Introduction
Epigenetic mechanisms, including microRNAs (miRNAs),
have been implicated in the pathogenesis and prognosis of
myelodysplastic syndrome (MDS) and have been exploited in the
successful treatment of this condition (1–2).
Single-agent epigenetic therapy (EGT) with demethylating agents,
including azacytidine (AZA) and decitabine, has been successfully
utilized in MDS to improve overall survival rate and time (1–3), whilst
clinical trials suggest combination EGT utilizing demethylating
agents and histone deacetylase inhibitors (HDACis) may result in
enhanced clinical responses over single-agent therapy (4). Despite successful therapeutic
intervention utilizing EGT in MDS, not all patients respond (~50%),
and treatment regimens are often lengthy, with responses frequently
only observed subsequent to several cycles of therapy (3). The problematic response to EGT in MDS
has generated a significant demand for molecular markers to improve
the early prediction of response to EGT.
miRNAs are small non-coding single stranded RNA
molecules of 20–25 base pairs that are responsible for
post-transcriptional regulation of gene expression (5). Epigenetic regulation of miRNA expression
has recently been implicated in the pathogenesis of MDS.
Demonstration of epigenetic silencing of the miRNA-124 promoter in
murine MDS studies (6) together with
significant inhibition of miRNA-124 expression and excessive
miRNA-124 promoter methylation in MDS patients (7) suggests miRNA-124 may not only be
implicated in the pathogenesis of MDS, but also potentially in the
molecular mechanisms associated with response to EGT. Evidence of
early upregulation of miRNA-124 expression in patients with MDS and
acute myeloid leukemia (AML) treated with and responding to
single-agent or combination EGT compared with non-responding
patients suggests a critical role for epigenetic regulation of
miRNA-124 in the response to EGT and indicates a potential use of
miRNA-124 as a biomarker of early response to EGT in MDS (8).
Investigation into downstream molecular targets of
EGT-mediated ‘re-expression’ of miRNA-124 may reveal additional
molecular mechanisms associated with the EGT-mediated miRNA-124
therapeutic response in MDS and afford the potential for
identification of additional biomarkers and therapeutic targets for
this condition. Previous studies have demonstrated that epigenetic
loss of miRNA-124 expression correlates with activation of the
oncogene cyclin-dependent kinase (CDK) 6, which in turn
phosphorylates and inactivates the tumor-suppressor protein Rb
(9). EGT-mediated induction of
miRNA-124 in MDS/AML is associated with attenuation of CDK6
messenger (m)RNA levels in treatment responders (8), suggesting a tumor suppressor role for
miRNA-124.
The present study aimed to evaluate the effects of
combination EGT with AZA and panobinostat (LBH589) in vitro
and in vivo on expression of additional downstream targets
of miRNA-124, including CDK4 and enhancer of zeste homolog 2 (EZH2)
in order to further elucidate the molecular mechanisms associated
with EGT-mediated regulation of miRNA-124 expression in MDS/AML.
The present study aimed to further qualify miRNA-124 as a possible
biomarker of early response to EGT and potentially a valid
therapeutic target, together with CDK4, CDK6 and EZH2.
Materials and methods
Cell culture
HL60 cells were cultured in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
heat-inactivated fetal calf serum (Thermo Fisher Scientific, Inc.)
and kept in a 5% CO2 incubator at 37°C. The agents added
to plates for 48 h were as follows: AZA, which was provided by
Celgene (Melbourne, Australia); and LBH589, which was provided by
Novartis (Sydney, Australia). AZA was dissolved in H2O
with 0.2% acetic acid and used at a final concentration of 1.0 µM.
LBH589 was dissolved in phosphate-buffered saline (PBS) with 1%
dimethyl sulfoxide and used at a final concentration of 20 nM.
RNA extraction
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was used to extract RNA from HL60 cells treated with AZA, LBH589 or
a combination of AZA and LBH589. TRIzol was also used to extract
RNA from the mononuclear cell fractions of blood samples obtained
from 9 patients in a phase Ib/II clinical trial (10) and healthy controls (n=6). The
open-label, phase Ib/II study (10)
was conducted at three centres: Alfred Hospital, Melbourne,
Australia; Princess Alexandra Hospital, Brisbane, Australia; and
Austin Hospital, Melbourne, Australia. The study was approved by
the ethics committees at the aforementioned institutions and was
performed in accordance with the principles of independent Human
Research and Ethics Committees, and registered with the Australian
and New Zealand Clinical Trials Registry (ACTRN12610000924055). All
participating patients and healthy controls were required to
provide written informed consent for participation in the
study.
