Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignancy and the third leading cause of cancer-associated
mortality. It is estimated that 500,000–1,000,000 new cases of HCC
develop every year (1,2). The disease is often fatal since HCC
shows aggressive metastasis and is often diagnosed at an advanced
stage (3). Despite advances in
surgical and chemotherapeutic approaches, the prognosis of HCC is
poor; the 5-year survival rate of patients with HCC is as low as
20–50%, even in early-stage HCC subsequent to radical resection
(4,5).
Recurrence following treatment remains one of the most important
causes of poor long-term survival. Molecular markers for the
prediction of prognosis of HCC may be able to aid the development
of more effective therapeutic strategies (6).
The hedgehog (Hh) signaling pathway is an important
pathway in the development of embryos and is essential for the
growth and differentiation of embryonic cells and the maintenance
of stem cells (7). However, abnormal
activation of this signaling pathway may cause excessive cell
proliferation, resulting in the development of cancer (8). Recently, abnormal activation of the Hh
pathway has been reported in diverse cancers, including skin,
gastrointestinal tract, breast, prostate, pancreas and lung cancer
(9–15). The Hh signaling pathway is composed of
three ligands, consisting of sonic Hh (Shh), Indian Hh, and desert
Hh, which bind to the transmembrane receptor Patched 1 (Ptch1)
(2). The Hh signaling cascade is
initiated as Hh binds to the 12 transmembrane proteins that form
Ptch1. This internalizes Ptch1 and relieves the suppression of
Smoothened (Smo), which activates the glioma-associated oncogene
Gli transcription factor. The Gli transcription factor exists in 3
forms, consisting of GLI family zinc finger 1 (Gli-1), GLI family
zinc finger 2 (Gli-2) and GLI family zinc finger 3 (Gli-3)
(11,16).
Although the detailed mechanism of this pathway has
yet to be established, it is known that full-length Gli-3 is
transported into the nucleus in order to activate the Hh target
genes (17). Among the Hh target
genes functioning as transactivators, Gli-1 is considered to be a
marker of the Hh pathway activation (17,18). In
addition, the Hh pathway cascade cross-talks with the WNT,
epidermal growth factor (EGF)/fibroblast growth factor (FGF) and
transforming growth factor (TGF)-β/Activin/Nodal/bone morphogenetic
protein (BMP) signaling cascades, which are implicated in
epithelial-to-mesenchymal transition (EMT) through repression of
E-cadherin and activation of N-cadherin. Therefore, the Hh
signaling pathway is involved in the invasion and metastasis of
cancers (19). EMT also plays an
important role in the invasion and metastasis of cancers.
Epithelial cells lose cell polarity and cell-cell adhesion
properties, and gain migratory and invasive properties to become
mesenchymal cells during the process of EMT (20). Cells that have undergone EMT behave
similarly to stem cells isolated from normal or neoplastic cell
populations in numerous ways (21).
The basic helix-loop-helix transcription factor Twist and the
zinc-finger transcriptional repressor Snail are important
regulators of EMT. These transcription factors induce changes in
the spreading ability and morphology of cancer cells through
suppression of E-cadherin expression, and epithelial cancer cells
then acquire mesenchymal markers (22). The Hh signaling pathway may be one of
the signaling pathways in the tumor microenvironment that involves
EMT (23). However, the association
between the Hh signaling pathway and EMT in HCC is remains poorly
understood.
In the present study, the expression of Gli-1 and
Gli-2, which are key transcriptional factors in the Hh signaling
pathway, and of Twist and E-cadherin, which are two factors
involved in EMT, was evaluated in patients with HCC, according to
immunohistochemical results. In addition, the association between
the expression of these factors and known clinicopathological
factors associated with prognosis was analyzed. The present
findings may uncover the clinical significance of the Hh signaling
pathway and EMT in HCC.
Materials and methods
Samples
In total, 42 samples of HCC tissue were obtained
from surgical resection, including lobectomy and segmentectomy,
performed at the Department of Surgery, Chosun University Hospital
(Gwangju, South Korea) between February 2006 and December 2012. The
Institutional Review Board of Chosun University Hospital waived the
requirement for written informed consent due to the nature of the
study (CHOSUN 2014-04-003). For the comparative analysis, 20
samples of non-tumorous liver (NTL) tissue were included.
Histopathological analysis
Each case was re-evaluated by retrospective analysis
of the medical records and the tissue slide files at the Department
of Pathology, College of Medicine, Chosun University (Gwangju,
South Korea). The age, gender, presence of hepatitis B surface
antigen (HBsAg) and level of serum α-fetoprotein (s-AFP) were
assessed. The examined tissues were fixed in 10% neutral formalin
and the prepared paraffin-embedded tissues were sectioned (4–5 µm
in thickness). Hematoxylin and eosin (H&E) staining (Ventana
Medical Systems, Tucson, AZ, USA) was performed according to the
standard procedure and the sections were examined under a light
microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan). By
review of the H&E slides, the histological diagnosis, tumor
size, T stage, Edmonson-Steiner grade (24), invasion of liver capsule and bile duct
and liver cirrhosis in the non-tumor liver tissue were reevaluated.
A representative area of tumor suitable for the purpose of the
present study was selected, and the slides were prepared for
immunohistochemical analysis.
Immunohistochemical staining
All specimens were tested using rabbit polyclonal
anti-Gli-1 (catalog no., ab92611; Abcam, Cambridge, MA, USA),
anti-Gli-2 (catalog no., ab7181) and anti-Twist (catalog no.,
ab50581; Abcam), and mouse monoclonal anti-E-cadherin (catalog no.,
NCL-L-E-Cad; Novocastra; Leica Biosystems, Milton Keynes, UK)
antibodies, according to the manufacturer's protocol.
Immunolocalization was performed using the ImmunoCruz Mouse
Staining System (catalog no., sc-2050; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), according to the manufacturer's protocol.
The staining process was performed according to the protocol
recommended by the manufacturer of the NexES autoimmunostainer
(Ventana Medical Systems).
