Introduction
Breast cancer is the most common type of cancer in
women. Over the past 30 years, the morbidity of breast cancer has
increased at a rate of 3% each year in china (1). Surgery combined with chemotherapy and
radiotherapy is the main therapeutic approach for this disease, but
severe side effects occur in the course of these treatments.
Therefore, effective antitumor therapeutic drugs with few side
effects need to be developed.
The dried flowers of Trollius chinensis is
used as a common traditional Chinese medicine. Modern
pharmacological studies have shown that it possesses antimicrobial,
antiviral (2), anti-oxidative and
anti-tumor activities and has been used widely to treat chronic
tonsillitis and upper respiratory infections in clinical therapy
(3).
Flavonoids as the major constituents of T.
chinensis (4) possess
antimicrobial, anti-inflammatory, hypotensive, antiviral,
spasmolysis and antioxidant effects (in vitro and in
vivo) (5–9). Currently, T. chinensis flavonoids
are used for their antibacterial and antioxidant properties
(10,11). A preliminary study demonstrated the
strong inhibitory effects of T. chinensis flavonoids in
K562, HeLa, EC-109 and NCI-H446 cells (12). T. chinensis flavonoids were
also demonstrated to inhibit the proliferation of A549 cells in a
dose-dependent manner, and the effect was associated with the
expression of apoptosis-related genes (13). Another previous study showed the
ability of T. chinensis flavonoids to dose-dependently
inhibit the proliferation of MCF-7 cells, and telomerase activity
decreased progressively with increasing drug concentrations
(14); however, cellular apoptosis
has not been verified from a morphological perspective, and the
mechanism of action has not been clarified. Therefore, the current
study was conducted to examine apoptosis induced by T.
chinensis flavonoids in MCF-7 cells by different methods,
including MTT assay, differential interference contrast (DIC),
Hoechst staining, scanning electron microscopy, hematoxylin and
eosin (HE) staining, Annexin-V/propidium iodide (PI)
double-labeling and western blot analysis, in order to clarify the
underlying biochemical mechanisms and facilitate clinical
anti-tumor drug development.
Materials and methods
Drugs and reagents
3-(4,5)-dimethlythiahiazo-z-y10-3,5-diphenytetrazoliumromide
(MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Annexin V-FITC/PI was manufactured by Beijing Zhaungmeng Science
and Technology Limited Company (Beijing, China). Hoechst 33258 dye
was obtained from Sigma-Aldrich. Total flavonoids were dissolved in
DMSO and diluted with culture media immediately prior to use.
Primary and secondary antibodies were manufactured by Beijing
Boaosen Biotechnology Limited Company (Beijing, China). The T.
chinensis flavonoids were prepared as previously described
(15) with a purity of ~68%.
Cell culture and experimental
groups
MCF-7 breast cancer cells maintained at the Medical
Genetics Department of Beijing Cancer Institute were cultured in
RPMI-1640 (Gibco, ThermoFisher Scientifc, Inc., Waltham, MA, USA)
medium supplemented with 10% fetal bovine serum (FBS), 100 µM/ml
gentamicin at 37°C in an atmosphere with 5% CO2. Cells
were subcultured and passaged at ~70–80% confluency. Cells in the
logarithmic phase were used in all experiments. Total flavonoids
were extracted from T. chinensis. Stock solutions, kept at
−20°C, were diluted to the final concentrations with culture medium
prior to use. The final DMSO concentration was below 0.2% in all
experiments.
Growth inhibition assay
The effect of total flavonoids on cell growth was
evaluated by using an MTT assay. Briefly, cells were seeded at
105 cells/ml in 96-well plates for the assay. After
culturing with flavonoids at different concentrations (0, 0.0991,
0.1982, 0.3964, 0.7928 or 1.5856 mg/ml) for various times (24, 48
or 72 h), 20 µl of MTT was added to each well. Plates were
incubated for another 4 h at 37°C, and then the medium was
aspirated by pipetting and replaced with 100 µl/well DMSO in order
to dissolve the formazan produced by viable cells exposed to MTT.
The absorbance reflecting the cell growth was measured at 490 nm
with a microplate reader. All data were obtained from 4 independent
experiments and expressed as the mean ± standard deviation.
DIC microscopy and Hoechst33258
staining
Morphological changes were detected with Hoechst dye
staining. Cells were seeded (105 cells/ml) on coverslips
overnight and then treated with flavonoids at different
concentrations (0, 0.0991, 0.1982, 0.3964, 0.7928 or 1.5856 mg/ml)
for 24 h. Thereafter, the coverslips were washed with PBS and fixed
with 4% paraformaldehyde for 1 h. After washing the cells with PBS,
images were captured with DIC software (Nikon, Tokyo, Japan). The
cells on the coverslips were incubated with Hoechst 33258 staining
solution for 30 min, washed 3 times for 2 min with PBS and mounted
on microscope slides. A fluorescence microscope (Nikon) was used to
capture images using an excitation wavelength of 340 nm. The entire
experiment was performed at room temperature.
Scanning electron microscopy
Scanning electron microscopy was used for
observation of morphological changes in apoptotic cells. MCF-7
cells were seeded in a 24-well plate at the density of
105 cells/ml. Overnight, cells were treated with culture
medium or total flavonoids at 0, 0.0991, 0.1982, 0.3964, 0.7928 and
1.5856 mg/ml. A total of 24 h later, the supernatant was discarded,
and the cells were washed with PBS, fixed with glutaraldehyde for
24 h, washed 3 times with PBS for 1 min and then dehydrated in a
gradient of alcohol solutions for 1 min at each concentration.
Finally, the cells were soaked in tert-butyl alcohol twice for 10
min each time, dried with a vacuum pump for 24 h and metal
spray-coated for 90 sec. The prepared specimen was observed under a
scanning electron microscope.