The clinical trial consisted of a 5-day schedule of
AZA administration followed by LBH589 administration in high-risk
MDS or AML patients. On days 1–5, 75 mg/m2 AZA was
injected subcutaneously and LBH589 was administered orally 3 times
a week (Monday/Wednesday/Friday), starting on day 5 of each 28-day
cycle. A total of 40 evaluable patients were enrolled in the
present study (10), of which, 9
patients, consisting of 5 patients with AML and 4 patients with
high-risk MDS (Table I), were
evaluated for miRNA-124, CDK4, CDK6 and EZH2 expression at screen
and prior to the end of cycle 1 (day 25). The association between
the expression of miRNA-124, CDK4, CDK6 and EZH2 and treatment
response were determined using bone marrow biopsies at 1, 3 and 6
months subsequent to treatment commencement (10). Bone marrow biopsies were performed at
1, 3 and 6 months after treatment initiation to evaluate the
clinical response to treatment, which may be delayed with
epigenetic treatment. Only end of first cycle (day 25) miRNA-124,
CDK4, CDK6 and EZH2 samples were evaluated, as the study was
investigating early markers of response to epigenetic treatment.
Treatment responses were defined according to International Working
Group criteria for AML and MDS (11).
 | Table I.Association between clinical and
molecular response and miRNA-124, CDK4, CDK6 and EZH2
expression. |
Table I.
Association between clinical and
molecular response and miRNA-124, CDK4, CDK6 and EZH2
expression.
| Patient ID | Age, years | Gender | Diagnosis | Best response | miRNA-124
inductiona | CDK4
inhibitionb | CDK6
inhibitionb | EZH2
inhibitionc |
|---|
| 3 | 72 | F | MDS | CR | Yes | Yes | Yes | Yes |
| 7 | 58 | M | AML | Resistant | No | No | No | No |
| 8 | 75 | M | AML | Resistant | No | No | No | No |
| 18 | 67 | F | MDS | PR | No | Yes | Yes | Yes |
| 22 | 78 | M | AML | CR | Yes | No | No | No |
| 23 | 69 | F | AML | Resistant | No | No | No | Yes |
| 26 | 75 | M | MDS | PD | No | No | No | Yes |
| 28 | 69 | M | AML | PR | Yes | Yes | Yes | Yes |
| 33 | 62 | F | MDS | Marrow CR | Yes | Yes | Yes | Yes |
Reverse transcription (RT)
RT of mRNA obtained from HL60 cells, healthy
volunteers and patients was performed using a 15 µl reaction mix
containing 100 mM deoxynucleotide triphosphates (dNTPs), 50 U/µl
MultiScribe Reverse Transcriptase, 10X RT buffer, 20 U/µl RNase
Inhibitor, nuclease-free water (Invitrogen; Thermo Fisher
Scientific, Inc.), oligo(dT) random primer (Promega Corporation,
Madison, WI, USA) and mRNA samples from HL60 cells, healthy
volunteers or patients.
RNA isolation and stem-loop
RT-polymerase chain reaction (PCR) for miRNA-124
Total RNA was isolated using TRIzol, according to
the manufacturer's protocol. RT was performed using TaqMan MicroRNA
RT kit and TaqMan MicroRNA Assay kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
Total RNA was reverse transcribed using 1 mM dNTPs, 50 U
MultiScribe Reverse Transcriptase, 1X RT Buffer, 3.8 U RNase
Inhibitor and 1X stem-loop RT primer (all obtained from Applied
Biosystems; Thermo Fisher Scientific, Inc.), under the following
thermal cycling conditions: 16°C for 30 min; 42°C for 30 min; and
85°C for 5 min. Quantitative PCR (qPCR) of miRNA-124 was performed
using 1.33 ml of 1:15 diluted RT product in 1X TaqMan Universal PCR
Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
1X TaqMan Assay (Applied Biosystems; Thermo Fisher Scientific,
Inc.) at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. U6 was used as a reference for data analysis
using the 2−∆∆Cq method (12).
qPCR
Reaction volumes of 20 µl contained SYBR Green 1
Buffer (Qiagen GmbH, Hilden, Germany) and forward and reverse
primers for the target genes CDK4, CDK6 and EZH2. Each PCR run also
included wells containing a no-template control. A melting point
dissociation curve generated by the instrument (Applied Biosystems
7500 Real-Time PCR System; Thermo Fisher Scientific, Inc.) was used
to confirm that only a single product was present. The fluorescence
data were quantitated using the threshold cycle value (12). Data were normalized to β-actin and
presented as the mean fold-change compared with the pre-treatment
screening sample. PCR was performed a minimum of 2 times for each
patient sample to ensure consistency (13). Forward and reverse primer sequences
for CDK4, CDK6 and EZH2 were as follows: CDK4 forward,
5′-AGTTCGTGAGGTGGCTTTA-3′ and reverse, 5′-GGGTGCCTTGTCCAGATA-3′;
CDK6 forward, 5′-GCCTATGGGAAGGTGTTCAA-3′ and reverse,
5′-CTGTCTGTTCGTGACACTGT-3′; and EZH2 forward,
5′-TTCATGCAACACCCAACACT-3′ and reverse, 5′-GAGAGCAAACTCCT-3′. The
primers used for β-actin were as follows: Forward,
5′-GACAGGATGCAGAAGGAGATTACT-3′; and reverse,
5′-TGATCCACATCTGCTGGAAGGT-3′.