Briefly, the 4-µm sections obtained following
formalin fixation and paraffin embedding were deparaffinized in
xylene and then rehydrated with distilled water through a graded
series of ethanol solutions. The sections were then placed in a
glass jar with 10 mmol/l citrate buffer (pH 6.0) and were
irradiated in a microwave oven for 15 min at 99°C. The sections
were allowed to cool in the jar at room temperature for 20 min. The
slides were then rinsed with Tris-buffered saline and, subsequent
to quenching the endogenous peroxidase activity in 0.3% hydrogen
peroxide for 10 min. The slides were then washed as described, and
immunohistochemistry was performed using the NexES
autoimmunostainer. Slides were incubated with primary antibodies
against Gli-1 (dilution, 1:100), Gli-2 (dilution, 1:100), Twist
(dilution, 1:100) and E-cadherin (dilution, 1:100) for 32 min. The
ultraView Universal DAB Detection kit (catalog no., 760-500;
Ventana Medical Systems) was used as the secondary detection
method. This kit includes the biotinylated immunoglobulin (Ig)
secondary purified goat anti-mouse IgG and IgM and goat anti-rabbit
IgG antibodies in phosphate-buffered saline with preservative.
Incubation was performed for 8 min and was followed by the addition
of conjugated streptavidin horseradish peroxidase for 8 min. Slides
were then counterstained with hematoxylin (catalog no., 760-2021;
Ventana Medical Systems).
Analysis and interpretation of
staining
Representative histological sections of the lesions
were immunohistochemically stained and the expression of the target
proteins was analyzed using antibodies against Gli-1, Gli-2, Twist
and E-cadherin.
To assess the expression of Gli-1 and Gli-2, a total
of 10 high-power fields were selected from each section, and
nuclear protein expression in ≥10% of cells was considered to
indicate positive expression (23).
E-cadherin was expressed in the cellular membrane of normal
hepatocytes and cancer cells. Expression intensity that was similar
or increased relative to that of adjacent normal hepatocytes was
defined as maintenance of expression; weak expression compared with
normal hepatocytes or loss of expression was defined as a decrease
in expression (25). Twist was
expressed in the nucleus and cytoplasm of cancer cells; positive
expression was defined as a staining intensity higher than that of
background staining.
Statistical analysis
Statistical analysis was performed using SPSS 12.0
software (SPSS, Inc., Chicago, IL, USA). The χ2 test and
Fisher's exact test were used to demonstrate the association
between the expression of proteins and clinicopathological factors.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical and histological
features
The clinicopathological characteristics of the
patients are summarized in Table I.
The mean age of the 42 patients with HCC at the time of surgery was
57.8 years, and the ratio of male to female patients was 35:7
(83.3:16.7%), showing a male predominance. In total, 26 patients
(61.9%) showed expression of HbsAg. The tumor size was <5 cm in
29 patients (69.0%) and ≥5 cm in 13 patients (31.0%). There were
prominent cirrhotic lesions of the background liver in 33 patients
with HCC (78.6%). The T stage, which was assessed according to
tumor size, tumor number and vascular invasion, was classified as
pT1 in 31 patients (73.8%), pT2 in 5 patients (11.9%) and pT3 in 6
patients (14.3%). Capsular invasion was found in 8 patients (19.0%)
and bile duct invasion was found in 3 patients (7.1%).
 | Table I.Clinicopathological characteristics
of patients with hepatocellular carcinoma. |
Table I.
Clinicopathological characteristics
of patients with hepatocellular carcinoma.
| Characteristic | Value, n (%) |
|---|
| Gender |
|
|
Male | 35 (83.3) |
|
Female | 7
(16.7) |
| Age |
|
| Mean,
years | 57.8 |
| <50
years | 5
(11.9) |
| ≥50
years | 37 (88.1) |
| HBsAg |
|
|
Present | 26 (61.9) |
|
Absent | 16 (38.1) |
| Tumor size |
|
| <5
cm | 29 (69.0) |
| ≥5
cm | 13 (31.0) |
| pT |
|
| 1 | 31 (73.8) |
| 2 | 5
(11.9) |
| 3 | 6
(14.3) |
| Cirrhosis |
|
|
Present | 33 (78.6) |
|
Absent | 9
(21.4) |
| E-S grade |
|
| 1 | 8
(19.0) |
| 2 | 32 (76.2) |
| 3 | 2 (4.8) |
| Capsule
invasion |
|
|
Present | 8
(19.0) |
|
Absent | 34 (81.0) |
| Bile duct
invasion |
|
|
Present | 3 (7.1) |
|
Absent | 39 (92.9) |
| Histology |
|
|
Trabecular | 20 (47.6) |
|
Mixeda | 18 (42.9) |
|
Solid | 4 (9.5) |
| s-AFP |
|
| <100
ng/ml | 31 (73.8) |
| ≥100
ng/ml | 11 (26.2) |
Histologically, tumors were trabecular type in 20
patients (47.6%), pseudoglandular or mixed type of trabecular and
pseudoglandular types in 18 patients (42.9%), and solid type in 4
patients (9.5%). The s-AFP level was <100 ng/ml in 31 patients
(73.8%) and ≥100 ng/ml in 11 patients (26.2%).
Immunohistochemistry results
The present study examined the expression of the Hh
signaling pathway-associated proteins Gli-1 and Gli-2 and the
EMT-associated proteins Twist and E-cadherin in 42 tissue samples
with HCC and 20 tissue samples of NTL.