HE staining
MCF-7 cells were seeded in a 6-well plate at
3×105 cells/well in RPMI-1640 with 10% FBS. After 24 h,
cells were treated with flavonoids at a concentration of 0, 0.0991,
0.1982, 0.3964, 0.7928 or 1.5856 mg/ml. Untreated cells served as
the control group. After 24 h, supernatants were discarded, and the
cells were fixed with 1% paraformaldehyde for 24 h. After washing
with PBS twice, cells were stained with hematoxylin for 15 min,
differentiated in hydrochloric acid alcohol for 10 sec, treated
with ammonium hydroxide for 20 sec and stained with eosin for 10
min, with rinses under running water after each step. Subsequently,
the cells were subjected to gradient alcohol dehydration, and
dimethylbenzene was used for rendering the slides transparent.
Finally, the cells were mounted with resin and observed under a
light microscope.
Laser scanning confocal
microscopy
Laser scanning confocal microscopy was used for
observation of cell morphological changes during apoptosis. MCF-7
cells were seeded in 6-well plates in RPMI-1640 media containing
10% FBS. After 24 h, the cells were exposed to flavonoids at
concentrations of 0, 0.0991, 0.1982, 0.3964, 0.7928 and 1.5856
mg/ml for 24 h. The supernatant was discarded, and the cells were
washed 3 times with PBS for 2 min each time. After incubation with
5 µl Annexin V and 10 µl PI for 10 min in the dark, cells were
washed with PBS for 2 min and images were immediately captured
under a laser scanning confocal microscope.
Western blot analysis
MCF-7 cells grown in RPMI medium with 10% FBS were
treated with flavonoids at different concentrations. After 24 h,
cells were harvested and disrupted in RIPA lysis buffer to extract
total cellular proteins. The protein content was determined with a
BAC protein determination kit (Wuhan Boshide Bio-engineering
Limited Company, Wuhan, China). Total cellular proteins (50 µg)
were separated in 15% SDS-PAGE gels and transferred into PVDF
membranes (Sigma-Aldrich) with a wet transfer system (Bio-Rad
Laboratories). Membranes were blocked in 5% non-fat milk for 1 h at
room temperature with 5% fat-free milk dissolved in PBST buffer.
Thereafter, the membranes were probed with β-actin (cat. no. ZA109;
