Introduction
Breast cancer is the most common malignant cancer
and the major cause of mortality due to cancer in females worldwide
(1), and the morbidity has increased
gradually over recent years (2).
Although the tremendous progress in diagnostic instruments and the
development of standardized systematic therapy, including various
combinations of surgery, radiation therapy, chemotherapy and
hormone therapy (3), the prognosis
for breast cancer patients is still not ideal. The high mortality
rate is associated with the ability of breast cancer cells to
metastasize to distant organs (4).
Metastasis is a multistep process requiring cellular and
environmental progresses to a secondary site (5). However, the regulatory mechanisms remain
poorly understood. Therefore, the identification of critical
pathways will help to discover novel therapeutic targets for breast
cancer.
Increased evidence suggests that the lipogenic
phenotype is a major characteristic of cancer (6,7). Current
research links aberrant lipogenesis and cholesterogenesis with
breast cancer development and progression (8). Sterol regulatory element-binding
proteins (SREBPs) are important in regulating the gene expression
of key enzymes involved in fatty acid and cholesterol biosynthesis
(9,10), including SREBP-1a, SREBP-1c and
SREBP-2. Previous studies have demonstrated that the aberrant
expression of SREBP-1 is upregulated in several metabolic diseases,
including diabetes mellitus, morbid obesity, hyperlipidemia and
atherosclerosis (11). In addition to
regulation by sterols, SREBP-1 has been reported to be stabilized
and activated by the phosphatidylinositol-4,5-bisphosphate
3-kinase/Akt oncogenic signaling pathway in cancer (12–14). The
suppressed expression of SREBP-1 could inhibit cell growth,
migration and invasion, and induce cell apoptosis in ovarian cancer
(15). Pharmacological and genetic
inhibition or reduction of SREBP-1 significantly induced
glioblastoma cell death (16).
Overexpression of SREBP-1 has been observed in atypically
hyperplastic endometrium and endometrial cancer tissues, while
knockdown of SREBP-1 dramatically inhibited the proliferating
potential of endometrial cancer cells (17). The expression level of SREBP-1 is also
elevated in certain types of tumors such as prostate and gastric
cancer (18,19). However, the expression and function of
SREBP-1 in human breast cancer remains to be fully elucidated.
In the present study, the expression levels SREBP-1
in breast cancer tissues were evaluated. The data indicated that
the SREBP-1 level was correlated with prognosis of breast cancer,
and suggested that it may serve as an independent prognostic factor
in breast cancer. SREBP-1 promoted cell migration and invasion
in vitro. Taken together, the present results demonstrated
that SREBP-1 is pivotal for the tumorigenesis of breast cancer.
Materials and methods
Ethical review
The Ethics Committee of Nanjing Medical University
Affiliated Wuxi Second Hospital (Wuxi, Jiangsu, China) approved the
current protocols, according to the 1975 Declaration of Helsinki.
Informed consent form was signed by each patient.
Clinical tissue specimens and cell
lines
The 82 female patients enrolled in the present study
underwent curative surgery for breast cancer without radiation or
chemotherapy prior to surgical treatment at the Nanjing Medical
University Affiliated Wuxi Second Hospital, between January 2008
and December 2009. The mean age of the patients was 54.8 years
(standard error of the mean, 3.2; range, 29–73 years). Tissue
specimens were confirmed separately by two experienced pathologists
under double-blinded conditions, and none of the patients received
any therapy prior to operation. The demographic features and
clinicopathological data were reviewed in the patients' medical
records. The specimens were collected and immediately stored in 4%
paraformaldehyde for immunohistochemistry (IHC), or in liquid
nitrogen for western blotting. The prognosis concerning
disease-specific survival and overall survival, which were defined
as the time from surgery to first recurrence or mortality,
respectively, were analyzed. A total of 60 months of clinical
follow-up data were available.
The human breast cancer cell lines MCF7 and SKBR3
were cultured in complete Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), while the human breast cancer cell line MDA-MB-231 was
cultured in L15 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
containing 10% FBS, in a humidified 5% CO2 incubator at
37°C. The cell lines were obtained from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China).