Statistical analysis
Results were expressed as the mean + standard error
of the mean, and analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA), using t-tests for
two-group comparisons and one-way analysis of variance for three or
more group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of AZA and LBH589 on miRNA-124,
CDK4, CDK6 and EZH2 expression in HL60 cells
LBH589 or AZA alone increased miRNA-124 expression,
with AZA being the more potent agent (Fig. 1A). The combination of AZA with LBH589
demonstrated a significant and additive increase in miRNA-124
expression compared with single-agent treatment (Fig. 1A). LBH589 or AZA alone decreased
expression of CDK4 expression and the combination of AZA and LBH589
significantly and additively decreased CDK4 expression compared
with single agent treatment (Fig.
1B). AZA and LBH589 inhibited CDK6 expression, with LBH589
being the more potent agent and demonstrating a significant
inhibition of CDK6 expression (Fig.
1C). The combination of AZA and LBH589 also resulted in
significant attenuation of CDK6 mRNA expression compared with
untreated cells (Fig. 1C). AZA,
LBH589 and the combination of AZA and LBH589 all significantly
inhibited EZH2 expression, with single and combination therapy
being equally efficacious (Fig.
1D).
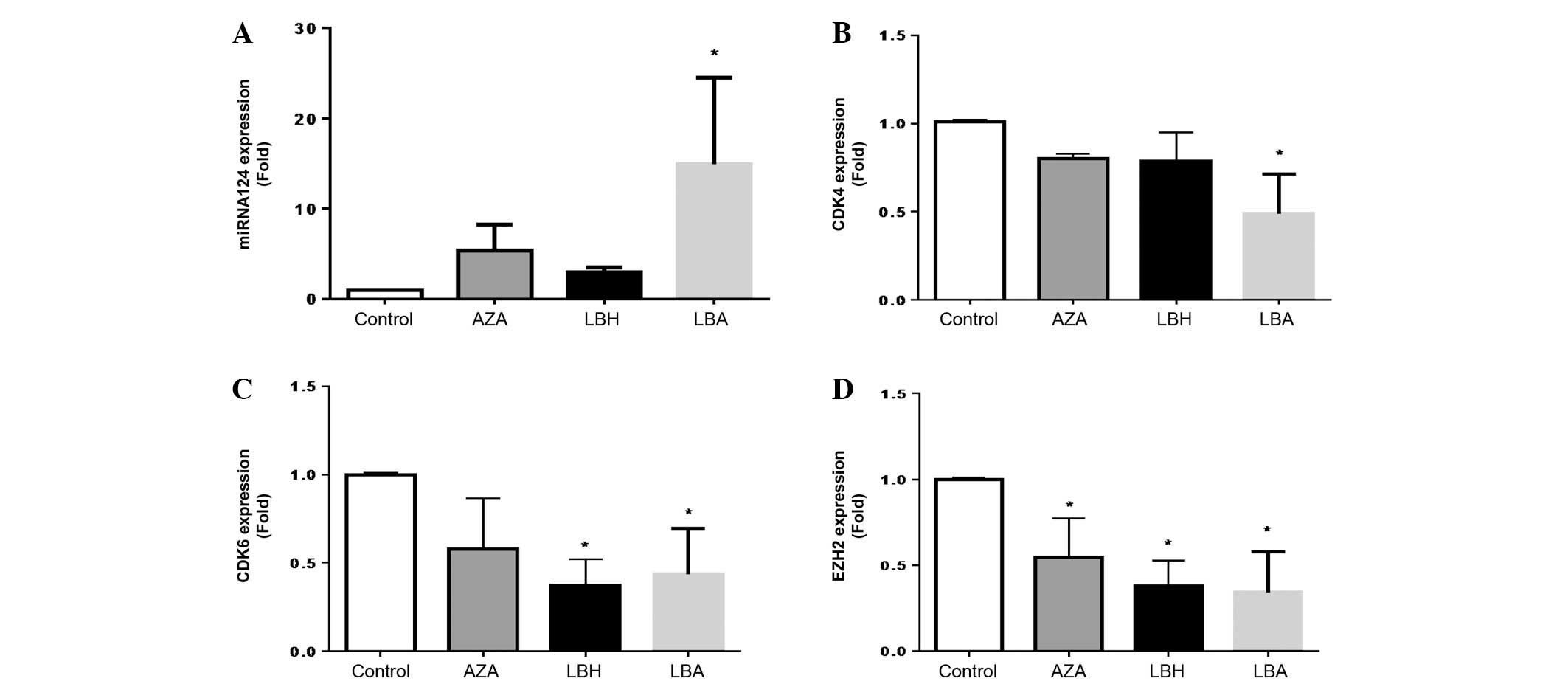 | Figure 1.In vitro effects of azacytidine