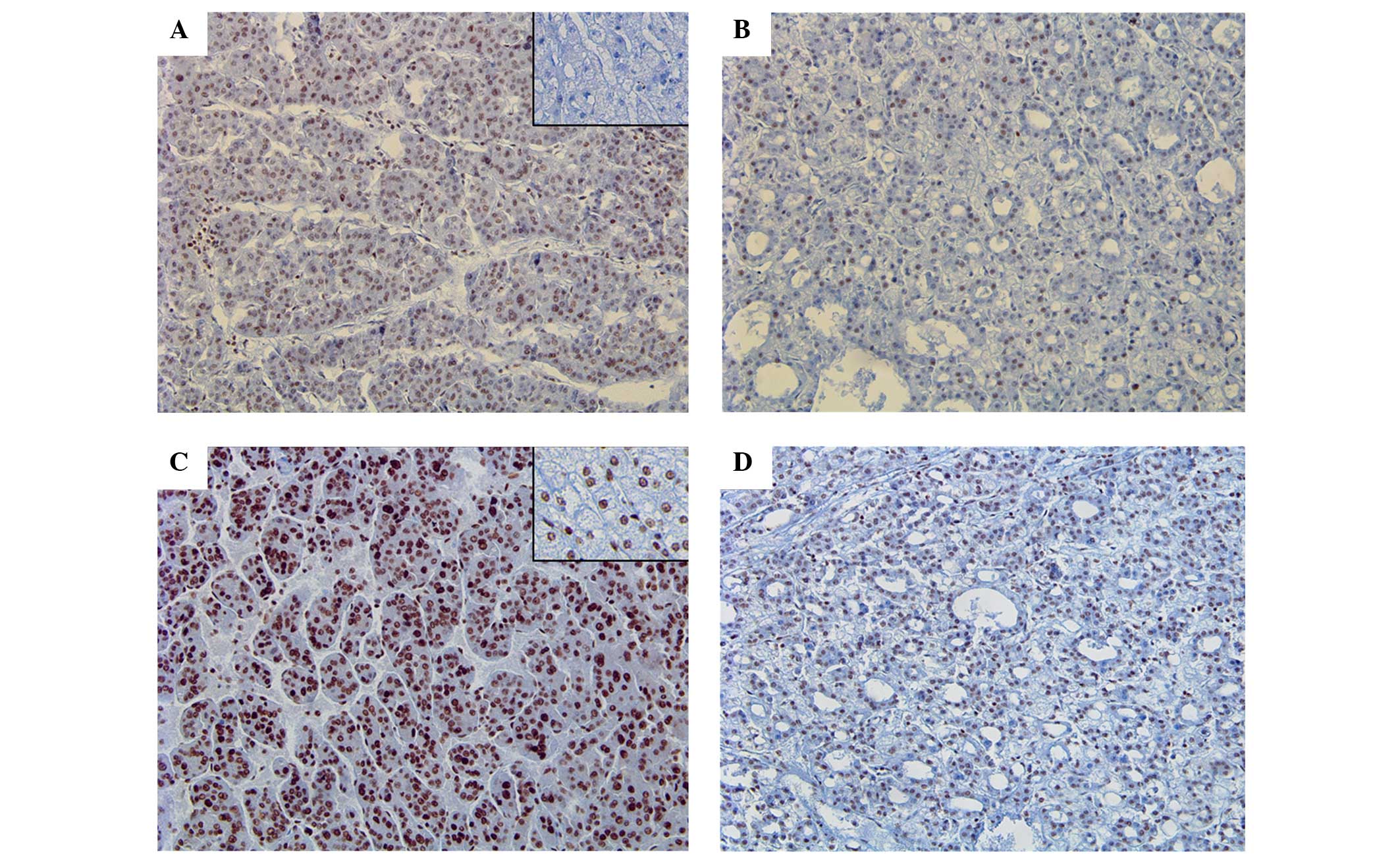
The expression of Gli-1 was identified in 2 NTL
tissues (10.0%) and 17 HCC tissues (40.5%), which showed a
statistically significant difference in expression between NTL and
HCC (P=0.0.19) (Fig. 1A and B).
However, Gli-2 expression was identified in 14 NTL tissues (70.0%)
and 33 HCC tissues (78.6%), which did not show any difference
between two groups (Fig. 1C and
D).
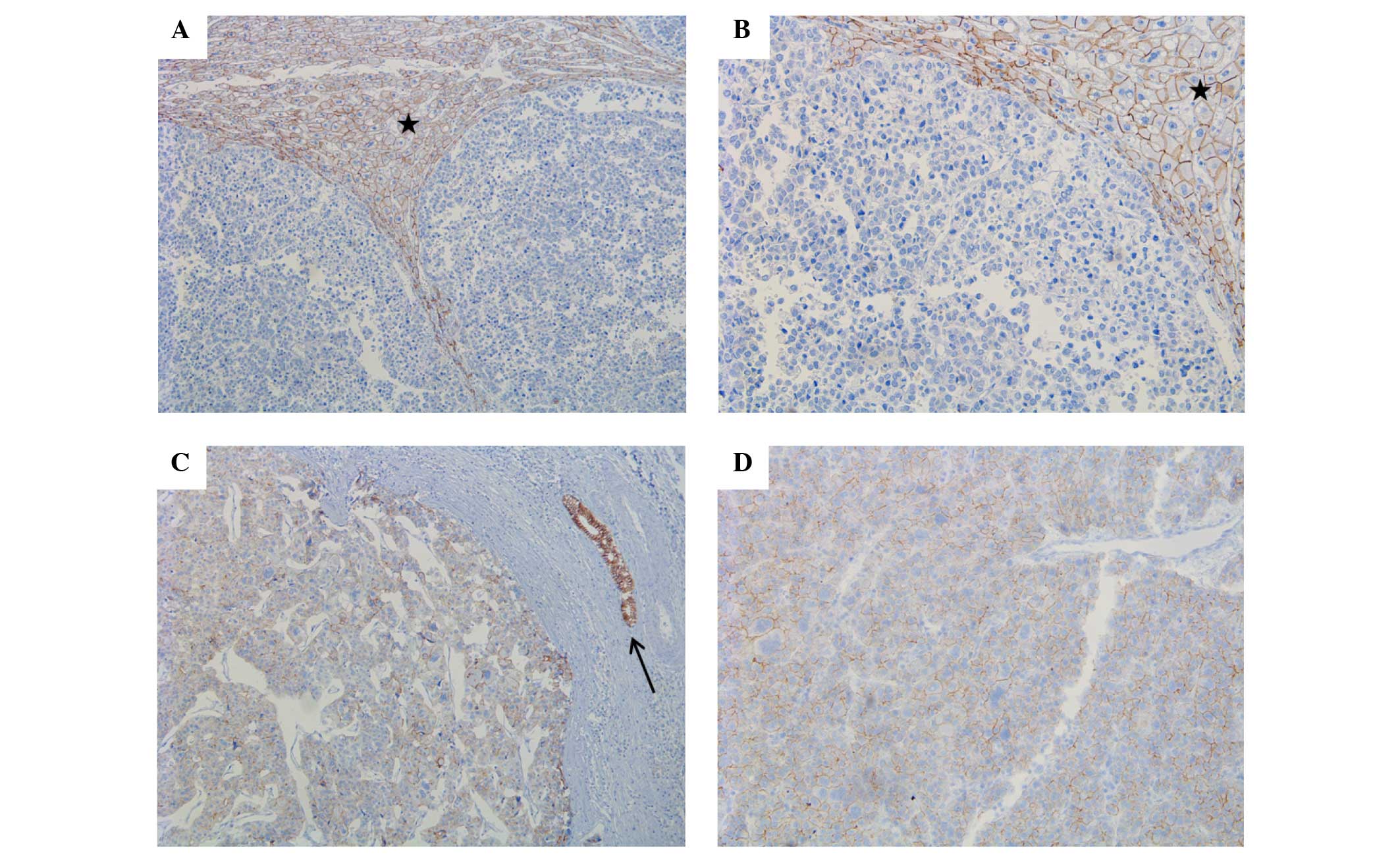
Strong E-cadherin expression was identified in all
NTL tissues (100%), whereas only 8 HCC tissues were positive for
E-cadherin expression, which showed a statistically significant
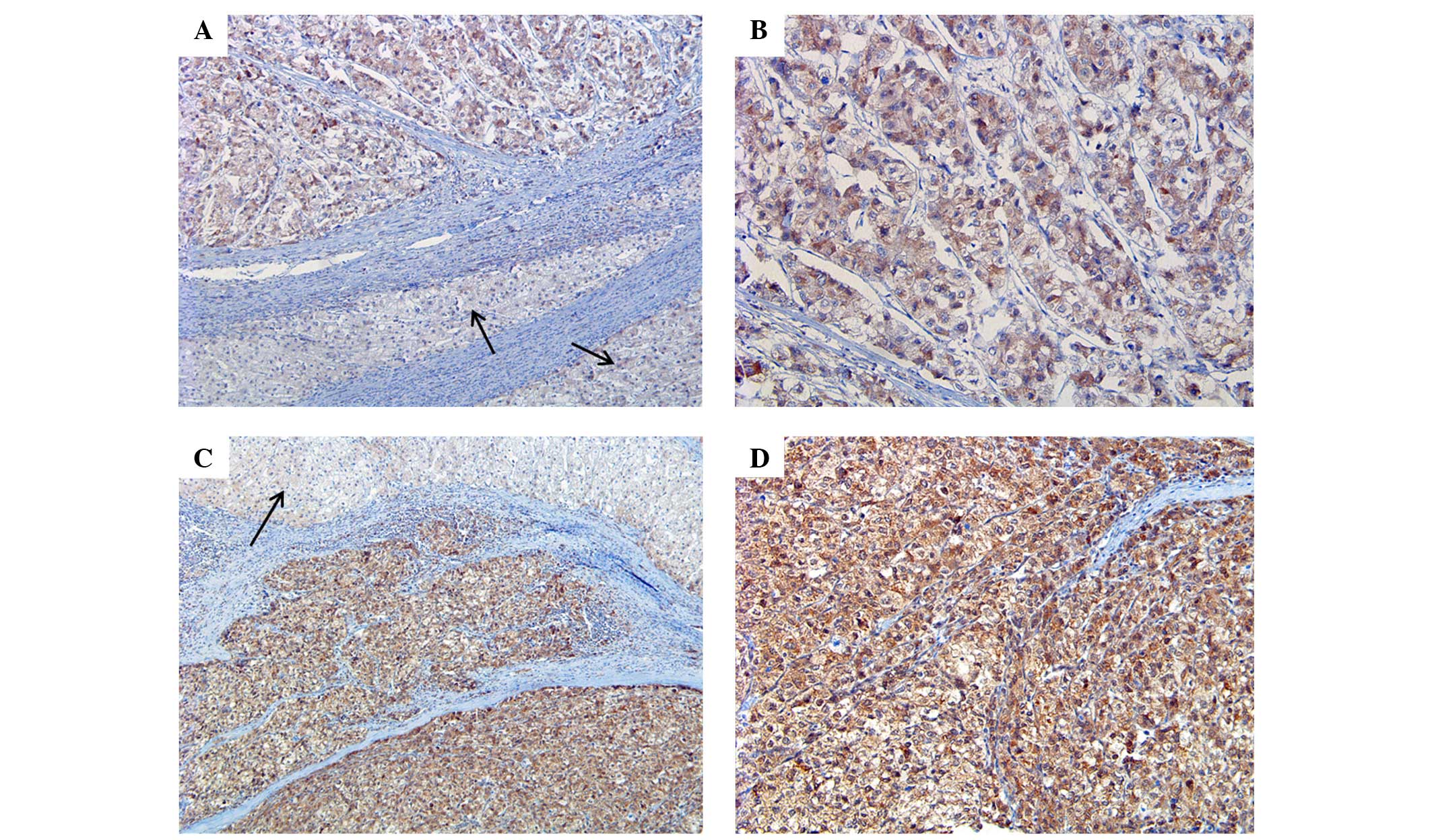
difference between the two groups (P<0.001) (Fig. 2). Twist expression was also
significantly different between the two groups, with Twist
expression identified in 8 NTL tissues (40.0%) and 34 HCC tissues
(81.0%) (P=0.003; Table II; Fig. 3).
 | Table II.Expresion of Gli-1, Gli-2, Twist and
E-cadherin in NTL and HCC tissues. |
Table II.
Expresion of Gli-1, Gli-2, Twist and
E-cadherin in NTL and HCC tissues.
| Protein | NTL, n (%) | HCC, n (%) | P-value |
|---|
| Gli-1 |
|
|
0.019 |
|
Total | 20
(100.0) | 42
(100.0) |