Beijing Zoman Biotechnology Co., Ltd., Beijing, China), bcl-2 (cat.
no. bs-0032R; BIOSS, Beijing, China), FasL (cat. no. bs-0216R;
BIOSS), caspase-3 (cat. no. ZA135; Beijing Zoman Biotechnology Co.,
Ltd.), caspase-9 (cat. no. ZA137; Beijing Zoman Biotechnology Co.,
Ltd.), p53 (cat. no. ZA120; Beijing Zoman Biotechnology Co., Ltd.)
and NF-κB (cat. no. ZA131; Beijing Zoman Biotechnology Co., Ltd.)
primary antibodies in TBST buffer containing 0.1% Tween-20 at
1:1,000 dilution for 24 h at 4°C, followed by exposure to
horseradish peroxidase-conjugated secondary antibodies (Beijing
Boaosen Corporation, Beijing, China) for 90 min at temperature. The
protein levels were visualized using a Western blot detection
system, and immunoreactivities were detected with an enhanced
colorimetric detection kit (Amplified Alkaline Phosphatase Goat
Anti-Rabbit Immun-Blot Assay Kit, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Results
Effect of flavonoids of T. chinensis
on MCF-7 cell viability
An MTT assay was performed to examine the effect of
total flavonoids on cell growth. MCF-7 cells were treated with
different concentrations (0, 0.0991, 0.1982, 0.3964, 0.7928 and
1.5856 mg/ml) of total flavonoids for 24, 48 and 72 h. As presented
in Fig. 1, the survival of MCF-7
cells reduced in a time-dependent and dose-dependent manner. At 72
h, the maximum inhibition ratio reached up to 81.31% at the drug
concentration of 1.5856 mg/ml (Table
I).
 | Table I.Inhibition effect of T.
chinensis flavonoids on MCF-7 cells detected by MTT method. |
Table I.
Inhibition effect of T.
chinensis flavonoids on MCF-7 cells detected by MTT method.
|
| Cell growth
inhibition rate by T. chinensis flavonoids (%) |
|---|
|
|
|
|---|
| Concentration
(mg/ml) | 0 h | 24 h | 48 h | 72 h |
|---|
| 0.0991 | 0 |
3.62±0.23a,d |
5.14±0.11a,e |
8.75±0.56a,f |
| 0.1982 | 0 |
24.8±0.55b,d |
27.61±0.54b,e |
31.76±0.69b,f |
| 0.3964 | 0 |
35.72±0.72c,d |
46.52±0.75c,e |
50.01±0.84c,f |
| 0.7928 | 0 |
48.03±1.34c,d |
73.49±1.51c,e |
77.92±1.61c,f |
| 1.5856 | 0 |
53.8±1.28c,d |
76.76±1.83c,e |
81.31±1.86c,f |
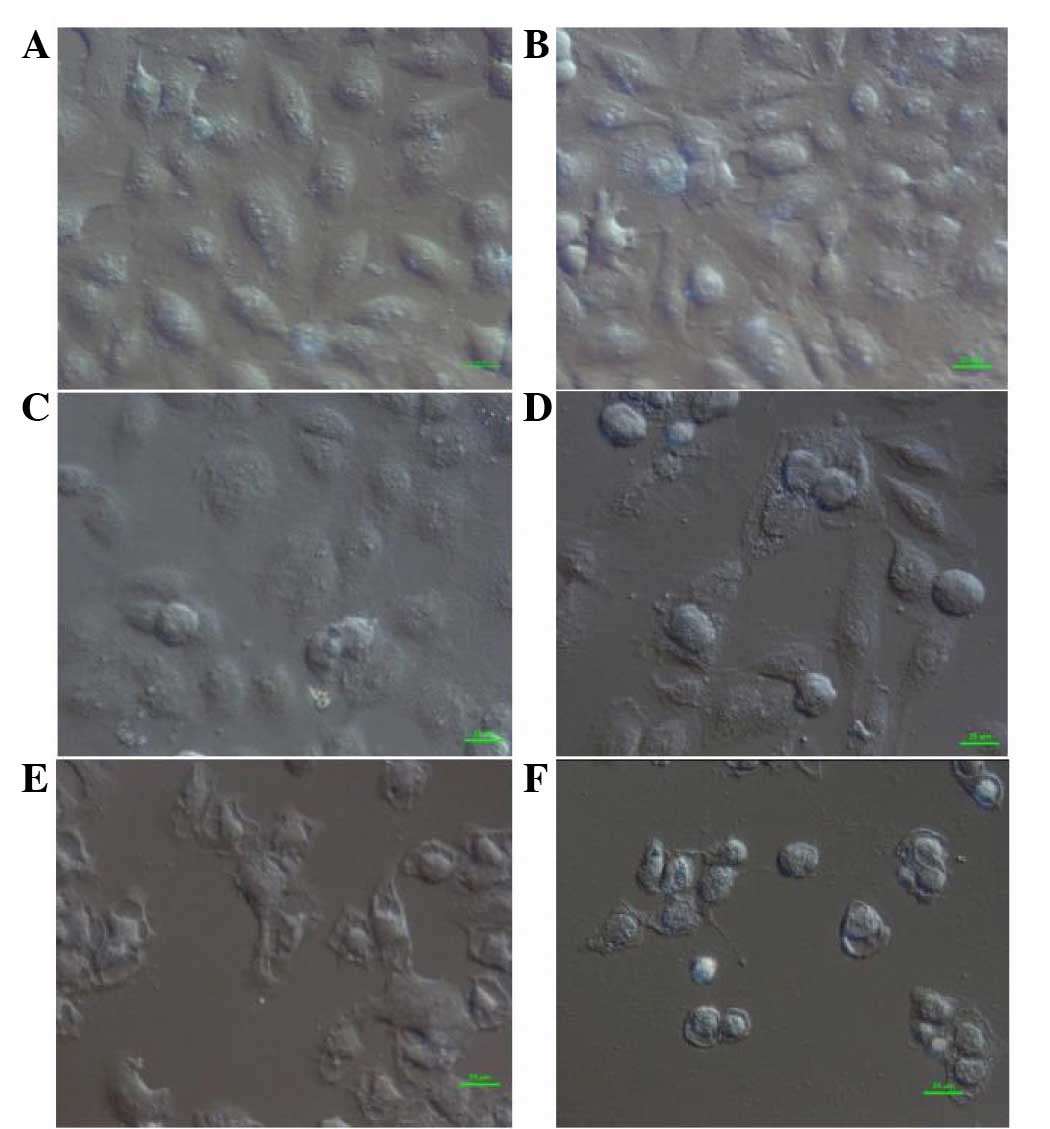
DIC microscopy analysis