and/or LBH589 on (A) miRNA-124, (B) CDK4, (C) CDK6 and (D) EZH2
mRNA expression in HL60 cells. *P<0.05 vs. Control (n=3). The
P-values obtained were as follows: (A) P=0.620, 0.740 and 0.032 for
AZA, LBH and LBA, respectively, vs. Control; (B) P=0.812, 0.511 and
0.025 for AZA, LBH and LBA, respectively, vs. Control; (C) P=0.519,
0.024 and 0.031 for AZA, LBH and LBA, respectively, vs. Control;
and (D) P=0.025, 0.002 and 0.008 for AZA, LBH and LBA,
respectively, vs. Control. LBH589, panobinostat; miRNA, microRNA;
CDK, cyclin-dependent kinase; EZH2, enhancer of zeste homolog 2;
AZA, cells treated with 1.0 µM 5-azacytidine for 48 h; LBH, cells
treated with 20 nM LBH589 for 48 h; LBA, cells treated with 20 nM
LBH589 + 1.0 µM AZA; Control, HL60 cells without treatment. |
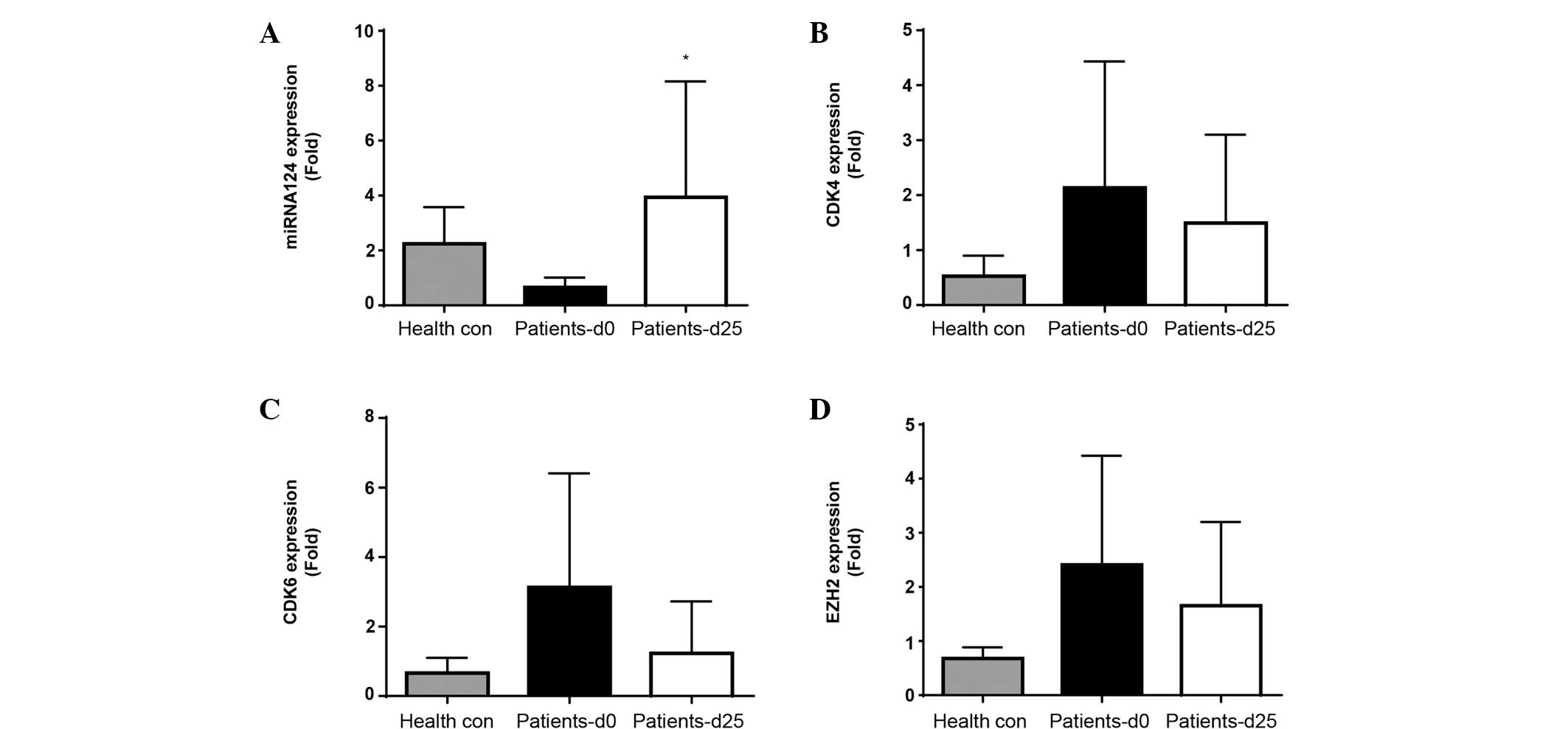
In vivo expression of miRNA-124, CDK4,
CDK6 and EZH2 in peripheral blood mononuclear cells
miRNA-124, CDK4, CDK6 and EZH2 mRNA expression
levels were determined at screening (prior to treatment
commencement, day 0) and on day 25, subsequent to the first cycle
of treatment. Screening levels of miRNA-124, CDK4, CDK6 and EZH2
expression were initially compared between patients and healthy
controls (Fig. 2A-D). A decrease in
miRNA-124 and increase in CDK4, CDK6 and EZH2 expression was
observed in patients compared with controls, suggesting
disease-associated inhibition of miRNA-124 and induction of CDK4,
CDK6 and EZH2.