|
| + | 2
(10.0) | 17 (40.5) |
|
| − | 18
(90.0) | 25 (59.5) |
|
| Gli-2 |
|
|
0.532 |
|
Total | 20
(100.0) | 42
(100.0) |
|
| + | 14 (70.0) | 33 (78.6) |
|
| − | 6
(30.0) | 9
(21.4) |
|
| Twist |
|
|
0.003 |
|
Total | 20
(100.0) | 42
(100.0) |
|
| + | 8
(40.0) | 34 (81.0) |
|
| − | 12
(60.00) | 8
(19.0) |
|
| E-cadherin |
|
| <0.001 |
|
Total | 20
(100.0) | 42
(100.0) |
|
| + | 20
(100.0) | 8
(19.0) |
|
| − | 0 (0.0) | 34
(810.0) |
|
Association between E-cadherin
expression and clinicopathological factors
Loss or decrease of E-cadherin expression was
identified in 33 out of 42 patients (78.6%) with HCC, which was
associated with the histological type of HCC, as loss or decrease
of expression was identified in 12/20 trabecular type tissues
(60%), 17/18 mixed type tissues (94.4%) and 4/4 solid type tissues
(100%). E-cadherin expression demonstrated a significant
association with histological differentiation of HCC (P=0.021)
(Fig. 2). However, other
clinicopathological factors were not associated with E-cadherin
expression (Table III).
 | Table III.Association between expression of
Twist and E-cadherin and clinicopathological features of
hepatocellular carcinoma. |
Table III.