In the control group, normal fusiform-shaped cells
were connected and radiated outward, adhering tightly to the cover
glass. With a low concentration of flavonoids (0.0991 mg/ml), the
shape of cells started to change. Upon exposure to increased
flavonoid concentrations (0.1982 and 0.3964 mg/ml), the cells
rounded up with loosened cell junctions and appeared to be
vacuolated. At high concentrations of flavonoids (0.7928 and 1.5856
mg/ml), fractured junctions, swelled nuclei, plasmolysis and a
surge in the number of desquamated cells was observed (Fig. 1).
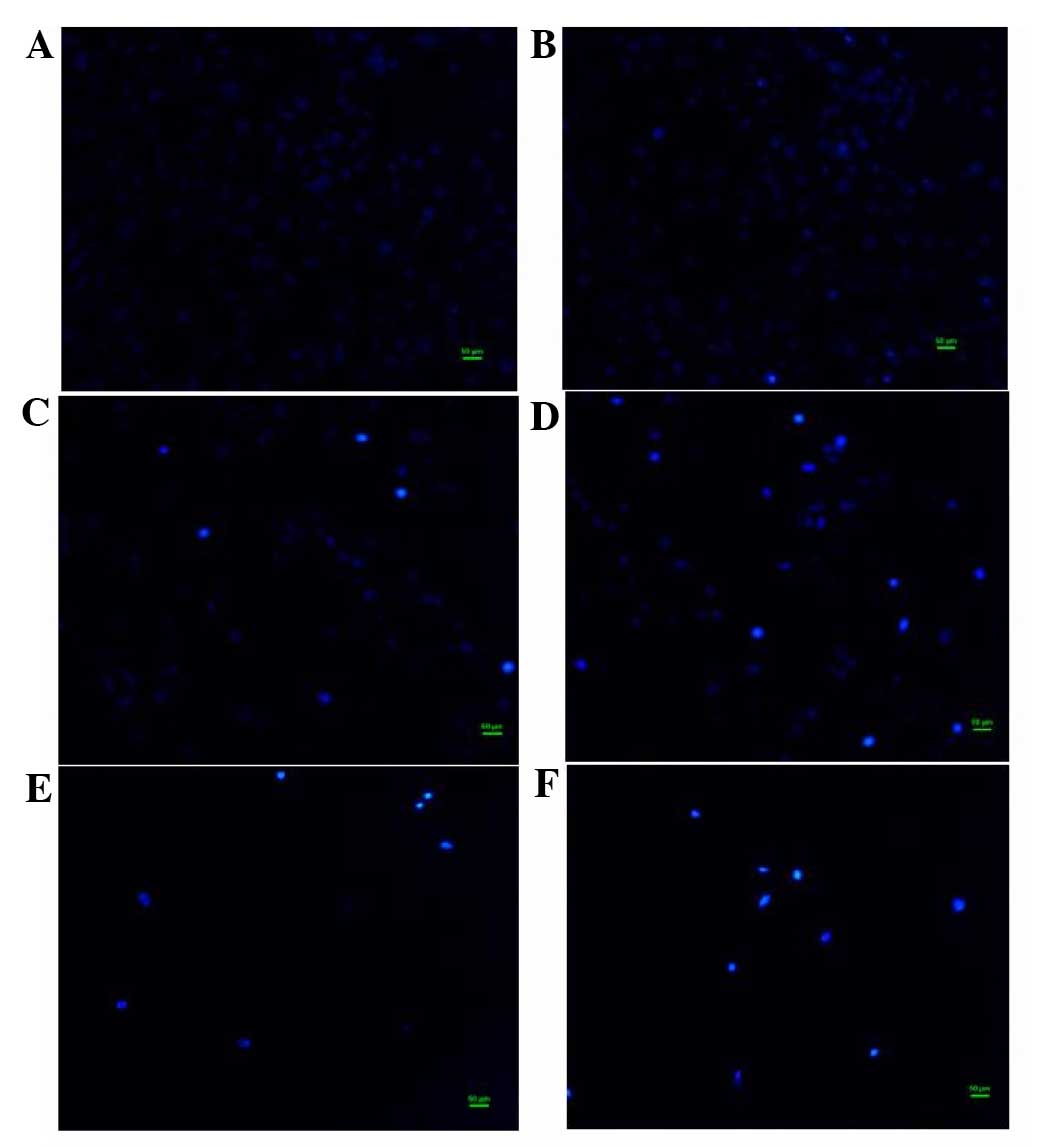
Effect of flavonoids of T. chinensis
on nuclear morphology of MCF-7 cells
To examine the cell death induced by total
flavonoids, the nuclear morphology of dying MCF-7 cells stained
with Hoechst 33258 dye was observed. In the control group, the
nuclei of cells had a regular shape and showed a uniform
distribution of low-density fluorescence. In the group exposed to a
low concentration of flavonoids (0.0991 mg/ml), the nuclei of a
proportion cells appeared darker than those of normal cells,
indicating apoptotic cell death. In groups treated with flavonoids
in the middle of the concentration range (0.1982 and 0.3964 mg/ml),
cell numbers gradually decreased, while the proportion of cells
with hyperchromatic nuclei increased. With high concentrations of
flavonoids (0.7928 and 1.5856 mg/ml), MCF-7 cell numbers fell
sharply, fragmented nuclei appeared and typical apoptotic
characteristics became more apparent (Fig. 2).
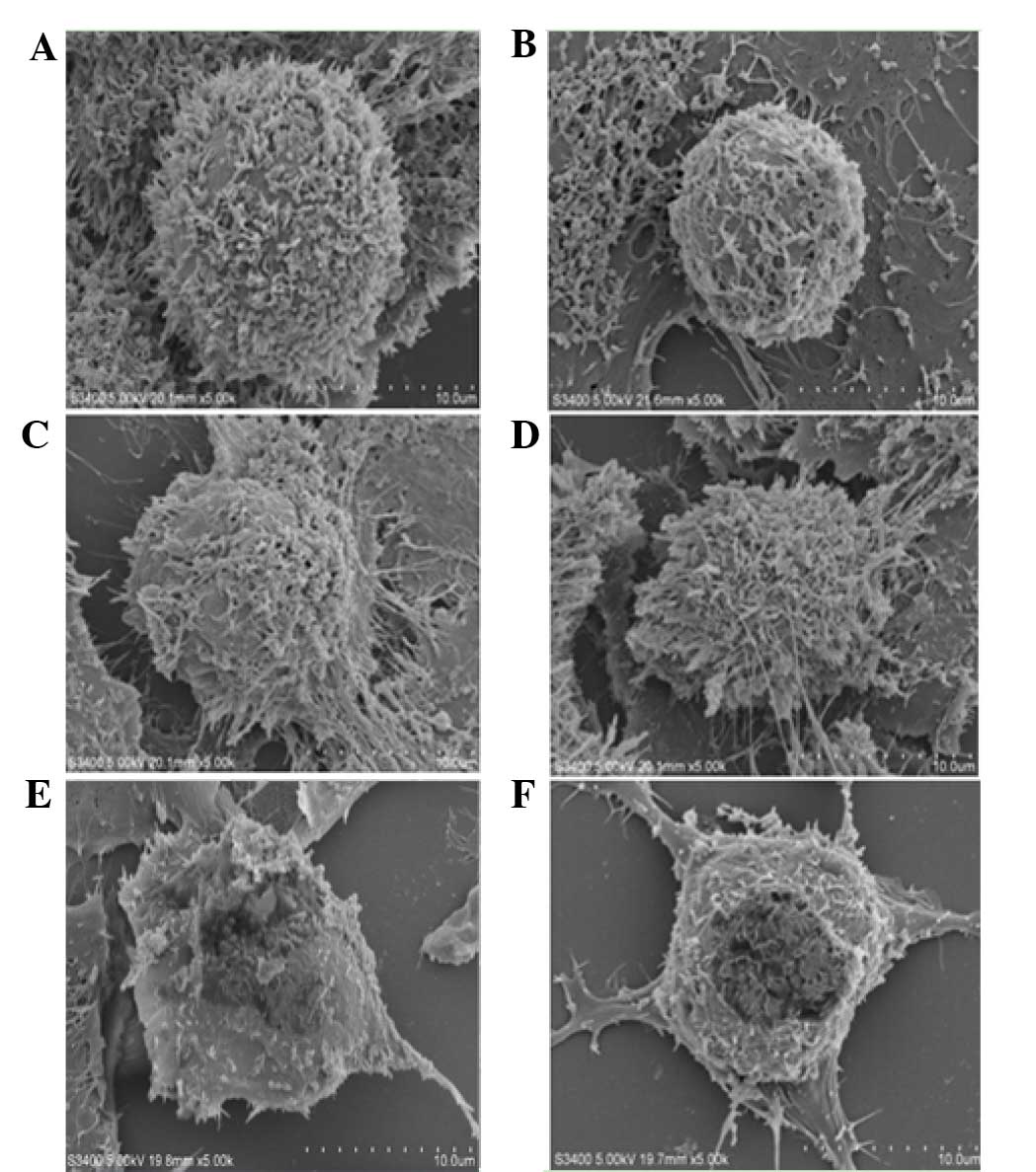
Scanning electron microscopy
analysis
MCF-7 cells in the control group were firmly
adherent and covered with abundant microvilli. Those cells were
connected tightly with neighboring cells and extended in all
directions. In the low flavonoid concentration group (0.0991
mg/ml), cells with gap junctions shrank and showed decreased
surface microvilli. In the middle concentration group (0.1982 and
0.3964 mg/ml), characteristics of apoptosis became marked. With
high concentrations of flavonoids (0.7928 and 1.5856 mg/ml), the
microvilli nearly completely disappeared, and cell membranes
collapsed (Fig. 3).
