidences of brain metastases. Regarding MPNSTs of
the lung, only 21 cases of metastases to the brain have been
reported previously (7). Of these 21
cases, the frontal lobe was the most common location for brain
metastasis (7). Metastasis to the
brainstem is uncommon and accounts for only 3–5% of all brain
metastases (8). Therefore, in the
present case, dysphagia and stagger were initially suspected to be
side effects of oxycodone treatment. The clinical presentation of
brain metastasis is characterized by the acute onset of
neurological symptoms (1,7). The clinical symptoms of medullary
metastasis may be similar to a medullary infarction, which include
dysphagia, dysarthria, dysphonia, vertigo, contralateral deficits
in pain and temperature sensation, and vomiting (9). The median survival time in patients
exhibiting untreated metastasis to the brain is 1–2 months
(10–12). Smalley et al (13) reported a median survival time of 11.7
months following resection of solitary brain metastases. Generally,
brainstem metastases are not treated with conventional neurosurgery
due to the risk of neurological damage (9,10), and
Gamma Knife surgery is typically considered for brainstem
metastasis (9) Gamma knife surgery
exhibits a local control rate of 83–94% with a median survival time
of 7–12 months (10). In the present
case, gamma knife surgery may have been an appropriate treatment
for tumor control. However, the patient's general condition rapidly
worsened and the patient succumbed 5 days subsequent to the initial
presentation with the clinical symptoms of brain metastasis.
Therefore, no treatment was administered. Although a plain brain CT
scan was performed prior to resection of the recurrent tumor, brain
metastasis was not detected. MRI is a useful examination tool for
the identification of brain metastasis; however, the high medical
cost of MRI as a routine examination must be considered due to the
rarity of such metastases. In conclusion, due to the rarity of
brain metastases and the lack of information regarding treatment,
follow-up treatment strategies for brain metastases arising from
soft tissue sarcoma require additional investigation.
Acknowledgements
The authors would like to thank Dr R Imai from the
Research Center Hospital for Charged Particle Therapy, National
Institute of Radiological Sciences (Chiba, Japan) for performing
carbon ion radiation.
References
|
1
|
Zhou W, Du X, Song F, Zheng H, Chen K,
Zhang W and Yang J: Prognostic roles for fibroblast growth factor
receptor family members in malignant peripheral nerve sheath tumor.
Oncotarget. Mar 14–2016.(Epub ahead of print).
|
|
2
|
Rawal A, Yin Q, Roebuck M, Sinopidis C,
Kalogrianitis S, Helliwell TR and Frostick S: Atypical and
malignant peripheral nerve-sheath tumors of the brachial plexus:
Report of three cases and review of the literature. Microsurgery.
26:80–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valentin T, Le Cesne A, Ray-Coquard I,
Italiano A, Decanter G, Bompas E, Isambert N, Thariat J, Linassier
C and Bertucci F: Management and prognosis of malignant peripheral
nerve sheath tumors: The experience of the French Sarcoma Group
(GSF-GETO). Eur J Cancer. 56:77–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Espat NJ, Bilsky M, Lewis JJ, Leung D and
Brennan MF: Soft tissue sarcoma brain metastases. Prevalence in a
cohort of 3829 patients. Cancer. 94:2706–2711. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura T, Matsumine A, Matsubara T,
Asanuma K, Niimi R, Uchida A and Sudo A: Retrospective analysis of
metastatic sarcoma patients. Oncol Lett. 2:315–318. 2011.PubMed/NCBI
|
|
6
|
Ogose A, Morita T, Hotta T, Kobayashi H,
Otsuka H, Hirata Y and Yoshida S: Brain metastases in
musculoskeletal sarcomas. Jpn J Clin Oncol. 29:245–247. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shweikeh F, Bukavina L, Saeed K, Sarkis R,
Suneja A, Sweiss F and Drazin D: Brain metastasis in bone and soft
tissue cancers: A review of incidence, interventions, and outcomes.
Sarcoma. 2014:4751752014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamm AF, Elaimy AL, Lamoreaux WT, Mackay
AR, Fairbanks RK, Demakas JJ, Cooke BS and Lee CM: A review of the
clinical outcomes for patients diagnosed with brainstem metastasis
and treated with stereotactic radiosurgery. ISRN Surg.
2013:6528952013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai-fung Li, Gilberto Ka-kit Leung, Ronnie
Sin-lun Ho and Wai-man Lui: Recurrent natural killer cell lymphoma
with central nervous system metastasis mimicking cerebellar
infarction. World Neurosurg. 84:2074.e5–9. 2015. View Article : Google Scholar
|
|
10
|
Yoo TW, Park ES, Kwon do H and Kim CJ:
Gamma knife radiosurgery for brainstem metastasis. J Korean
Neurosurg Soc. 50:299–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lang EF and Slater J: Metastatic brain
tumors: results of surgical and non-surgical treatment. Surg Clin
North Am. 44:865–872. 1964.PubMed/NCBI
|
|
12
|
Markesbery WR, Brooks WH, Gupta GD and
Young AB: Treatment for patients with cerebral metastases. Arch
Neurol. 35:754–756. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smalley SR, Laws ER Jr, O'Fallon JR, Shaw
EG and Schray MF: Resection for solitary brain metastasis. Role of
adjuvant radiation and prognostic variables in 229 patients. J
Neurosurg. 77:531–540. 1992. View Article : Google Scholar : PubMed/NCBI
|