Introduction
Renal cell carcinoma (RCC) is one of the most common
tumors of the urological system in humans (1). Treatment for this disease is difficult
due to its high metastatic tendency, as well as its resistance to
radiotherapy and chemotherapy (2).
Patients with RCC develop metastasis in ~33% of cases (3), which causes ~35% of mortality (4–6). Recently,
patients with RCC are being diagnosed at increasingly younger ages
(7). Approximately 20% of patients
with RCC lose the possibility of radical treatment approaches due
to metastasis (3,8). Furthermore, 40–50% of those patients
with localized advanced disease will ultimately progress to
metastatic disease (9). Therefore, it
is important to determine the molecular mechanism of invasion and
metastasis in RCC.
The epithelial-mesenchymal transition (EMT) is an
important process in tumor metastasis (10). Tumor EMT is a phenotypic switch that
promotes the acquisition of a fibroblastoid-like morphology by
epithelial tumor cells, increased expression of
mesenchymal-associated proteins, decreased expression of epithelial
markers, and enhanced tumor cell motility and invasiveness
(11–13). A previous study demonstrated that EMT
contributes to the metastasis of RCC (14). However, the underlying cellular and
molecular mechanisms have not been clarified yet.
As a critical chemoattractant, interleukin (IL)-8 is
known to participate in cancer progression (15). In recent years, an association between
IL-8, tumor EMT and tumor stemness has been demonstrated (11,13,16).
Previous research has indicated that renal cancer cells in
vitro are able to secrete IL-8, particularly those cell lines
that are undergoing EMT and have metastatic potential (17). However, the role of IL-8 in renal
cancer progression and in the induction of EMT in RCC remain
unknown. The serine-threonine kinase AKT has been demonstrated to
participate in signal transmission pathways in numerous types of
cancer (18). The potential role of
the activation of AKT in RCC remains unclear. The present study
aimed to identify the potential role of IL-8 as well as that of AKT
activation in RCC to demonstrate a possible molecular mechanism for
RCC metastasis.
Materials and methods
Materials
The renal carcinoma 786-O cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA). IL-8
and Super ECL Plus hypersensitivity light-emitting solution were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-IL-8
antibody was purchased from Abnova (rabbit; polyclonal; catalog no.
ABIN453704; Taipei City, Taiwan), while anti-phospho-AKT (mouse;
monoclonal; catalog no. 12694) and anti-AKT (rabbit; monoclonal;
catalog no. 4691) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA) (all 1:1,000 dilution).
Anti-β-actin (mouse; monoclonal; catalog no. sc8432),
anti-E-cadherin (mouse; monoclonal; catalog no. sc8426) and
anti-N-cadherin (mouse; monoclonal; catalog no. sc8424) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA) (all 1:1,000 dilution). LY294002 (S1737), a
phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) inhibitor,
was purchased from Beyotime Institute of Biotechnology (Haimen,
China). RPMI 1640 was purchased from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was
purchased from HyClone (GE Healthcare Life Sciences, Logan, UT,
USA). The culture plates were purchased from Corning Incorporated
(Corning, NY, USA). Phenylmethane sulfonyl fluoride and bovine
serum albumin were purchased from Gen-View Scientific Inc. (El
Monte, CA, USA). Polyvinylidene fluoride membranes were purchased
from EMD Millipore (Billerica, MA, USA). Western blot and
immunoprecipitation cell lysates were prepared in the
laboratory.
Cell culture
The human RCC 786-O cell line was maintained in RPMI
1640 medium supplemented with 10% FBS at 37°C and 5%
CO2. To determine the cellular growth curve,
5×104 cells suspended in 2 ml medium were seeded into a
6-well plate and cultured under normal conditions. At 24 or 48 h
after seeding, the cells in each well were trypsinized and
counted.
Clinical data and renal cancer
tissues
A total of 20 fresh RCC tissues and their
corresponding paired adjacent non-cancerous tissue samples were
randomly selected from patients undergoing laparoscopic radical
nephrectomy at the Sun Yat-sen Memorial Hospital (Guangzhou, China)
from January 2009 to December 2011. The tissues were collected and
processed immediately within 15 min. Each sample was frozen and
stored at −80°C. The paired non-cancerous tissues were isolated
from ≥1 cm away from the tumor border and were demonstrated to lack
tumor cells by microscopy. All patients in the present study met
the following inclusion criteria: The resected mass was identified
as RCC by pathological examination; no anti-cancer treatments were
administered prior to surgery; and complete resection of all tumor
nodules was verified by the cut surface being free of cancer by
pathological examination. Enzyme-linked immunosorbent assay was
performed to detect the supernatant prepared for determining the
IL-8 according to the manufacturer's protocol (Bray Leino Group
Ltd., Chicago, IL, USA).