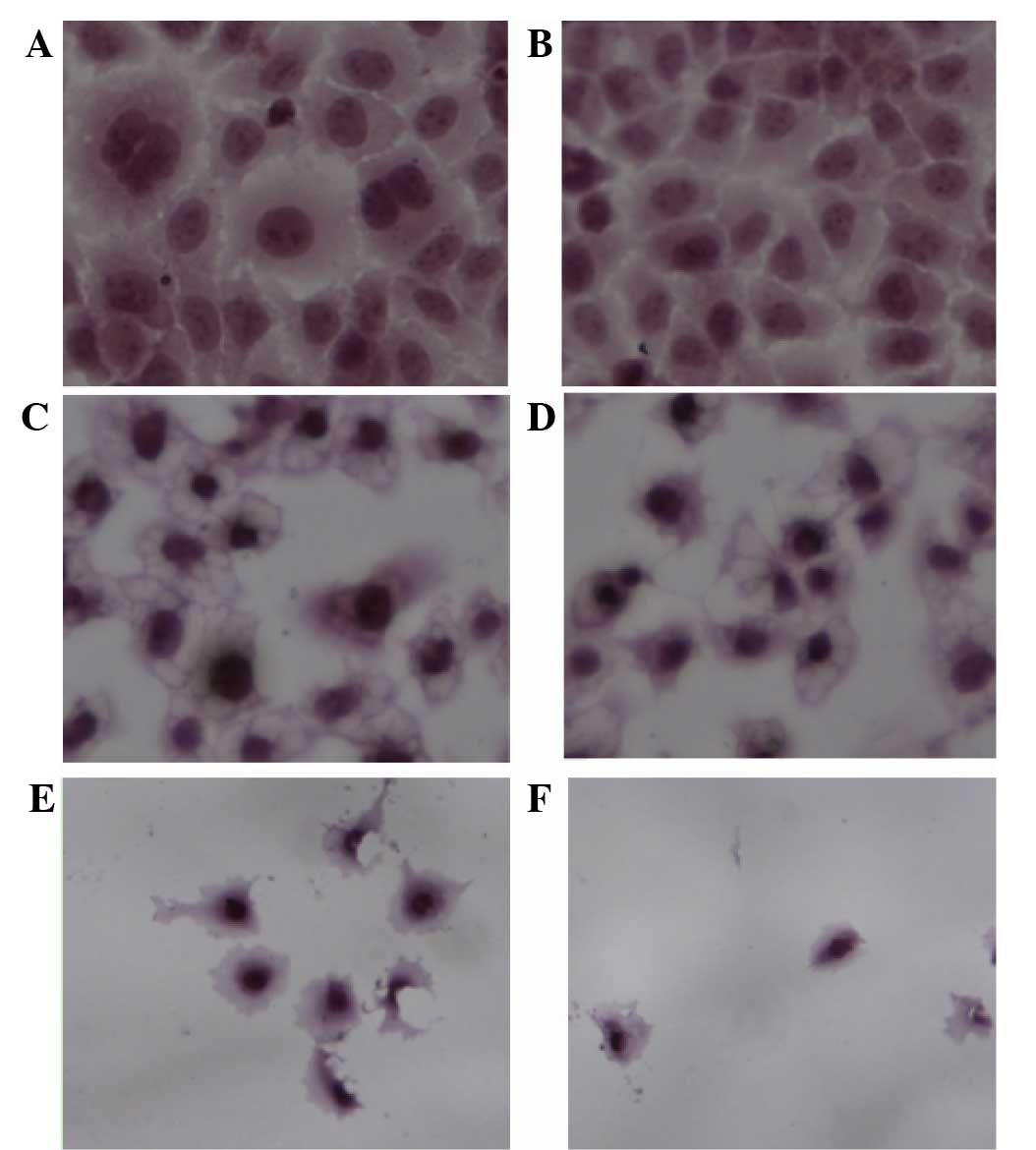
HE staining analysis
In the control group, cells were in good condition
and able to grow adhering to the cover glass. The nucleoplasm was
uniformly stained, and mitoses could be observed. The nucleolus and
nuclear membrane profiles also could be seen. At a low
concentration (0.0991 mg/ml), treatment with total flavonoids
halted cell division. With flavonoids in the middle range of
concentrations (0.1982 and 0.3964 mg/ml), intercellular spaces
dilated. However, cells with condensed cytoplasm maintained their
plasma membrane integrity. The cells shrank and appeared
increasingly deformed or dehydrated. At high concentrations of
flavonoids (0.7928 and 1.5856 mg/ml), adherent cells were
drastically reduced in number and cells were no longer intact.
Cells treated with high flavonoid concentrations (0.7928 and 1.5856
mg/ml) were more hyperchromatic than the cells of the middle
concentration group (0.1982 and 0.3964 mg/ml). The HE result
revealed shows swollen nucleoli and fractured junctions, which was
in accordance with what DIC and scanning electron microscopy
results demonstrated (Fig. 4).