IHC staining
Paraformaldehyde-fixed, paraffin-embedded tissue
sections were used for IHC with an anti-SREBP-1 (1:500; catalog no.
sc-8984; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibody,
according to the streptavidin-peroxidase method (15). The staining results for the SREBP-1
protein were evaluated by the staining intensity and the percentage
of positive cells. The staining intensity was scored as follows:
0=none; 1=weak; 2=moderate; and 3=strong. The percentage of
positive cells was scored as follows: 0, <5%; 1, 6–25%; 2,
26–50%; 3, 51–75%; and 4, >75%. A total of 10 independent,
high-magnification (×400) fields were observed to calculate a mean
score. A total score >1 was defined as positive staining.
Small interfering (si)RNA
transfection
siRNA specific against SREBP-1 and non-silencing
scrambled sequence siRNA (used as the negative control) were
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China).
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
used for transfection in accordance with the manufacturer's
protocol. Further experiments were performed after 48 h. The
specific SREBP-1 siRNA sequence was: Sense
5′-GGAAGAGUCAGUGCCACUGTT-3′ and anti-sense
5′-CAGUGGCACUGACUCUUCCTT-3′.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from breast cancer tissues
and cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA
concentration was quantified by a NanoDrop spectrophotometer
(NanoDrop Technologies; Thermo Fisher Scientific, Inc., Wilmington,
DE, USA). Complementary (c)DNA was synthesized using a first-strand
cDNA synthesis kit (GE Healthcare Life Sciences; Chalfont, UK).
cDNA (2 µl) was amplified and quantified by RT-qPCR using SYBR
Premix Ex Taq II (Takara Biotechnology Co., Ltd., Dalian, China)
with the following cycling conditions: 94°C for 3 min, followed by
35 cycles of 94°C for 30 sec and 60°C for 30 sec. The human
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was regarded
as the internal control. The primers used for PCR were synthesized
by Shanghai GenePharma Co., Ltd., and their sequences were as
follows: 5′-CAGTCCAGCCTTTGAGGATA-3′ (forward) and
5′-CAAAGGATTGCAGGTCAGAC-3′ (reverse) for SREBP-1, and
5′-CAAGCTCATTTCCTGGTATGAC-3′ (forward) and
5′-CAGTGAGGGTCTCTCTCTTCCT-3′ (reverse) for GAPDH. All samples were
normalized to GAPDH and calculated with the relative
2−ΔΔCq method (17).
Protein extraction and western
blotting
A modified radioimmunoprecipitation assay buffer
with protease inhibitor was used for protein isolation
(Sigma-Aldrich, St. Louis, MO, USA). Protein concentrations were
measured using the Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc., Rockford, IL, USA). Total proteins (50 µg) were
resolved on 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA), which were blocked
with 5% non-fat dry milk (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) in phosphate-buffered saline (PBS) with 0.1% Tween 20. Next,
the membranes were incubated with primary anti-SREBP-1 (1:300;
catalog no. sc-365513; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and anti-β-actin (1:5,000; catalog no. 3700; Cell Signaling
Technology, Inc.) antibodies at 4°C overnight, followed by
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
catalog no. bs12471; Bioworld Technology, Inc., St. Louis Park, MN,
USA) for 2 h at room temperature. Bands were detected with an
enhanced chemiluminescence reagent (EMD Millipore).
Wound healing assay
Cells (5×105 cells/well) were seeded into
a 6-well plate and incubated to a confluent monolayer. Scratch
wounds were created using a pipette tip, and washed twice with
sterile PBS. Cells were then cultured in serum-free medium in a
humidified 5% CO2 incubator at 37°C for 48 h, and
visualized under an inverted microscope.
Transwell assay
Transwell chambers (8 µM pore-sized; Nalge Nunc
International; Thermo Fisher Scientific, Inc.) were coated with
matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at 1 mg/ml on
the inner layer. Cells transfected with control siRNA or SREBP-1
siRNA were seeded in the upper chamber at a density of
1×105 cells/chamber in 100 µl serum-free medium. The
lower chamber was filled with 600 µl DMEM containing 10% FBS.