IL-8-mediated induction of EMT in
786-O cells
786-O cells were cultured for 24 h in RPMI 1640
containing 10% FBS at 37°C and 5% CO2. When the cells
reached a density of 30–50%, the medium was replaced with
serum-free medium for 12 h. Subsequently, the medium was replaced
again in the experimental group, which had IL-8 added at a
concentration of 100 µg/l, whereas the control group had normal
medium culture without additional IL-8. In accordance with the
experimental design, cells were collected at 96 h after the
follow-up test, as shown in Fig.
1.
Migration and invasion assays
Migration and invasion assays were performed as
described in the BD Biosciences Operations Guide (19). Briefly, 100 µl Matrigel was added to
the upper filters of the cell culture inserts, and immediately
placed in a culture plate. Subsequently, the 786-O cell density was
adjusted to 5×105 cells/ml. RPMI 1640 medium with or
without IL-8 was then added to the lower filters, and
1×105 cells were added into the upper filters and
incubated for the indicated time. The migrated or invaded cells in
the lower filters were fixed and counted under a microscope.
Proliferation assay
786-O cells were collected, and the cell suspension
was adjusted to a concentration of 5×104 cells/ml. The
cell culture medium, with or without IL-8, was added to the
experimental and control treatment groups, and incubated for the
indicated time. Subsequently, 20 µl
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution was added into each well and later removed. Dimethyl
sulfoxide was then added into each hole of the 96-well plates,
incubated for 10 min, and the absorbance was detected in a
microplate reader immediately after the crystals were fully
dissolved.
Western blotting
MSCs at ~75% confluence were harvested and the cell
density was adjusted to 1×106/ml. The cell suspension
was added to 6-well plates (3 ml/well, 3×106/well). The
cells were maintained in serum-free medium overnight. In the IL-8
group, the cells were treated with IL-8 for 15, 30 and 60 min,
while in the anti-CXCR2 group, the cells were pretreated with
anti-CXCR2 antibodies for 30 min and then with IL-8 for 15, 30 and
60 min. Next, these cells were washed twice with cold
phosphate-buffered saline and were transferred into centrifuge
tubes, followed by centrifugation at 1,800 × g for 4 min. The
supernatant was removed. The cells were mixed with lysis buffer (80
µl) and pipetted. The lysate was kept on ice for 15 min, followed
by centrifugation at 12,000 × g at 4°C for 10 min. The supernatant
was collected (60 µl) and mixed with 4% sodium dodecyl sulfate (20
µl), followed by heating at 95°C for 5 min. Proteins were subjected
to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and then were transferred onto a polyvinyldifluoride (PVDF)
membrane. The PVDF membrane was blocked in 5% skimmed milk in 1X
Tris-buffered saline containing 0.05% Tween-20 (TBST) at room
temperature for 60 min. The membrane was incubated with the
corresponding antibody (dilution, 1:1,000) at 4°C overnight (10 ml
of 1X TBST, 0.5 g bovine serum albumin, and 10 µl of 1-mg/ml
antibody). The membrane was washed with TBST and then treated with
horseradish peroxidase-conju-gated goat anti-rabbit secondary
antibody (dilution, 1:5,000) at room temperature for 1 h.
Subsequent to washing in TBST, visualization was performed with an
enhanced chemiluminescence kit, and bands were observed with a gel
image system. Similar procedures were employed to detect the
expression of β-actin. The expression of target proteins was
normalized to that of β-actin.
Statistical analysis
Experimental data are presented as the mean ±
standard deviation. SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Student's t test was
used to compare two independent groups of data. P<0.05 was
considered to indicate a statistically significant difference.