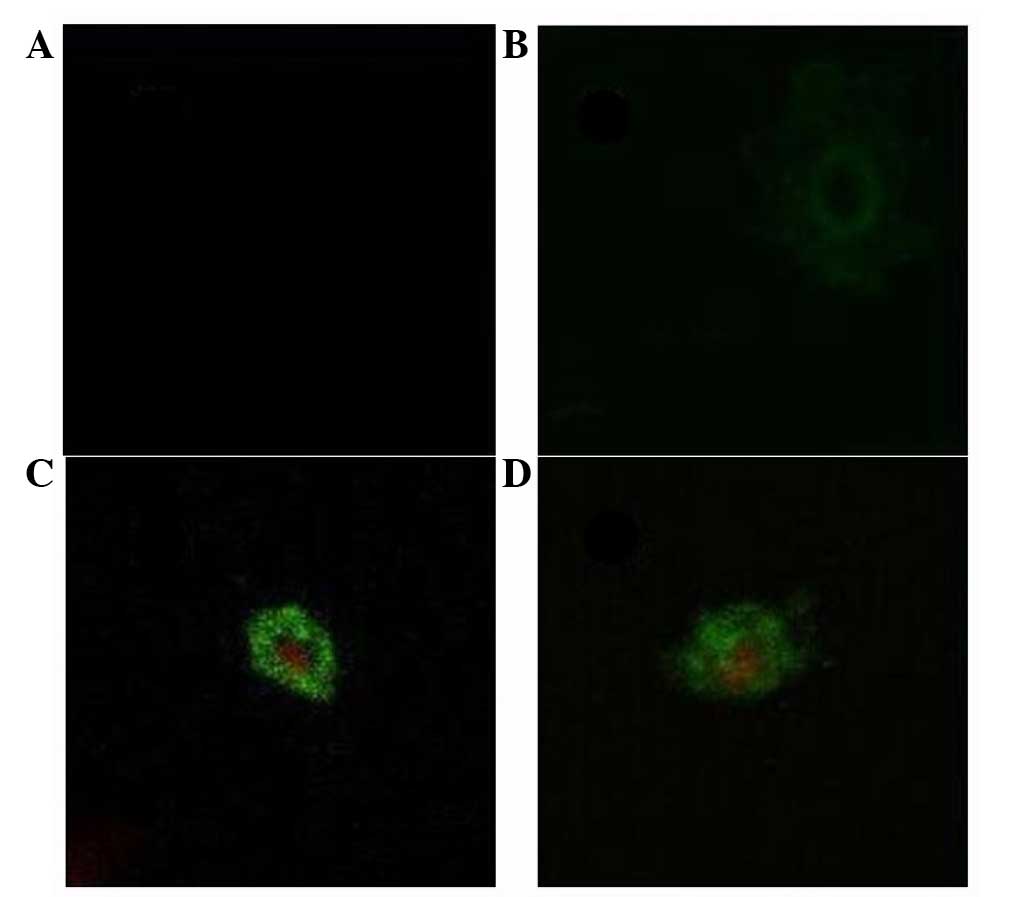
Detection of apoptosis by Annexin
V-FITC/PI staining
In the control group, MCF-7 cells were not readily
stained by Annexin V or PI. In the low flavonoid concentration
group (0.0991 mg/ml), cells in the early stage of apoptosis were
seen. Plasma cell membranes labeled green by Annexin V appeared
gradually. In the middle concentration range of flavonoids (0.1982
and 0.3964 mg/ml), the integrity of the cytomembrane was no longer
intact and stained green, while the nucleus was dyed red. In the
high flavonoid concentration group (0.7928 and 1.5856 mg/ml), both
the cytomembrane and nucleus were fragmented (Fig. 5).
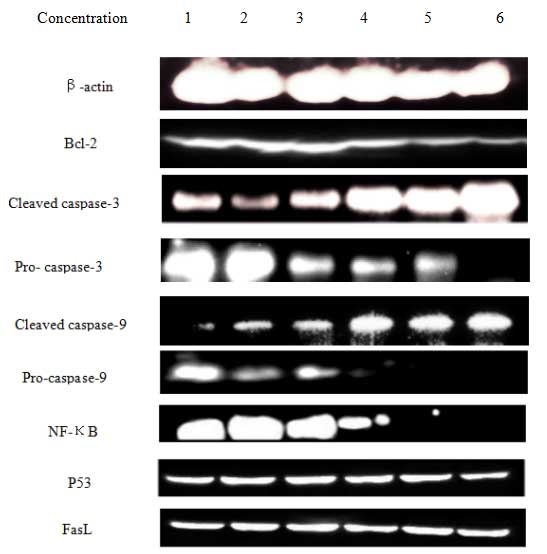
Effect of T. chinensis flavonoids on
levels of apoptosis-associated proteins
In order to study the mechanism of flavonoid-induced
apoptosis in MCF-7 cells, the expression of caspases, fasL, p53 and
bcl-2 were determined. After 24 h of exposure to flavonoids, the
expression of cleaved caspase-3 and caspase-9 increased in a
dose-dependent manner. Bcl-2 is a crucial determinant of cell
apoptosis, and a decrease in its expression was observed in the
flavonoid-treated cells. This observation suggests that an
intrinsic apoptotic signaling contributed to the damaging effects
of flavonoids on MCF-7 cells. Total flavonoids also down-regulated
the expression of NF-κB, which in turn could down-regulate the
expression of bcl-2. By contrast, the expression levels of p53 and
fasL were not markedly changed. These findings suggest that
induction of the mitochondrial pathway is crucial for
flavonoid-induced apoptosis of MCF-7 cells (Fig. 6).
Discussion
In the present study, T. chinensis flavonoids
were shown to clearly inhibit the growth and proliferation of MCF-7
cells within a certain range of concentrations (0.0991–1.5856
mg/ml) by the MTT assay. The inhibitory effect increased as the
concentration or treatment time of flavonoids increased. Although
the inhibition of MCF-7 cells increased with time (24 h ≤48 h ≤72
h) when treated with flavonoids at the same concentration, a direct
proportional relationship between the inhibition ratio and time was
not observed. According to previous evaluation criterion (16), the effect is weak when inhibition rate
is <30%; The effect is strong when inhibition rate is >50%.
And the effect is modest when inhibition rate is between 30 and
50%. In the present study, the inhibition rate was 58.3% when the
concentration was 1.5856 mg/ml at 24 h. The effect may therefore be
described as strong and the typical apoptosis characteristics were
apparent at 24 h, therefore 24 h was selected as an appropriate
time-point for further analyses.
The findings of the present study are in line with
those of Sun et al (14)
reporting that total flavonoids in T. chenensis could
inhibit the proliferation of MCF-7 cells. This finding was
confirmed in the present study with additional morphological
observations and the corresponding mechanism was investigated.