Association between expression of
Twist and E-cadherin and clinicopathological features of
hepatocellular carcinoma.
|
|
| E-cadherin, n | Twist, n |
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n (%) | + | − | P-value | + | − | P-value |
|---|
| HBsAg |
|
|
| 0.265 |
|
| 1.000 |
|
Present | 26 (61.9) | 4 | 22 |
| 21 | 5 |
|
|
Absent | 16 (38.1) | 5 | 11 |
| 13 | 3 |
|
| Tumor size |
|
|
| 0.232 |
|
|
0.043b |
| <50
mm | 29 (69.0) | 8 | 21 |
| 21 | 8 |
|
| ≥50
mm | 13 (31.0) | 1 | 12 |
| 13 | 0 |
|
| pT |
|
|
| 0.660 |
|
| 0.655 |
| 1 | 31 (73.8) | 6 | 26 |
| 25 | 7 |
|
|
2a | 11 (26.2) | 3 | 7 |
| 9 | 1 |
|
| Cirrhosis |
|
|
| 1.000 |
|
| 0.168 |
|
Present | 33 (78.6) | 7 | 26 |
| 25 | 8 |
|
|
Absent | 9
(21.4) | 2 | 7 |
| 9 | 0 |
|
| E-S grade |
|
|
| 0.558 |
|
| 0.651 |
| 1 | 8
(19.0) | 1 | 7 |
| 7 | 1 |
|
| 2 | 32 (76.2) | 8 | 24 |
| 25 | 7 |
|
| 3 | 2 (4.8) | 0 | 2 |
| 2 | 0 |
|
| Capsule
invasion |
|
|
| 1.000 |
|
| 0.635 |
|
Present | 8
(19.0) | 2 | 6 |
| 6 | 2 |
|
|
Absent | 34 (81.0) | 7 | 27 |
| 26 | 8 |
|
| Bile duct
invasion |
|
|
| 0.525 |
|
| 1.000 |
|
Present | 3 (7.1) | 1 | 2 |
| 3 | 0 |
|
|
Absent | 39 (92.9) | 8 | 31 |
| 31 | 8 |
|
| Histology |
|
|
|
0.021b |
|
| 0.297 |
|
Trabecular | 20 (47.6) | 8 | 12 |
| 14 | 6 |
|
|
Mixedb | 18 (42.9) | 1 | 17 |
| 16 | 2 |
|
|
Solid | 4 (9.5) | 0 | 4 |
| 4 | 0 |
|
| s-AFP |
|
|
| 0.403 |
|
| 0.657 |
| <100
ng/ml | 31 (73.8) | 8 | 23 |
| 24 | 7 |
|
| ≥100
ng/ml | 11 (26.2) | 1 | 10 |
| 10 | 1 |
|
Association between Twist expression
and clinicopathological factors
Twist expression was identified in 34/42 HCC tissues
(81.0%) and was associated with tumor size, as Twist expression was
identified in 21/29 tumors <5 cm in size (72.4%) and in 13/13
tumors ≥5 cm in size (100%) (P=0.043). However, other
clinicopathological factors were not significantly associated with
E-cadherin expression (Table
III).
Association between the expression of
Gli-1 and Gli-2 and clinicopathological factors
Gli-1 expression and Gli-2 expression did not show a
significant association with any clinicopathological factors
(Table IV).
 | Table IV.Association between the expression of
Gli-1 and Gli-2 and clinicopathological features of hepatocellular
carcinoma. |
Table IV.
Association between the expression of
Gli-1 and Gli-2 and clinicopathological features of hepatocellular
carcinoma.
|
|
| Gli-1, n | Gli-2, n |
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n (%) | + | − | P-value | + | − | P-value |
|---|
| HBsAg |
|
|
| 0.195 |
|
| 0.202 |
|
Present | 26 (61.9) | 13 | 13 |
| 22 | 4 |
|
|
Absent | 16 (38.1) | 4 | 12 |
| 11 | 5 |
|
| Tumor size |
|
|
| 0.738 |
|
| 0.421 |
| <50
mm | 29 (69.0) | 11 | 18 |
| 22 | 4 |
|
| ≥50
mm | 13 (31.0) | 6 | 7 |
| 11 | 2 |
|
| pT |
|
|
| 0.714 |
|
| 1.000 |
| 1 | 31 (73.8) | 12 | 20 |
| 25 | 7 |
|
|
2a | 11 (26.2) | 5 | 5 |
| 8 | 2 |
|
| Cirrhosis |
|
|
| 0.446 |
|
| 0.375 |
|
Present | 33 (78.6) | 12 | 21 |
| 27 | 6 |
|
|
Absent | 9
(21.4) | 5 | 4 |
| 6 | 3 |
|
| E-S grade |
|
|
| 0.603 |
|
| 0.443 |
| 1 | 8
(19.0) | 2 | 6 |
| 7 | 1 |
|
| 2 | 32 (76.2) | 14 | 18 |
| 24 | 8 |
|
| 3 | 2 (4.8) | 1 | 1 |
| 2 | 0 |
|
| Capsule
invasion |
|
|
| 1.0 |
|
| 0.336 |
|
Present | 8
(19.0) | 3 | 5 |
| 5 | 3 |
|
|
Absent | 34 (81.0) | 14 | 20 |
| 28 | 6 |
|
| Bile duct
invasion |
|
|
| 0.260 |
|
| 1.000 |
|
Present | 3 (7.1) | 0 | 3 |
| 3 | 0 |
|
|
Absent | 39 (92.9) | 17 | 22 |
| 30 | 9 |
|
| Histology |
|
|
| 0.704 |
|
| 0.462 |
|
Trabecular | 20 (47.6) | 9 | 11 |
| 16 | 4 |
|
|
Mixedb | 18 (42.9) | 6 | 12 |
| 13 | 5 |
|
|
Solid | 4 (9.5) | 2 | 2 |
| 4 | 0 |
|
| s-AFP |
|
|
| 0.268 |
|
| 0.209 |
| <100
ng/ml | 31 (73.8) | 11 | 20 |
| 26 | 5 |
|
| ≥100
ng/ml | 12 (26.2) | 6 | 5 |
| 7 | 4 |
|
Correlation between E-cadherin
expression and Twist expression
A significant inverse association was identified
between Twist and E-cadherin expression (P=0.006), as 34/42 HCC
tissues (81.0%) showed Twist expression, and 30/34 of these
Twist-positive HCC tissues showed a loss of or prominent decrease
in E-cadherin expression (Table
V).
 | Table V.Association between Twist and
E-cadherin expression in hepatocellular carcinoma tissues. |
Table V.