Subsequent evaluation of miRNA-124, CDK4, CDK6 and
EZH2 expression levels was performed to compare treatment
non-responders and responders at screening (Fig. 3A-D). No significant differences were
observed between responder and non-responder expression levels of
miRNA-124, CDK4, CDK6 and EZH2 at screening (Fig. 3).
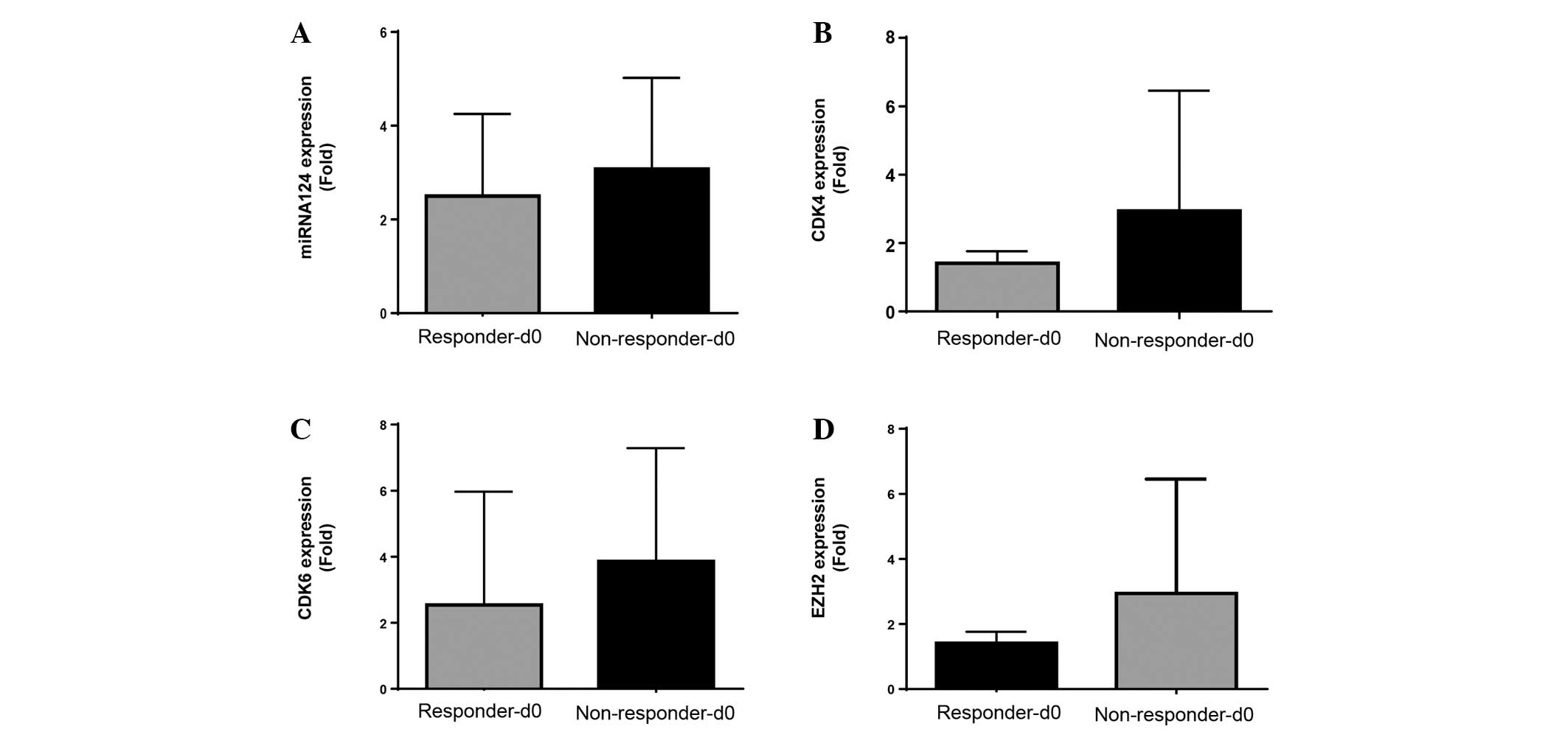 | Figure 3.In vivo expression at screening
of miRNA-124, CDK4, CDK6 and EZH2 from responder and non-responder
MDS/AML patients treated with a combination azacytidine and
panobinostat. Pre-treatment screening mRNA expression levels of (A)
miRNA-124, (B) CDK4, (C) CDK6 and (D) EZH2 for responders
(Responders-d0) and non-responders (Non-responder-d0), with
clinical response to treatment determined at 1, 3 and 6 months
(n=9). Responses were defined according to International Working
Group criteria for AML and MDS (11).
AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; miRNA,
microRNA; CDK, cyclin-dependent kinase; EZH2, enhancer of zeste
homolog 2. |
Finally, post-first treatment cycle evaluation of
miRNA-124, CDK4, CDK6 and EZH2 mRNA expression levels between
treatment responders and non-responders was performed (Fig. 4A-D). A significant increase in the
expression of miRNA-124 compared with screening levels (Figs. 3A and 4A), together with a significant association
with treatment response, compared with non-responders at day 25,
was observed in the responders (Fig.
4A). CDK4, CDK6 and EZH2 mRNA expression in responders at day
25 subsequent to the first cycle of treatment was decreased
compared with screening levels in responders (Figs. 3B-D and 4B-D). Notably, a significant decrease in
CDK4 and CDK6 was observed, although significant inhibition of EZH2
expression was not observed, in treatment responders compared to
non-responders at day 25 (Fig. 4B-D;
Table I).
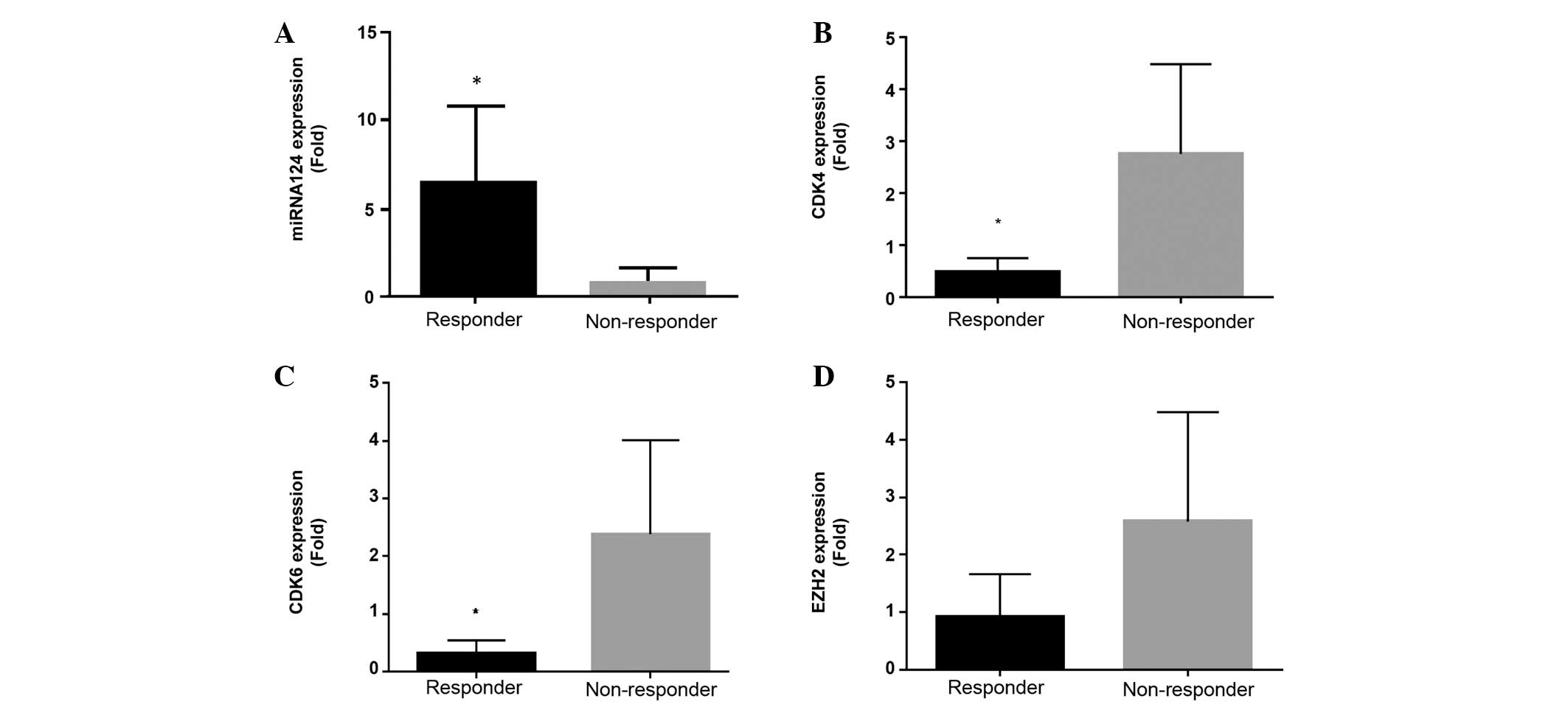 | Figure 4.In vivo expression at Day 25 of
miRNA-124, CDK4, CDK 6 and EZH2 from responder and non-responder
MDS/AML patients treated with a combination of azacytidine and
panobinostat. mRNA expression levels of (A) miRNA-124, (B) CDK4,
(C) CDK6 and (D) EZH2 subsequent to first cycle (day 25) for
responders and non-responders, with clinical response to treatment
determined at 1, 3 and 6 months (n=9). *P<0.05, responder vs.
non-responder. The P-values were as follows: (A), P=0.036; (B)
P=0.036; (C) P=0.025; and (D) P=0.111. Responses were defined
according to International Working Group criteria for AML and MDS
(11). MDS, myelodysplastic syndrome;
AML, acute myeloid leukemia; miRNA, microRNA; CDK, cyclin-dependent
kinase; EZH2, enhancer of zeste homolog 2. |
Discussion
Epigenetic dysregulation of miRNAs is an emerging
mechanism of action implicated in the pathogenesis, response to
therapy and prognosis of myeloid malignancies (1). miRNA-124, a potential tumor suppressor
gene with a hypothesized epigenetic role in the development of MDS
and AML, has previously been shown to respond to EGT and may have a
role in the therapeutic response to EGT in MDS/AML, with potential
to act as a biomarker of early treatment response (1,6–8).