The DIC results demonstrated that particles appeared
on the surface of cells treated with drugs. ‘Bubble up’
demonstrated that cytoskeletal structure was damaged. Ji et
al (17) observed the
morphological changes in MCF-7 and MDA-MB-231 cells treated with
Huaier Granule (a traditional Chinese medicine) by DIC software,
and observed morphological changes induced by flavonoids that were
indicative of apoptosis in MCF-7 cells.
The Hoechst results demonstrated that the nuclei of
cells treated with drugs appeared darker than that of normal cells
and fragmented nuclei appeared. Wang et al (18) studied the apoptotic effect of
genistein on breast cancer cells by the Hoechst 33258 method and
similarly to the present study, the authors observed that MCF-7
cells treated with drugs shows apoptotic morphological
characteristics.
The scanning electron microscope results indicated
that the microvilli on the surface of cells treated with drug of
different concentrations were reduced. In the high dose group
(0.7982, 1.5856 mg/ml), cell membrane collapse was observed,
appearing as a hole-like structure. The disruption of the cell
membrane indicates cells necrosis. The typical apoptosis
characteristics observed in the present study are in line with what
Li et al (19) observed, in a
previous study on the apoptosis of breast cancer cells induced by
Herceptin. Therefore, flavonoids could induce the apoptosis of
MCF-7 cells.
The HE results indicated that in the low and middle
flavanoid dose group (0.0991, 0.1982, 0.3964 mg/ml), there were
shrunken nuclei of cells dyed black-blue and intercellular gap
junctions were fractured, indicating the apoptosis of cells. In the
high dose group (0.7928, 1.5856 mg/ml), the cells were no longer
intact and the cell membrane was disrupted indicating cell
necrosis. The typical apoptosis characteristics that were observed
using HE methods were in accordance with what Li observed (20), in a previous study investigating the
apoptosis of lung adenocarcinoma A549 cells induced by soy
isoflavone and vinorelbine. These results provide further evidence
that flavonoids may induce apoptosis in MCF-7 cells.
The results of laser confocal microscopy indicated
that T. chinensis flavonoids at the concentration of 0.0991,
0.1982 and 0.3964 mg/ml inhibit MCF-7 cell proliferation by
inducing the apoptosis of cells. However, T. chinensis
flavonoids at the concentration of 0.7982 and 1.5856 mg/ml inhibit
the proliferation of MCF-7 cells by inducing the necrosis of
cells.
The abovementioned results reveal that morphological
changes in MCF-7 cells occur via multiple pathways and from
multiple perspectives, leading to the apoptosis of cells. Cell
apoptosis is affected by precise control and interaction of
regulatory factors, and may be activated by extracellular and
intracellular stimulation. Although the apoptotic signals of cells
were different, the apoptotic morphology of the cells were the same
(21). A large proportion of
antineoplastic drugs kill cancer cells via starting the cell
apoptosis mechanism (22).
Mitochondria are the central control sites for one
mechanism of apoptosis (23). DNA
injury activates the mitochondrial pathway of apoptosis, and NF-κB
inhibits cellular apoptosis via down-regulation of bcl-2 (24). Furthermore, bcl-2 expression is able
to influence mitochondrial membrane depolarization (25). Caspases form a proteolytic network
within the cell whereby upstream initiator caspases are activated
early in the apoptotic process (e.g., caspase-8 and caspase-9) and
then activate other downstream caspases (e.g., caspase-3) (26). The activation of caspase-9 is an
indicator of the activation of the mitochondrial pathway of
apoptosis.
FasL, ligand of FasL, combined with FasL on the
surface of cells activating caspase-8 and −10, when exogenous
signals stimulate. Then downstream caspase-6 and −7 are activated,
caspase-3 activation is also induced triggering the apoptosis of
cells (27). p53 serves a prominent
role in cell apoptosis and is an important cancer suppressor gene.
The main biological activity of p53 is to identify DNA injury and
to repair DNA damage. If the repair fails, p53 induces cell
apoptosis to prevent the development of tumor (28,29). p53
down-regulates the expression of bcl-2 to induce the apoptosis of
MCF-7 cells (30).
The results of the present study demonstrate that
FasL and p53 protein expression in MCF-7 cells was not changed
significantly as drug concentration increased. These results
indicate that flavonoids do not influence the expression of FasL
and p53 protein.
In the present study, flavonoids may have
down-regulated the expression of NF-κB and bcl-2 and no expression
of NF-κB protein was observed in flavanoid-treated breast cancer
cells. These results indicate that the decrease in NF-κB and bcl-2
protein levels promotes the expression of caspase-9 and caspase-3.
In addition, the expression levels of caspase-9 and- 3 were
up-regulated, which suggests that upstream caspase-9 protein
increased to increase the expression of downstream caspase-3, the
activation of caspase-3 resulting in the degradation of
cytoskeleton and DNA strand breaks inducing MCF-7 apoptosis.
In conclusion, T. chinensis flavonoids were
shown to suppress the growth and induce apoptosis of MCF-7 cells.
Their effects in down-regulating the expression of NF-κB may serve
to down-regulate expression of Bcl-2, which participates in the
mitochondrial pathway involving caspase-3 and caspase-9 to induce
cell apoptosis. There is no change in FasL and p53 protein
expression, so the FasL and p53 protein could not lead to the
apoptosis of EC-109 cells. Therefore, T. chinensis
flavonoids may have the potential to be developed as a novel
anti-breast cancer drug.
Acknowledgements
The present study was financially supported by the
major scientific projects of Hebei North University (grant no.
ZD1314) and partly supported by the Technology Bureau of
Zhangjiakou city (grant no. 11110015D).
References
|
1
|
Li SP and Jiao W: Exercise and Prevention
of breast cancer. Wuhan Tiyuxue Yuan Xue Bao. 48:71–77. 2014.(In
Chinese).
|
|
2
|
Lin FQ, Feng SQ, Li WL, et al: Antiviral
ingredients research of Trollius chinensis. Zhejiang Daxue Xue Bao.