Plates were incubated for 24 h at 37°C, and cells in the top
surface of the matrigel membrane were swabbed carefully. The
adherent cells on the undersurface of the insert were stained with
0.3% crystal violet and counted under a light microscope. A total
of four fields were randomly selected to calculate the mean cell
number.
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS version 13 (SPSS, Inc., Chicago, IL, USA) was used
for Pearson χ2 test and multivariate Cox regression
analysis. Two-tailed Student's t test or Kaplan-Meier method
was used to calculate the survival, and log-rank test or analysis
of variance was used to analyze the difference in multivariate Cox
regression using GraphPad Prism 5 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased SREBP-1 protein expression
in breast cancer and its correlation with clinicopathological
features
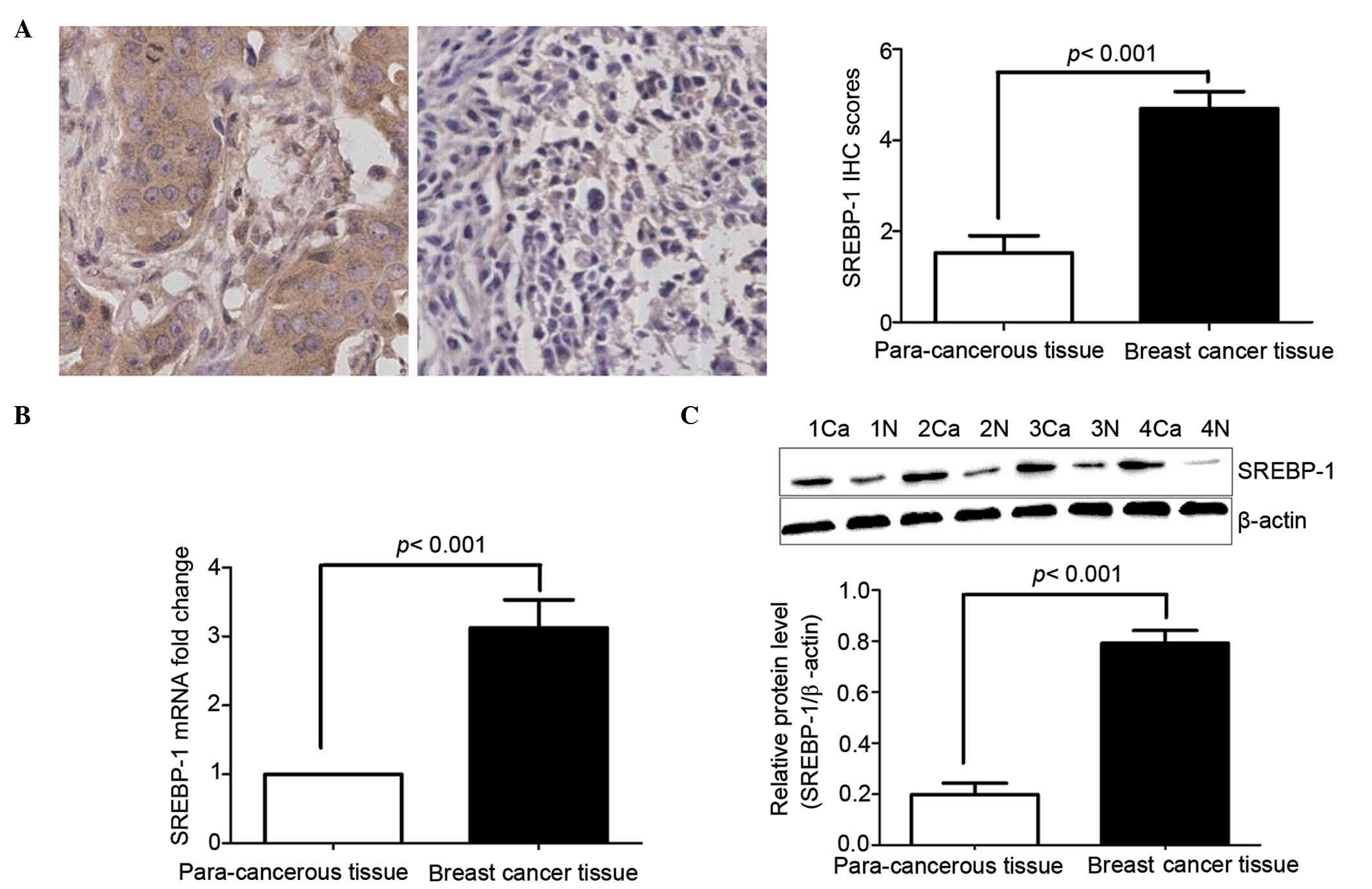
To evaluate the potential role of SREBP-1 in human
breast cancer, RT-qPCR, IHC staining and western blotting were
performed to investigate SREBP-1 messenger (m)RNA and protein
expression in tissue samples of 82 patients with primary breast
cancer, together with matched para-cancerous tissue samples.