Association between Twist and
E-cadherin expression in hepatocellular carcinoma tissues.
|
| E-cadherin, n
(%) |
|
|---|
|
|
|
|
|---|
| Twist | + | − | P-value |
|---|
| + | 4 (9.6) | 30 (71.4) | 0.006 |
| − | 5
(11.9) | 3 (7.1) |
|
Association between the Hh signaling
pathway-associated proteins Gli-1 and Gli-2 and the EMT-associated
proteins Twist and E-cadherin
There was no significant association between the
expression of the Hh signaling pathway-associated proteins and the
EMT-associated proteins.
Discussion
The Hh signaling pathway plays an important role in
embryonic development, organ maturation and morphological
maintenance (26). This pathway is
inactivated when embryos become mature. Abnormal activation of the
Hh signaling pathway is closely associated with the occurrence,
invasion and metastasis of certain malignant tumors, including
gastric cancer (19,20). Out of several Hh target genes, Gli-1
is a major transcription factor of the Hh signaling pathway
(6). High expression of Gli-1 has
been reported in several cancers, including gastric and colon
cancer, in contrast to normal cells (6,23). Wang
et al (23) reported that
Gli-1 expression increased markedly in progressive gastric cancer
and was closely associated with increased Snail (EMT marker)
expression and decreased E-cadherin expression. The positive Gli-1
expression group had a significantly increased invasion depth and a
higher percentage of patients with lymph node (LN) invasion
compared with the negative Gli-1 expression group. In the study of
colon cancer and melanosis coli by Wang et al (27), it was also reported that the protein
and mRNA levels of Gli-1 are aberrantly elevated in colon cancer
compared with normal tissues.
Gli-2 is one of the transcription factors of the Hh
pathway and regulates the expression of downstream target genes,
including Gli-1, Bcl-2, c-FLIP, cyclin D1, c-Myc and vascular
endothelial growth factor (6,28,29). Gli-2
overexpression has also been reported in several malignant tumors,
such as medulloblastoma, breast cancer and HCC (6,30), and
Gli-2 overexpression is associated with poor survival (6).
Im et al (30)
analyzed the expression of Hh signaling proteins, including Shh,
Ptch, Smo, Gli-1, Gli-2 and Gli-3. The expression of Hh signaling
proteins demonstrated a statistically significant correlation with
certain prognostic factors, such as LN metastasis and tumor stage.
LN metastasis was associated with Shh and Ptch expression and tumor
stage was associated with Shh and Gli-3 expression. Gli-2
expression was associated with a poorer overall survival time. In
addition, in a study of 68 cases of HCC and 20 cases of NTL by
Zhang et al (6), high
expression of Gil-2 was identified in HCC tissue and was associated
with poor prognostic factors, including differentiation of cancer
cells, capsular invasion, tumor recurrence and intra-hepatic
metastasis subsequent to hepatectomy.
EMT, which is one of the characteristics of the
tumor microenvironment, also plays an important role in the
invasion and metastasis of cancers. Through EMT, epithelial cells
lose cell polarity and adhesion properties between epithelial
cells, and gain migratory and invasive properties to become
mesenchymal cells (20). A hallmark
of EMT is downregulation of the cell adhesion molecule E-cadherin.
E-cadherin is a transmembrane protein that is essential for the
formation of adherent cell junctions and the upregulation of
mesenchymal molecules, including vimentin, fibronectin and
N-cadherin. It has been reported that suppression of E-cadherin is
associated with dedifferentiation of cancer cells, infiltrative
growth of tumors and the high incidence of LN metastasis in several
cancers (31–33). This suppression is induced by several
routes, including gene mutation, promoter hypermethylation or
promoter repression by transcription repressors during tumor
progression. A variety of transcription factors all interact with
the E-box element within the proximal region of the E-cadherin
promoter, including the zinc finger Snail homologs Snail1,
Snail2/Slug and Snail3, and several basic helix-loop-helix factors,
such as Twist, ZEB-1 and ZEB-2 (32,34–36). Of
these crucial factors regulating EMT, the Twist family shares
homology in structure across the basic helix-loop-helix domain and
is expressed during mesoderm and muscle development (37). Similar to other EMT-associated
transcription factors, such as Snail, Slug and SIP, Twist binds to
DNA using similar E-box sequence motifs and suppresses E-cadherin
expression (38).
In the present study, the expression of the
Hh-associated proteins Gli-1 and Gli-2 and the EMT-associated
proteins Twist and E-cadherin were examined in 42 HCC tissues and
20 NTL tissues by immunohistochemistry, in order to determine the
role of the Hh signaling pathway and EMT in HCC. Based on the
results of immunohistochemistry, the present study analyzed whether
these proteins show different expression in HCC compared with NTL,
the association between the expression of these proteins and
various clinicopathological factors, and whether the expression of
Hh signaling pathway-associated proteins is associated with the
expression of EMT-associated proteins in HCC.
In contrast to the results of the study by Zhang
et al (6), Gli-2 was expressed
in NTL and HCC tissues in the present study, and there was no
significant difference between the expression in the two groups.
However, Gli-1 was expressed in 40.5% of HCC tissues and 10% of NTL
tissues, showing a significant difference between the two groups.