31:4122004.(In Chinese).
|
|
3
|
Li YL, Ye SM, Wang LY, et al: The
isolation and bioactivity of proglobeflowery acid in Trollius
macropetalus. Jinan Daxue Xue Bao. 23:124–126. 2002.(In
Chinese).
|
|
4
|
Li LQ: The geographical distribution of
Helleboroideae plants of buttercup family. Zhi Wu Fen Lei Xue Bao.
33:535–537. 1995.(In Chinese).
|
|
5
|
Yang GD, Rao N, Wang SH, et al: The
research on antioxidant of orientin and vitexin in Trollius
chinensis. Shizhen Guo Yi Guo Yao. 22:2172–3. 2011.(In
Chinese).
|
|
6
|
Yan J, Hu H, An F, et al: The research on
antioxidant of trollius flavonoids. Shizhen Guo Yi Guo Yao.
22:386–7. 2010.(In Chinese).
|
|
7
|
Fu XC, Li SP, Wang MW, et al: The relaxant
effect and the mechanism of orientin on aorta smooth muscle.
Nanfang Yi Ke Daxue Xue Bao. 27:1173–5. 2007.(In Chinese).
|
|
8
|
Fu XC, Li SP, Wang XG, et al: The research
on antithrombotic effect of orientin. Zhong Guo Yao Fang.
17:1292–3. 2006.(In Chinese).
|
|
9
|
Fu XC, Li SP, Qiu YF, et al: The research
on Anti-anoxia of orientin on mice of anoxia model. Zhong Guo Yao
Fang. 17:654–35. 2006.(In Chinese).
|
|
10
|
Liu P, Chen GH, Deng SH, Liu YL and Tong
JM: The anti-microbial activity research of trollius flavonoids.
Zhonguo Shi Yan Fang Ji Xue Zazhi. 19:207–210. 2013.(In
Chinese).
|
|
11
|
Yan J: The antioxidant effects research of
trollius flavonoids. Shizhen Guo Yi Guo Yao. 21:386–387. 2010.(In
Chinese).
|
|
12
|
Sun L and Cheng JZ: The effect of trollius
flavonoids on K562, Hela, EC-109 and NCI-H446 cells proliferation.
Zhong Guo Laonian Xue Zazhi. 44:981–983. 2009.(In Chinese).
|
|
13
|
Sun L and Luo Q: The effect of trollius
flavonoids on proliferation and apoptosis of A549 cells. Zhong Guo
Laonian Xue Zazhi. 31:82–83. 2011.(In Chinese).
|
|
14
|
Sun Li: The effect of trollius flavonoids
on breast cancer cells. Zhong Guo Laonian Xue Zazhi. 29:1098–9.
2009.(In Chinese).
|
|
15
|
Yan J, Qu CH, Tian JM, An F and Wang SH:
The study on purification of trollius flavonoids. Hebei Bei Fang
Xue Yuan Xue Bao. 26:20–2. 2009.
|
|
16
|
Lu Y: The activity study of fermentative
soy isoflavone. Dong Bei Nong Ye Da Xue. 2005.(In Chinese).
|
|
17
|
Ji CY: The research on effect of huaier
granule on breast cancer MCF-7 (ER+) and MDA-MB-231 (ER-) cells.
Nanhua Daxue. 2013.(In Chinese).
|
|
18
|
Wang H: The study on apoptosis of breast
cancerMDA-MB-231 cells induced by genistein. Zhong Hua Ruxian Bing
Zazhi. 7:322–328. 2013.(In Chinese).
|
|
19
|
Li HZ, Zheng Y, Lin P, Dou CM, Dong JY and
Wang TW: The effect of herceptin on cell cycle and apoptosis of
breast cancer cells. Ji Chuyi Xue Yu Lin Chuang. 27:1251–1256.
2007.(In Chinese).
|
|
20
|
Li XL: The inhibition effect of soy
isoflavone and vinorelbine on lung adenocarcinoma A 549 cells. Yan
Bian Daxue. 2006.(In Chinese).
|
|
21
|
Jimingjie. The research on cancer cell
apoptosis induced by ar-curcumene. Shandong Daxue. (In
Chinese).
|
|
22
|
Biswas DK, Martin KJ, McAlister C, Cruz
AP, Graner E, Dai SC and Pardee AB: Apoptosis caused by
chemotherapeutic inhibition of nuclear factor-kappaB activation.
Cancer Res. 63:290–295. 2003.PubMed/NCBI
|
|
23
|
Lee CY, Chien YS, Chiu TH, Huang WW, Lu
CC, Chiang JH and Yang JS: Apoptosis triggered by vitexin in U937
human leukemia cells via a mitochondrial signaling pathway. Oncol
Rep. 28:1883–1888. 2012.PubMed/NCBI
|
|
24
|
Chen DY, Zhai ZH and Shu HB: The
regulating mechanism of NF-κB activation. Ke Xue Tong Bao.
48:1893–911. 2003.(In Chinese).
|
|
25
|
Orrenius S: Reactive oxygen species in
mitochondrial-mediated cell death. Drug Meteb Rev. 39:443–455.
2007. View Article : Google Scholar
|
|
26
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|
|
27
|
Reesink-Peters N, Hougardy BM, Hoor KA,
van den Heuvel FA, Ten Hoor KA, Hollema H, Boezen HM, de Vries EG,
de Jong S and van der Zee AG: Death recePtors and ligands in
cervical carcinogenesis: An immunohistochemical study. Gynecol
Oncol. 96:705–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asker C, Wiman KG and Selivanova G:
P53-induced apoptosis as a safeguard against cancer. Biochem
Biophys Res Commun. 265:1–6. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheikh MS and Furnace AJ Jr: Role of p53
family members in apoptosis. J Cell Phvsiol. 182:171–181. 2000.
View Article : Google Scholar
|
|
30
|
Zhangping. The research on cancer cell
apoptosis induced by flavonoids from seed residues and the
corresponding mechanism. Hua Dong Shi Fan Da Xue. 2004.(In
Chinese).
|