Downstream targets of EGT-mediated miRNA-124
re-expression identified using either single agent or combined EGT
include repression of CDK4, CDK6 and EZH2 mRNA, resulting in in
vitro and in vivo inhibition of cell growth in
hematological malignancies and solid tumors (8,9,14–16). The
present study has confirmed and extended these observations to
delineate multiple downstream targets of increased miRNA-124
expression in MDS/AML and associate this with response to
combination EGT.
In vitro studies in the HL60 cell line
confirm that EGT-mediated re-expression of miRNA-124 with either
single-agent or combination EGT is associated with repression of
multiple targets critical to cell cycle progression, such as CDK4
and CDK6, and the oncoprotein EZH2. Notably, EZH2, a histone methyl
transferase, has been identified to mediate oncogenic and tumor
suppressor effects in myeloid malignancies (1,17).
Inactivating mutations of EZH2 have been identified in MDS
(1), whilst loss of function
mutations in EZH2 are also associated with a decreased rate of
progression to AML (18). EZH2
upregulation has also been identified in myeloproliferative disease
(19) and AML (20). EZH2 (21), CDK4 and CDK6 (22) have all been identified as potential
therapeutic targets in hematological malignancies and may therefore
have a potential mechanistic role in EGT treatment response.
Additional studies are required to fully elucidate the roles of
CDK4, CDK6 and EZH2 in this setting.
In vivo analysis of miRNA-124 expression
together with its downstream targets CDK4, CDK6 and EZH2 in
patients identified significantly reduced expression of miRNA-124
in patients compared with healthy controls (Fig. 2), in addition to upregulation of CDK4,
CDK6 and EZH2, suggesting these expression changes are integral to
disease pathogenesis. Screening levels of miRNA-124, CDK4, CDK6 or
EZH2 demonstrated no significant association with clinical response
to therapy (Fig. 3), similar to
several previous studies undertaking analysis of other epigenetic
markers in combination EGT (10,23).
Determination of miRNA-124, CDK4 and CDK6 expression levels from
the post-first cycle treatment time point (day 25), demonstrated a
significant association with clinical response, although EZH2
showed a trend to but no significant association (Fig. 4; Table
I). CDK4, CDK6 and EZH2 have been previously identified as
potential targets of upregulation of miRNA-124 expression in uveal
melanoma (16). This suggests a
canonical pathway in response to miRNA-124 upregulation in the
setting of oncogenic transformation. The lack of association
between clinical response to EGT and miRNA-124, CDK4, CDK6 and EZH2
expression levels at screening suggests that these molecules would
not be useful predictors of clinical response if used prior to
commencement of EGT. However, they may be useful as early as
subsequent to the first cycle of treatment, resulting in
significant improvement over current predictive strategies for
determining early response to EGT.
Overall, the present observations provide a
potential molecular mechanism for miRNA-124-mediated response to
EGT in patients receiving combination treatment with a
demethylating agent and HDACi for high-risk MDS/AML, further
qualifying miRNA-124 as a possible marker of early response to EGT
and potentially a valid therapeutic target, together with CDK4,
CDK6 and EZH2. Future studies may include evaluation of miRNA-124
in comparison to other potential markers of early treatment
response, such as orphan nuclear receptor NUR77, and
confirmation of EZH2 expression and subsequent prognosis in
response to EGT.
References
|
1
|
Vasilatou D, Papageorgiou SG, Dimitriadis
G and Pappa V: Epigenetic alterations and microRNAs: New players in
the pathogenesis of myelodysplastic syndromes. Epigenetics.
8:561–570. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan PT and Wei AH: The epigenomics
revolution in myelodysplasia: A clinico-pathological perspective.