This finding suggests that Gli-1 expression has diagnostic value
for HCC. Gli-1 and Gli-2 expression were not associated with
various clinicopathological factors. The EMT-associated proteins
E-cadherin and Twist showed significantly decreased expression in
HCC compared with NTL. E-cadherin was expressed at the cellular
membrane in all NTL tissues (100%). However, 34/42 HCC tissues
(81.0%) showed a total loss or prominent decrease in E-cadherin
expression, which was a statistically significant difference
(P<0.0001). Twist was expressed in 8/20 NTL tissues (40.0%) and
34/40 HCC tissues (81.0%), showing significantly different
expression between the two groups. These results suggest that
E-cadherin and Twist also have crucial diagnostic value in the
histological examination of suspected HCC. Among the various
clinicopathological factors assessed, E-cadherin expression was
associated with the histological type of HCC, and Twist was
associated with the tumor size.
In the study by Katoh et al (19), it was found that the Hh signaling
cascade cross-talks with the WNT, EGF/FGF and
TGF-β/Activin/Nodal/BMP signaling cascades, which are involved in
EMT through E-cadherin suppression. The present study examined the
association between the Hh signaling pathway-associated proteins
Gli-1 and Gli-2 and the EMT-associated proteins Twist and
E-cadherin. However, there was no significant association
identified. By contrast, E-cadherin expression showed an inverse
association with Twist expression (P=0.006). An association between
high expression of Twist and loss of E-cadherin expression has been
reported in previous studies of endometrial cancer, HCC and colon
cancer (39–41). The study by Lee et al (40) reported a correlation between HCC
metastasis and Twist-induced HCC invasiveness through EMT by
suppression of E-cadherin expression. Yang et al (22) first reported the role of Twist in
cancer metastasis. This study suggested that Twist induced EMT,
resulting in the promotion of tumor invasion (22). However, the role of EMT-associated
proteins in cancer metastasis is controversial. Certain studies
have shown that Twist overexpression is correlated with
EMT-mediated metastasis in prostate and breast cancers (22,42). By
contrast, other studies have reported that there is no correlation
between Twist overexpression and EMT-mediated metastasis in gastric
and colon cancers (43,44). Twist, similar to other EMT-inducing
transcription factors, such as Snail, Slug and SIP, bind DNA using
similar E-box sequence motifs, repressing E-cadherin expression
(38). In the study of breast cancer
by Fackler et al (45), Twist
was hypermethylated less often in infiltrating lobular carcinoma
(ILC) than in infiltrating duct carcinoma (IDC), and exhibited
increased expression in IDC compared with ILC. Promoter methylation
of the Twist gene has been reported in breast cancer, particularly
in metastatic lesions (46). Although
increasing evidence has shown the importance of Twist in the
development and progression of human cancers, the underlying
function is controversial. These contrasting data suggest that the
role of Twist in tumor progression may be cell type-specific, and
the role of Twist in cancer progression thus requires additional
investigation (40).
The expression of E-cadherin is regulated at the
genetic level through gene mutation, loss of heterozygosity and
hypermethylation of its promoter in various cancers (39,46).
However, Tamura et al (47)
reported that E-cadherin promoter methylation is not closely
associated with the loss of E-cadherin expression. The regulatory
mechanism of E-cadherin is controversial and consensus has not been
reached (40).
In summary, the present study showed that expression
of Gli-1, Twist and E-cadherin in HCC was significantly different
compared with NTL, which suggests a diagnostic value of these
proteins in HCC. In addition, increased expression of Gli-1 and
Twist plays a role in the pathogenesis of HCC. Twist expression is
associated with reduced expression of E-cadherin, which suggests
that Twist provides tumoral invasiveness through EMT by loss of
E-cadherin expression. These results may be used to inform
guidelines regarding therapeutic approaches for HCC, such as the
regulation of E-cadherin. The present study aimed to analyze the
association between the Hh signaling pathway and EMT in HCC.
However, there was no significant difference in the expression of
proteins in the two groups. Additional investigation of the
interaction between the Hh signaling pathway and EMT in HCC and the
prognostic value of these proteins may be required.
Acknowledgements
The present study was supported by research funds
from the Institute of Medical Science, Chosun University, Republic
of Korea, 2012.
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lau WY and Lai EC: Hepatocellular
carcinoma: Current management and recent advances. Hepatobiliary
Pancreat Dis Int. 7:237–257. 2008.PubMed/NCBI
|
|
3
|
Zhou YM, Cao L, Li B, Zhang RX, Sui CJ,
Yin ZF and Yang JM: Clinicopathological significance of ZEB1
protein in patients with hepatocellular carcinoma. Ann Sung Oncol.
19:1700–1706. 2012. View Article : Google Scholar
|
|
4
|
Hubert C, Sempoux C, Rahier J, Horsmans Y,
Geubel A, Van Beers BE, Annet L, Zech F, Leonard D and Gigot JF:
Prognostic risk factors of survival after resection of
hepatocellular carcinoma. Hepatogastroenterology. 54:1791–1797.
2007.PubMed/NCBI
|
|
5
|
Sherman M: Recurrence of hepatocellular
carcinoma. N Engl J Med. 359:2045–2047. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang D, Cao L, Li Y, Lu H, Yang X and Xue
P: Expression of glioma-associated oncogene 2 (Gli2) is correlated
with poor prognosis in patients with hepatocellular carcinoma
undergoing hepatectomy. World J Surg Oncol. 29:252013. View Article : Google Scholar
|
|
7
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wicking C, Smyth I and Bale A: The
hedgehog signaling pathway in tumorigenesis and development.
Oncogene. 18:7844–7851. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berman DM, Karhadkar SS, Maitra A, De
Montes Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y,
Eshleman JR, Watkins DN and Beachy PA: Widespread requirement for
Hedgehog ligand stimulation in growth of digestive tract tumours.