Results
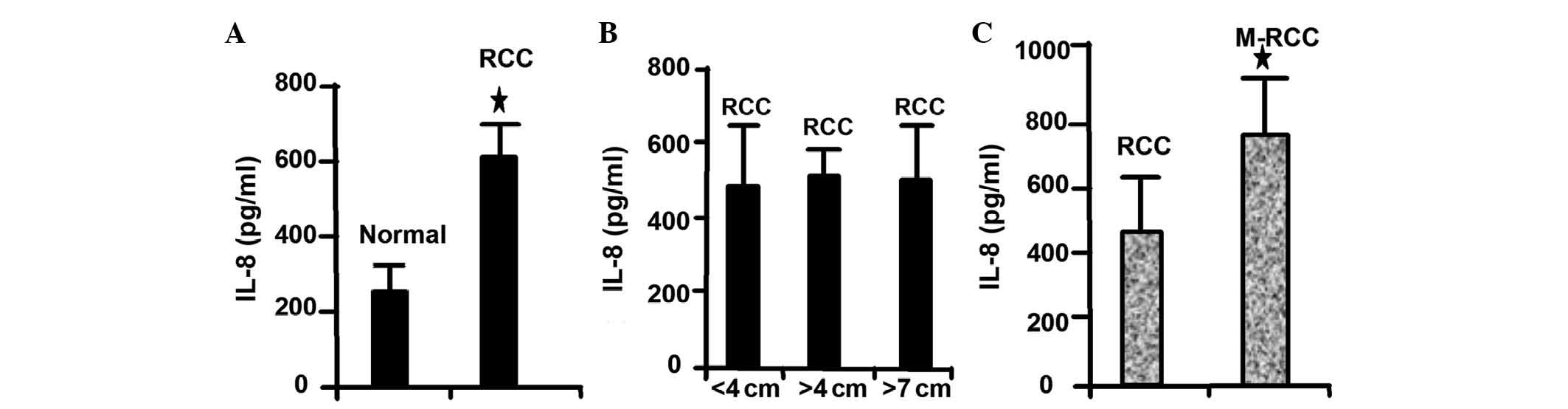
IL-8 is highly expressed in RCC
In several tumor types, including lung, colon and
breast cancer, the expression of IL-8 is elevated in tumor tissues
compared with normal tissues (20,21). In
agreement with these results, the present study revealed that IL-8
is highly expressed in RCC compared with normal tissues (Fig. 2A). Notably, high IL-8 expression was
not correlated with the size of the tumor (Fig. 2B). In addition, the present study
revealed that IL-8 is highly expressed in metastatic RCC (Fig. 2C). These results demonstrated that
IL-8 expression level is associated with the metastatic ability of
RCC cells.
IL-8 can induce EMT in RCC cells
Tumor EMT involves a phenotypic switch that promotes
the acquisition of a fibroblastoid-like morphology by epithelial
tumor cells, and it is an important step for cancer cells to
acquire metastatic capability (11,22). To
study the potential role of IL-8 in the metastatic ability of RCC,
the induction of RCC EMT induced by IL-8 was tested. The 786-O
cells at ~75% confluence were divided into two groups, the control
group (cultured without IL-8) and the IL-8-treated group
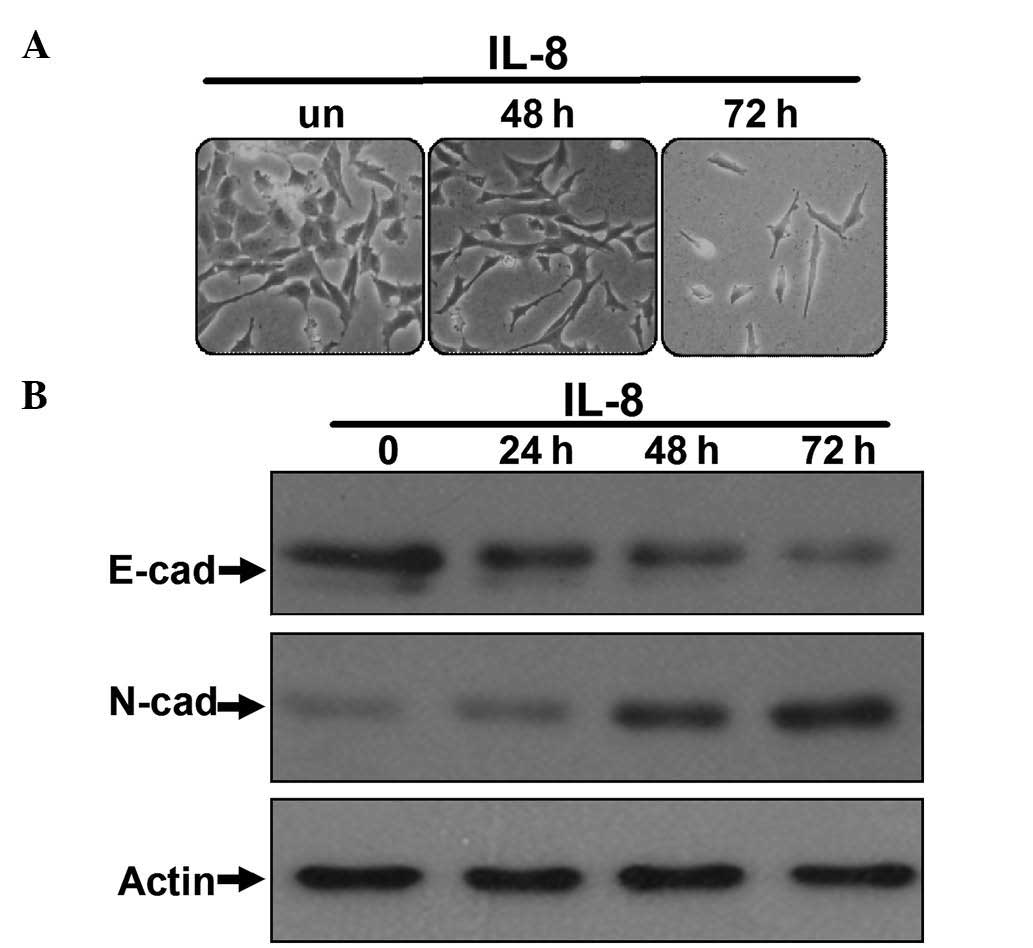
(experimental group). As shown in Fig.