Pathology. 43:536–546. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fenaux P, Mufti GJ, Hellstrom-Lindberg E,
Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz
G, List A, et al: Efficacy of azacitidine compared with that of
conventional care regimens in the treatment of higher-risk
myelodysplastic syndromes: A randomised, open-label, phase III
study. Lancet Oncol. 10:223–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ornstein MC, Mukherjee S and Sekeres MA:
More is better: Combination therapies for myelodysplastic
syndromes. Best Pract Res Clin Haematol. 28:22–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dickstein J, Senyuk V, Premanand K,
Laricchia-Robbio L, Xu P, Cattaneo F, Fazzina R and Nucifora G:
Methylation and silencing of miRNA-124 by EVI1 and self-renewal
exhaustion of hematopoietic stem cells in murine myelodysplastic
syndrome. Proc Natl Acad Sci USA. 107:9783–9788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia Q, Hu J and Meng YS: Abnormal
expression of microRNA-124 in patients with leukemia or
myelodysplastic syndrome and its significance. Zhongguo Shi Yan Xue
Ye Xue Za Zhi. 20:358–361. 2012.PubMed/NCBI
|
|
8
|
Castoro RJ, Dekmezian M, Saraf AJ,
Watanabe Y, Chung W, Adhab SE, Jelinek J and Issa JP: MicroRNA 124
and its role in response to epigenetic therapy in patients with
acute myelogenous leukemia and myelodysplastic syndrome. Blood.
112:5982008.
|
|
9
|
Lujambio A, Ropero S, Ballestar E, Fraga
MF, Cerrato C, Setién F, Casado S, Suarez-Gauthier A,
Sanchez-Cespedes M, Git A, et al: Genetic unmasking of an
epigenetically silenced microRNA in human cancer cells. Cancer Res.
67:1424–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan P, Wei A, Mithraprabhu S, Cummings N,
Liu HB, Perugini M, Reed K, Avery S, Patil S, Walker P, et al: Dual
epigenetic targeting with panobinostat and azacitidine in acute
myeloid leukemia and high-risk myelodysplastic syndrome. Blood
Cancer J. 4:e1702014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheson BD, Greenberg PL, Bennett JM,
Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM,
Stone RM, et al: Clinical application and proposal for modification
of the International Working Group (IWG) response criteria in
myelodysplasia. Blood. 108:419–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu HB, Mayes PA, Perlmutter P, McKendrick
JJ and Dear AE: The anti-leukemic effect and molecular mechanisms
of novel hydroxamate and benzamide histone deacetylase inhibitors
with 5-aza-cytidine. Int J Oncol. 38:1421–1425. 2011.PubMed/NCBI
|
|
14
|
Wong KY, So CC, Loong F, Chung LP, Lam WW,
Liang R, Li GK, Jin DY and Chim CS: Epigenetic inactivation of the
miR-124-1 in haematological malignancies. PLoS One. 6:e190272011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agirre X, Vilas-Zornoza A, Jiménez-Velasco
A, Martin-Subero JI, Cordeu L, Gárate L, San José-Eneriz E,
Abizanda G, Rodríguez-Otero P, Fortes P, et al: Epigenetic
silencing of the tumor suppressor microRNA Hsa-miR-124a regulates
CDK6 expression and confers a poor prognosis in acute lymphoblastic
leukemia. Cancer Res. 69:4443–4453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen X, He D, Dong XD, Dong F, Wang J,
Wang L, Tang J, Hu DN, Yan D and Tu L: MicroRNA-124a is
epigenetically regulated and acts as a tumor suppressor by
controlling multiple targets in uveal melanoma. Invest Ophthalmol
Vis Sci. 54:2248–2256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CJ and Hung MC: The role of EZH2 in
tumour progression. Br J Cancer. 106:243–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sashida G, Harada H, Matsui H, Oshima M,
Yui M, Harada Y, Tanaka S, Mochizuki-Kashio M, Wang C, Saraya A, et
al: Ezh2 loss promotes development of myelodysplastic syndrome but
attenuates its predisposition to leukaemic transformation. Nat
Commun. 5:41772014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herrera-Merchan A, Arranz L, Ligos JM, de
Molina A, Dominguez O and Gonzalez S: Ectopic expression of the
histone methyltransferase Ezh2 in haematopoietic stem cells causes
myeloproliferative disease. Nat Commun. 3:6232012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka S, Miyagi S, Sashida G, Chiba T,
Yuan J, Mochizuki-Kashio M, Suzuki Y, Sugano S, Nakaseko C, Yokote
K, et al: Ezh2 augments leukemogenicity by reinforcing
differentiation blockage in acute myeloid leukemia. Blood.
120:1107–1117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agarwal P, Alzrigat M, Párraga AA, Enroth
S, Singh U, Ungerstedt J, Österborg A, Brown PJ, Ma A, Jin J, et
al: Genome-wide profiling of histone H3 lysine 27 and lysine 4
trimethylation in multiple myeloma reveals the importance of
Polycomb gene targeting and highlights EZH2 as a potential
therapeutic target. Oncotarget. 7:6809–6823. 2016.PubMed/NCBI
|
|
22
|
Bose P, Simmons GL and Grant S:
Cyclin-dependent kinase inhibitor therapy for hematologic
malignancies. Expert Opin Investig Drugs. 22:723–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fandy TE, Herman JG, Kerns P, Jiemjit A,
Sugar EA, Choi SH, Yang AS, Aucott T, Dauses T, Odchimar-Reissig R,
et al: Early epigenetic changes and DNA damage do not predict
clinical response in an overlapping schedule of 5-azacytidine and
entinostat in patients with myeloid malignancies. Blood.
114:2764–2773. 2009. View Article : Google Scholar : PubMed/NCBI
|