Nature. 425:846–851. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji J, Kump E, Wernli M and Erb P: Gene
silencing of transcription factor Gli2 inhibits basal cell
carcinomalike tumor growth in vivo. Int J Cancer. 122:50–56. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubo M, Nakamura M, Tasaki A, Yamanaka N,
Nakashima H, Nomura M, Kuroki S and Katano M: Hedgehog signaling
pathway is a new therapeutic target for patients with breast
cancer. Cancer Res. 64:6071–6074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiyagarajan S, Bhatia N, Reagan-Shaw S,
Cozma D, Thomas-Tikhonenko A, Ahmad N and Spiegelman VS: Role of
GLI2 transcription factor in growth and tumorigenicity of prostate
cells. Cancer Res. 67:10642–10646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Velcheti V and Govindan R: Hedgehog
signaling pathway and lung cancer. J Thorac Oncol. 2:7–10. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Von Hoff DD, LoRusso PM, Rudin CM, Reddy
JC, Yauch RL, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, et
al: Inhibition of the hedgehog pathway in advanced basal-cell
carcinoma. N Engl J Med. 361:1164–1172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta S, Takebe N and Lorusso P: Targeting
the hedgehog pathway in cancer. Ther Adv Med Oncol. 2:237–250.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katano M: Hedgehog signaling pathway as a
therapeutic target in breast cancer. Cancer Lett. 227:99–104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karlstrom RO, Tyurina OV, Kawakami A,
Nishioka N, Talbot WS, Sasaki H and Schier AF: Genetic analysis of
zebrafish gli1 and gli2 reveals divergent requirements for gli
genes in vertebrate development. Development. 130:1549–1564. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katoh Y and Katoh M: Hedgehog signaling,
epithelial-to-mesenchymal transition and miRNA (review). Int J Mol
Med. 22:271–275. 2008.PubMed/NCBI
|
|
20
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang ZS, Shen Y, Li X, Zhou CZ, Wen YG,
Jin YB and Li JK: Significance and prognostic value of Gli-1 and
Snail/E-cadherin expression in progressive gastric cancer. Tumour
Biol. 35:1357–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Odze RD and Goldblum JR: Surgical
pathology of the GI tract, Liver, Biliary Tract and Pancreas (2nd).
Saunders. Philadelphia: 13032009.
|
|
25
|
Hashiguchi M, Ueno S, Sakoda M, Iino S,
Hiwatashi K, Minami K, Ando K, Mataki Y, Maemura K, Shinchi H, et
al: Clinical implication of ZEB-1 and E-cadherin expression in
hepatocellular carcinoma (HCC). BMC Cancer. 13:5722013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dessaud E, McMahon AP and Briscoe J:
Pattern formation in the vertebrate neural tube: A sonic hedgehog
morphogen-regulated transcriptional network. Development.
135:2489–2503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang ZC, Gao J, Zi SM, Yang M, Du P and
Cui L: Aberrant expression of sonic hedgehog pathway in colon
cancer and melanosis coli. J Dig Dis. 14:417–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kump E, Ji J, Wernli M, Häusermann P and
Erb P: Gli2 upregulates cFlip and renders basal cell carcinoma
cells resistant to death ligand-mediated apoptosis. Oncogene.
27:3856–3864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eichberger T, Sander V, Schnidar H, Regl
G, Kasper M, Schmid C, Plamberger S, Kaser A, Aberger F and
Frischauf AM: Overlapping and distinct transcriptional regulator
properties of the GLI1 and GLI2 oncogenes. Genomics. 87:616–632.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Im S, Choi HJ, Yoo C, Jung JH, Jeon YW,
Suh YJ and Kang CS: Hedgehog related protein expression in breast
cancer: Gli-2 is associated with poor overall survival. Korean J
Pathol. 47:116–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karayiannakis AJ, Syrigos KN, Chatzigianni
E, Papanikolaou S, Alexiou D, Kalahanis N, Rosenberg T and
Bastounis E: Aberrant E-cadherin expression associated with loss of
differentiation and advanced stage in human pancreatic cancer.
Anticancer Res. 18:4177–4180. 1998.PubMed/NCBI
|
|
32
|
Winter JM, Ting AH, Vilardell F, Gallmeier
E, Baylin SB, Hruban RH, Kern SE and Iacobuzio-Donahue CA: Absence
of E-cadherin expression distinguishes noncohesive from cohesive
pancreatic cancer. Clin Cancer Res. 14:412–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Joo YE, Rew JS, Park CS and Kim SJ:
Expression of E-cadherin, alpha- and beta-catenins in patients with
pancreatic adenocarcinoma. Pancreatology. 2:129–137. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Castanon I and Baylies MK: A Twist in
fate: Evolutionary comparison of Twist structure and function.
Gene. 287:11–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kyo S, Sakaguchi J, Ohno S, Mizumoto Y,
Maida Y, Hashimoto M, Nakamura M, Takakura M, Nakajima M, Masutomi
K and Inoue M: High Twist expression is involved in infiltrative
endometrial cancer and affects patient survival. Hum Pathol.
37:431–438. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL and Fan ST: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ran Hong and Sung-Chul Lim: Overexpression
of Twist in colorectal adenocarcinoma. Basic and Applied Pathology.
2:15–20. 2009. View Article : Google Scholar
|
|
42
|
Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C,
Zhang X, Chua CW, Chan KW, Chan FL, Glackin C, et al: Up-regulation
of Twist in prostate cancer and its implication as a therapeutic
target. Cancer Res. 65:5153–5162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rosivatz E, Becker I, Specht K, Fricke E,
Luber B, Busch R, Höfler H and Becker KF: Differential expression
of the epithelial-mesenchymal transition regulators snail, SIP1,
and twist in gastric cancer. Am J Pathol. 161:1881–1891. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rosivatz E, Becker I, Bamba M, Schott C,
Diebold J, Mayr D, Höfler H and Becker KF: Neoexpression of
N-cadherin in E-cadherin positive colon cancers. Int J Cancer.
111:711–719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fackler MJ, McVeigh M, Evron E, Garrett E,
Mehrotra J, Polyak K, Sukumar S and Argani P: DNA methylation of
RASSF1A, HIN-1, RAR-beta, Cyclin D2 and Twist in in situ and
invasive lobular breast carcinoma. Int J Cancer. 107:970–975. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mehrotra J, Vali M, McVeigh M, Kominsky
SL, Fackler MJ, Lahti-Domenici J, Polyak K and Sacchi N: Very high
frequency of hypermethylated genes in breast cancer metastasis to
the bone, brain and lung. Clin Cancer Res. 10:3104–3109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tamura G, Yin J, Wang S, Fleisher AS, Zou
T, Abraham JM, Kong D, Smolinski KN, Wilson KT, James SP, et al:
E-Cadherin gene promoter hypermethylation in rimary human gastric
carcinomas. J Natl Cancer Inst. 92:569–573. 2000. View Article : Google Scholar : PubMed/NCBI
|