1A, after being cultured for the indicated time, the morphology
of the 786-O cells in the IL-8 group had changed, switching from
epithelial tumor cell morphology to fibroblastoid-like morphology,
and reducing cell polarity and cell-to-cell contacts. In contrast,
the morphology of 786-O cells cultured without IL-8 remained
unchanged. Western blot analyses demonstrated reduced expression of
the E-cadherin epithelial marker and upregulation of the N-cadherin
mesenchymal marker in the IL-8-stimulated 786-O cells (Fig. 1B), which is the most important
characteristic of cancer cells undergoing EMT (23). These observations suggested that IL-8
may aid 786-O cells to reduce their polarity, enhance their
motility and acquire a fibroblastoid-like morphology. Furthermore,
IL-8 was able to upregulate mesenchymal markers expression and
downregulate epithelial markers expression in these cells,
indicating that IL-8 can induce EMT in RCC cells.
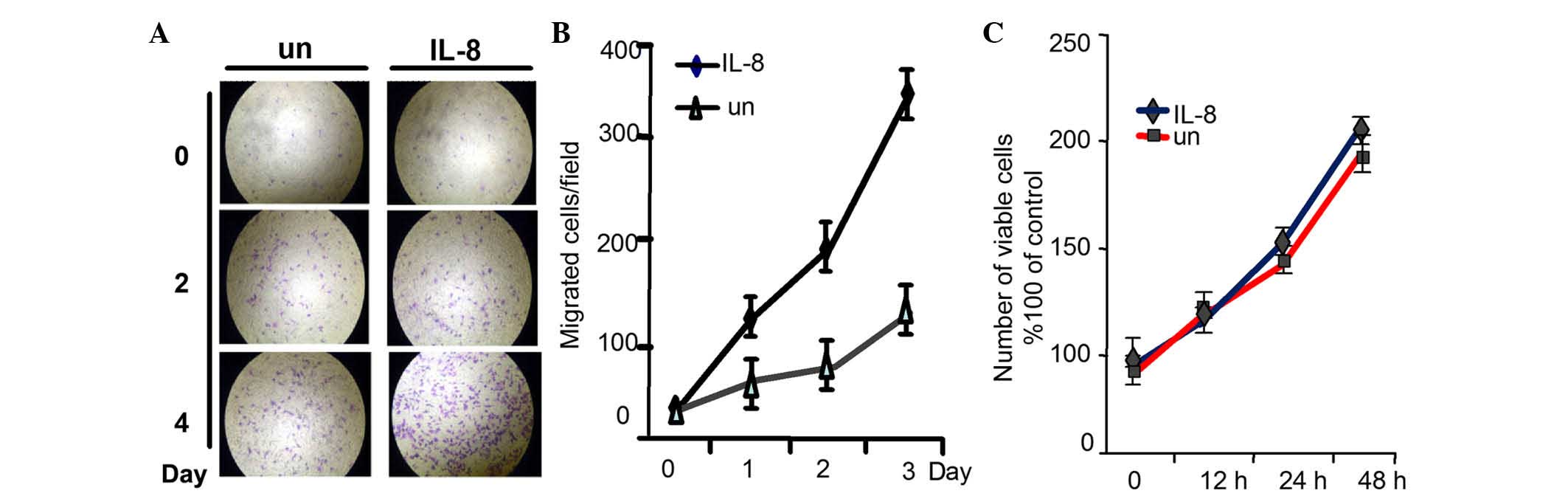
IL-8 promotes migration and invasion
of RCC cells without affecting cell proliferation
Tumor cells undergoing EMT reduce their polarity and
cell-to-cell contacts, and increase their motility and
invasiveness, which may be important in tumor metastasis and
progression (24). To determine the
effect of IL-8 on 786-O cell migratory ability and invasiveness
propensity, migration and invasion assays were used. As shown in
Fig. 3A and B, the quantity of cells
that penetrated through the matrigel was much larger in the
IL-8-stimulated group than in the normal group. However, the
cellular growth was not influenced when evaluated by MTT assay
(Fig. 3C). These results demonstrated
that IL-8 can promote RCC cells migration and invasion, and as a
result, it may also promote RCC metastasis, although it had no
effect on the proliferation of RCC cells.
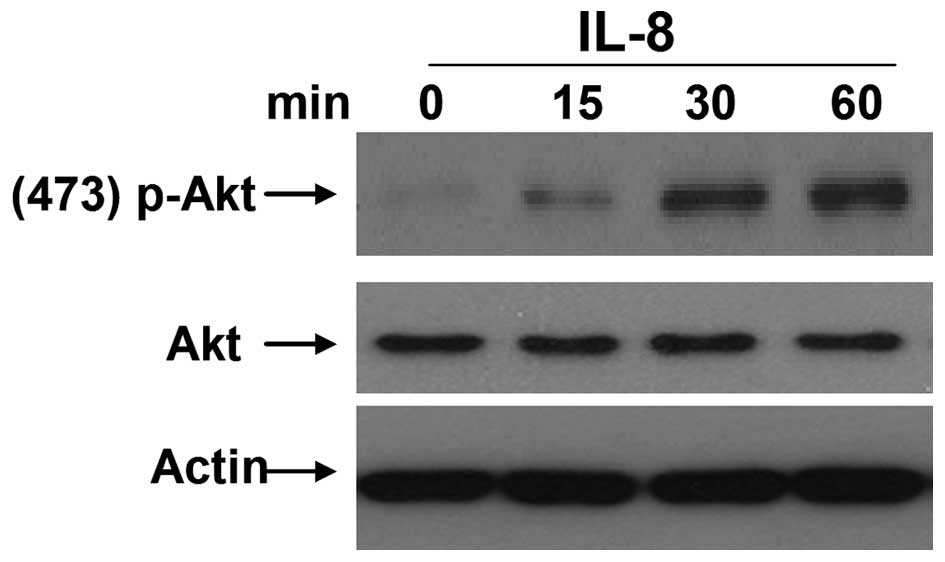
IL-8 can enhance the levels of
phosphorylated AKT in RCC cells
The PI3K/AKT is an important signaling pathway and
correlates with the progression of multiple malignant tumor types
(25). AKT, also called protein
kinase B, is regarded as the most important downstream signaling
molecule of PI3K, and is activated following phosphorylation
(26). According to recent research,
the PI3K/AKT signaling pathway is involved in EMT of numerous tumor
cells (27,28). To study the mechanism by which IL-8
promotes EMT in RCC, the levels of phosphorylated AKT were examined
in 786-O cells following stimulation with different concentrations
of IL-8. It was observed that appropriate IL-8 stimulation (100
µg/l) for 15, 30 or 60 min could noticeably enhance the levels of
phosphorylated AKT (Fig. 4). These
results suggested that IL-8 may be a critical factor to activate
AKT, and this may be one of the important mechanisms for regulating
EMT of RCC cells.
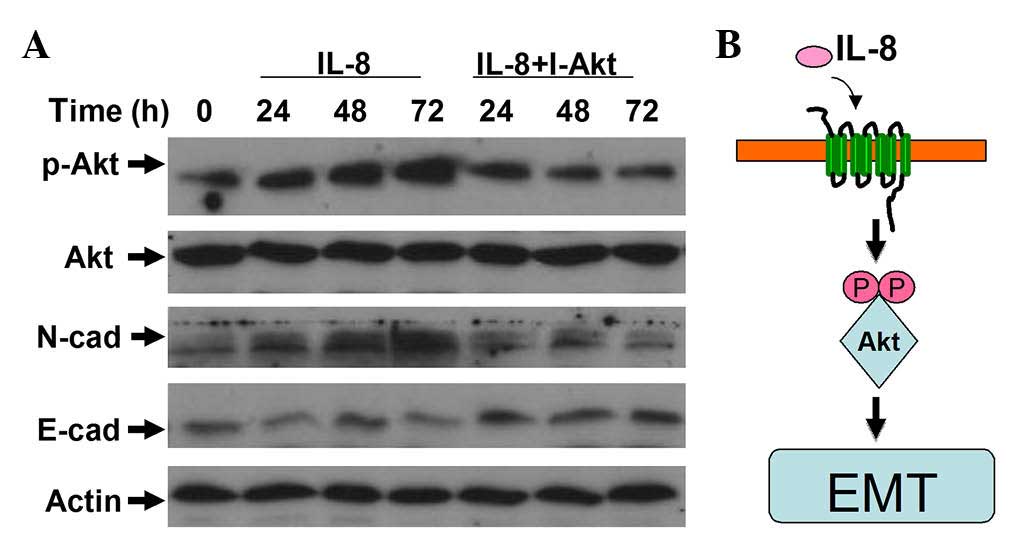
IL-8 participates in regulating the
EMT of RCC cells through the activation of AKT signaling
As mentioned above, IL-8 can elevate the phospho-AKT
level, which activates the PI3K/AKT signaling pathway.
Consequently, IL-8 may promote the migration and invasion of RCC
cells. To further examine the role of IL-8 in regulating the EMT of
RCC, LY294002 was used to inhibit AKT activation. The induction of
EMT caused by IL-8 was suppressed by LY294002 (Fig. 5). These results indicate that AKT
signaling is essential for IL-8 promotion of cellular motility in
RCC cells.
Discussion
The metastasis of RCC remains an intractable problem
in the clinic, mainly because it is not known exactly how RCC cells
acquire their invasive ability (29).
The present study demonstrated that IL-8 was highly expressed in
metastatic RCC and it could induce the EMT of RCC cells, which was
associated with AKT phosphorylation, and suggested that IL-8 may
induce the EMT of RCC by AKT signaling activation. This may be a
potential mechanism for RCC metastasis.
Malignant tumor progression consists of distinct
steps, including tumor growth, angiogenesis and EMT (30). RCC is one of the most common malignant
tumors of the urinary system, and the main obstacle that remains in
the current clinical management is its metastatic propensity and
resistance to chemotherapy, radiotherapy and immunological therapy
(31). Multiple studies have revealed
that EMT induction in human carcinoma cells is closely associated
with the enhanced secretion of numerous cytokines and chemokines,
as well as with the metastasis of several solid carcinomas,
including renal cancer (32,33). However, the exact mechanism of these
pathological processes is not clear. Elucidating the mechanism
underlying RCC metastasis, which could lead to improved treatments,
is of considerable significance.
IL-8 is a key chemokine that is important in
inflammation and cancer (34).
Increasing evidence in the literature has supported the role of
IL-8 as a critical factor for tumor growth, angiogenesis,
metastasis and development of tumors (35). Furthermore, it may be the connecting
factor between tumor cells and the tumor microenvironment. Tumor
cells tend to undergo EMT within the tumor microenvironment, and
then gain motility and invasive properties, and IL-8 is considered
to play a significant role in this process (36). In melanoma, tumor-derived IL-8 has
been demonstrated to promote tumor cell proliferation, survival and
migration via its autocrine activity (34). The present study revealed that IL-8
was highly expressed in metastatic RCC cells, and was able to
promote 786-O cell migration and invasion. These results suggest an
important potential mechanism for renal cancer metastasis, and IL-8
may be a potential drug target for preventing and inhibiting RCC
metastasis.
In recent years, the importance of EMT in the
progression of carcinomas has been demonstrated. It has been
revealed that tumor cells undergoing EMT have the potential to
reduce the expression of basement membrane constituents (collagen
type IV and laminins) and to augment the secretion of extracellular
matrix constituents (osteonectin and collagen type I) (37). Consequently, tumor epithelial cells
actively downregulate cell-cell adhesion systems, lose polarity and
acquire a mesenchymal phenotype with reduced intercellular
interactions (38). In the present
study, the motility and invasiveness of 786-O RCC cells undergoing
EMT were noticeably enhanced, and this phenomenon was positively
correlated with IL-8. Upon IL-8 stimulation, 786-O cells reduced
the expression of epithelial markers (E-cadherin) and upregulated
the expression of mesenchymal markers (N-cadherin), acquired a
phenotypic switch of EMT, and improved their ability to penetrate
through matrigel. The present data demonstrated that IL-8 could
induce EMT in renal cancer cells and may promote RCC
metastasis.
Activation of the PI3K signaling pathway is highly
prevalent in tumor growth, and it serves as a relay where signals
that emanate from the cell membrane are received and are converted
into intracellular signals that promote proliferation and survival
(39). The phospho-AKT level is a
classic indicator to evaluate AKT activity (40). Okui et al demonstrated that the
PI3K/AKT signaling pathway is closely associated with EMT induction
and human tumor development (41). In
the present study, IL-8 stimulation could noticeably enhance the
phosphorylated AKT levels as well as the motility and invasiveness
of RCC cells. Furthermore, when AKT signaling was blocked using
LY294002 to inhibit AKT activation, the induction of EMT mediated
by IL-8 stimulation could be eliminated. This observation indicated
that AKT is a key signaling pathway by which IL-8 regulates the
migratory and invasive abilities of RCC cells. Taken together,
these data indicate that IL-8 may induce EMT of RCC through the
activation of AKT signaling.
In conclusion, the results of the present study
demonstrated that IL-8 is highly expressed in metastatic RCC, and
it can promote 786-O cell migration and invasion by inducing EMT
via the activation of AKT signaling. However, there are various
other signaling pathways such as the nuclear factor-κB signaling
pathway that also are important in the induction and maintenance of
EMT (42). The impact that EMT plays
on tumor metastasis is still controversial (43–45). To
better understand the exact mechanisms of tumor metastasis and its
association with EMT and IL-8, further studies are required to
characterize other signaling pathways, as well as the phenotype of
RCC cells, in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant no.
81572507), and the Natural Science Foundation of Anhui Province
(grant no. 1508085SQH225).
References
|
1
|
Moch H: An overview of renal cell cancer:
Pathology and genetics. Semin Cancer Biol. 23:3–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pecuchet N, Fournier LS and Oudard S: New
insights into the management of renal cell cancer. Oncology.
84:22–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flanigan RC, Campbell SC, Clark JI and
Picken MM and Picken MM: Metastatic renal cell carcinoma. Curr
Treat Options Oncol. 4:385–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao X, Qi L, Chen X, Du J, Zhang Z and Liu
S: Expression of CX3CR1 associates with cellular migration,
metastasis, and prognosis in human clear cell renal cell carcinoma?
Urol Oncol. 32:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Escudier B, Albiges L and Sonpavde G:
Optimal management of metastatic renal cell carcinoma: Current
status. Drugs. 73:427–438. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun M, Shariat SF, Trinh QD, Meskawi M,
Bianchi M, Hansen J, Abdollah F, Perrotte P and Karakiewicz PI: An
evidence-based guide to the selection of sequential therapies in
metastatic renal cell carcinoma. Ther Adv Urol. 5:121–128. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Washio M, Mori M, Mikami K, Miki T,
Watanabe Y, Nakao M, Kubo T, Suzuki K, Ozasa K, Wakai K and
Tamakoshi A: Risk factors for renal cell carcinoma in a Japanese
population. Asian Pac J Cancer Prev. 15:9065–9070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Syrios J, Kechagias G and Tsavaris N:
Treatment of patients with metastatic renal cell carcinoma
undergoing hemodialysis: Case report of two patients and short
literature review. BMC Nephrol. 14:842013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ljungberg B: The role of metastasectomy in
renal cell carcinoma in the era of targeted therapy. Curr Urol Rep.
14:19–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JC, Liao SK, Lee EH, Hung MS, Sayion
Y, Chen HC, Kang CC, Huang LS and Cherng JM: Molecular events
associated with epithelial to mesenchymal transition of
nasopharyngeal carcinoma cells in the absence of Epstein-Barr virus
genome. J Biomed Sci. 16:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palena C, Hamilton DH and Fernando RI:
Influence of IL-8 on the epithelial-mesenchymal transition and the
tumor microenvironment. Future Oncol. 8:713–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bates RC, DeLeo MJ III and Mercurio AM:
The epithelial-mesenchymal transition of colon carcinoma involves
expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res.
299:315–324. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esteban MA, Bao X, Zhuang Q, Zhou T, Qin B
and Pei D: The mesenchymal-to-epithelial transition in somatic cell
reprogramming. Curr Opin Genet Dev. 22:423–428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mikami S, Oya M, Mizuno R, Kosaka T,
Katsube K and Okada Y: Invasion and metastasis of renal cell
carcinoma. Med Mol Morphol. 47:63–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Todorović-Raković N and Milovanović J:
Interleukin-8 in breast cancer progression. J Interferon Cytokine
Res. 33:563–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prud'homme GJ: Cancer stem cells and novel
targets for antitumor strategies. Curr Pharm Des. 18:2838–2849.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang D, Ding Y, Zhou M, Rini BI, Petillo
D, Qian CN, Kahnoski R, Futreal PA, Furge KA and Teh BT:
Interleukin-8 mediates resistance to antiangiogenic agent
sunitinibin renal cell carcinoma. Cancer Res. 70:1063–1071. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radisavljevic Z: AKT as locus of cancer
angiogenic robustness and fragility. J Cell Physiol. 228:21–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hasan MK, Nafady A, Takatori A, Kishida S,
Ohira M, Suenaga Y, Hossain S, Akter J, Ogura A, Nakamura Y, et al:
ALK is regulates a MYCN target gene and cell migration and invasion
in neuroblastoma. Sci Rep. 3:34502013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bai Z, Tai Y, Li W, Zhen C, Gu W, Jian Z,
Wang Q, Lin JE, Zhao Q, Gong W, et al: Gankyrin activates IL-8 to
promote hepatic metastasis of colorectal cancer. Cancer Res.
73:4548–4558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Umekawa K, Kimura T, Kudoh S, Suzumura T,
Oka T, Nagata M, Mitsuoka S, Matsuura K, Nakai T, Yoshimura N, et
al: Plasma RANTES, IL-10 and IL-8 levels in non-small-cell lung
cancer patients treated with EGFR-TKIs. BMC Res Notes. 6:1392013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu C, Li J, Cheng G, Zhou H, Tao L, Cai
H, Li P, Cao Q, Ju X, Meng X, et al: miR-154 inhibits EMT by
targeting HMGA2 in prostate cancer cells. Mol Cell Biochem.
379:69–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Simic P, Williams EO, Bell EL, Gong JJ,
Bonkowski M and Guarente L: SIRT1 suppresses the
epithelial-to-mesenchymal transition in cancer metastasis and organ
fibrosis. Cell Rep. 3:1175–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carpenter RL and Jiang BH: Roles of EGFR,
PI3K, AKT, and mTOR in heavy metal-induced cancer. Curr Cancer Drug
Targets. 13:252–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tong X and Pelling JC: Targeting the
PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anticancer
Agents Med Chem. 13:971–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dobbin Zc and Landen CN: The Importance of
the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int
J Mol Sci. 14:8213–8227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu H, Bhaijee F, Ishaq N, Pepper DJ,
Backus K, Brown AS, Zhou X and Miele L: Correlation of Notch1, pAKT
and nuclear NF-κB expression in triple negative breast cancer. Am J
Cancer Res. 3:230–239. 2013.PubMed/NCBI
|
|
29
|
Larkin J, Swanton C and Pickering L:
Optimizing treatment of metastatic renal cell carcinoma by changing
mechanism of action. Expert Rev Anticancer Ther. 11:639–649. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li F, Yin X, Luo X, Li HY, Su X, Wang XY,
Chen L, Zheng K and Ren GS: Livin promotes progression of breast
cancer through induction of epithelial-mesenchymal transition and
activation of AKT signaling. Cell Signal. 25:1413–1422. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gamelin E, Mertins SD, Regis JT, Mickley
L, Abati A, Worrell RA, Linehan WM and Bates SE: Intrinsic drug
resistance in primary and metastatic renal cell carcinoma. J Urol.
162:217–224. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M and Ben-Baruch A: Inflammatory
mediators in breast cancer: Coordinated expression of TNFα &
IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal
transition. BMC Cancer. 11:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chuang MJ, Sun KH, Tang SJ, Deng MW, Wu
YH, Sung JS, Cha TL and Sun GH: Tumor-derived tumor necrosis
factor-alpha promotes progression and epithelial-mesenchymal
transition in renal cell carcinoma cells. Cancer Sci. 99:905–913.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen XH, Xu SJ, Jin CY, Ding F, Zhou YC
and Fu GS: Interleukin-8 prevents oxidative stress-induced human
endothelial cell senescence via telomerase activation. Int
Immunopharmacol. 16:261–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fernando RI, Castillo MD, Litzinger M,
Hamilton DH and Palena C: IL-8 signaling plays a critical role in
the epithelial-mesenchymal transition of human carcinoma cells.
Cancer Res. 71:5296–5306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mathias RA, Chen YS, Wang B, Ji H, Kapp
EA, Moritz RL, Zhu HJ and Simpson RJ: Extracellular remodelling
during oncogenic Ras-induced epithelial-mesenchymal transition
facilitates MDCK cell migration. J Proteome Res. 9:1007–1019. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arima Y, Hayashi N, Hayashi H, Sasaki M,
Kai K, Sugihara E, Abe E, Yoshida A, Mikami S, Nakamura S and Saya
H: Loss of p16 expression is associated with the stem cell
characteristics of surface markers and therapeutic resistance in
estrogen receptor negative breast cancer. Int J Cancer.
130:2568–2579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Juvekar A and Wulf GM: Closing escape
routes: Inhibition of IL-8 signaling enhances the anti-tumor
efficacy of PI3K inhibitors. Breast Cancer Res. 15:3082013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kohn AD, Takeuchi F and Roth RA: Akt, a
pleckstrin homology domain containing kinase, is activated
primarily by phosphorylation. J Biol Chem. 271:21920–21926. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okui G, Tobiume K, Rizqiawan A, Yamamoto
K, Shigeishi H, Ono S, Higashikawa K and Kamata N: AKT primes
snail-induced EMT concomitantly with the collective migration of
squamous cells. J Cell Biochem. 114:2039–2049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Grund EM, Kagan D, Tran CA, Zeitvogel A,
Starzinski-Powitz A, Nataraja S and Palmer SS: Tumor necrosis
factor-alpha regulates inflammatory and mesenchymal responses via
mitogen-activated protein kinase kinase, p38, and nuclear factor
kappaB in human endometriotic epithelial cells. Mol Pharmacol.
73:1394–1404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li G, Zhang Y, Qian Y, Zhang H, Guo S,
Sunagawa M, Hisamitsu T and Liu Y: Interleukin-17A promotes
rheumatoid arthritis synoviocytes migration and invasion under
hypoxia by increasing MMP2 and MMP9 expression through NF-κB/HIF-1α
pathway. Mol Immunol. 53:227–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kiefel H, Bondong S, Pfeifer M, Schirmer
U, Erbe-Hoffmann N, Schäfer H, Sebens S and Altevogt P:
EMT-associated up-regulation of L1CAM provides insights into
L1CAM-mediated integrin signalling and NF-κB activation.
Carcinogenesis. 33:1919–1929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kang YH, Ji NY, Han SR, Lee CI, Kim JW,
Yeom YI, Kim YH, Chun HK, Kim JW, Chung JW, et al: ESM-1 regulates
cell growth and metastatic process through activation of NF-κB in
colorectal cancer. Cell Signal. 24:1940–1949. 2012. View Article : Google Scholar : PubMed/NCBI
|