Notably, the SREBP-1 mRNA and protein level was robustly increased
in the majority of the cancer samples compared with para-cancerous
tissues (P<0.01; Fig. 1A and B).
SREBP-1 was positively stained in both the nucleus and the
cytoplasm. Compared with malignant cells, SREBP-1 immunostaining in
benign cells was negative or relatively weak (Fig. 1A). According to the criteria of
semi-quantitative assessment employed, SREBP-1 was highly expressed
in 58 (70.7%) of 82 breast cancers and in 24 (29.3%) of 82
para-cancerous tissues. Statistical analysis of SREBP-1 staining
scores confirmed increased staining in malignant cells compared
with benign cells (P<0.01; Fig.
1A). Western blotting further confirmed the IHC results
(P<0.01; Fig. 1C).
Next, the association between the expression of
SREBP-1 protein and clinicopathological features was analyzed
(Table I). Pearson χ2 test
suggested that high SREBP-1 expression was strongly correlated with
tumor differentiation, tumor-node-metastasis (TNM) stage and lymph
node metastasis (P<0.01). However, there was no significant
association between SREBP-1 expression and other
clinicopathological variables. Taken together, these data reveal
that the expression of SREBP-1 in breast cancer is elevated, and
increased SREBP-1 expression is correlated with poor
clinicopathological features in breast cancer.
 | Table I.Correlation between relative SREBP-1
expression level and clinicopathological parameters in breast
cancer (n=82). |
Table I.
Correlation between relative SREBP-1
expression level and clinicopathological parameters in breast
cancer (n=82).
|
|
| SREBP-1
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases (n) | Positive (n=58) | Negative(n=24) | P-value |
|---|
| Age (years) |
|
|
| 0.071 |
|
<50 | 50 | 39 | 11 |
|
| ≥50 | 32 | 19 | 13 |
|
| Tumor size (cm) |
|
|
| 0.776 |
|
<2 | 39 | 27 | 12 |
|
| ≥2 | 43 | 31 | 12 |
|
| Tumor location |
|
|
| 0.953 |
| Left | 44 | 31 | 13 |
|
|
Right | 38 | 27 | 11 |
|
| Differentiation |
|
|
|
<0.001a |
|
Moderate/high | 34 | 11 | 23 |
|
| Poor | 48 | 47 | 1 |
|
| T stage |
|
|
| 0.806 |
| I/II | 67 | 47 | 20 |
|
|
III/IV | 15 | 11 | 4 |
|
| Lymph node
metastasis |
|
|
|
<0.001a |
|
Negative | 29 | 6 | 23 |
|
|
Positive | 53 | 52 | 1 |
|
| TNM stage |
|
|
| 0.044a |
|
I/II | 55 | 35 | 20 |
|
|
III/IV | 27 | 23 | 4 |
|
| ER status |
|
|
| 0.585 |
|
Negative | 44 | 30 | 14 |
|
|
Positive | 38 | 28 | 10 |
|
| HER2 status |
|
|
| 0.161 |
|
Negative | 38 | 24 | 14 |
|
|
Positive | 44 | 34 | 10 |
|
| PR status |
|
|
| 0.866 |
|
Negative | 49 | 35 | 14 |
|
|
Positive | 33 | 23 | 10 |
|
High SREBP-1 expression correlates
with a poor 5-year survival for breast cancer patients
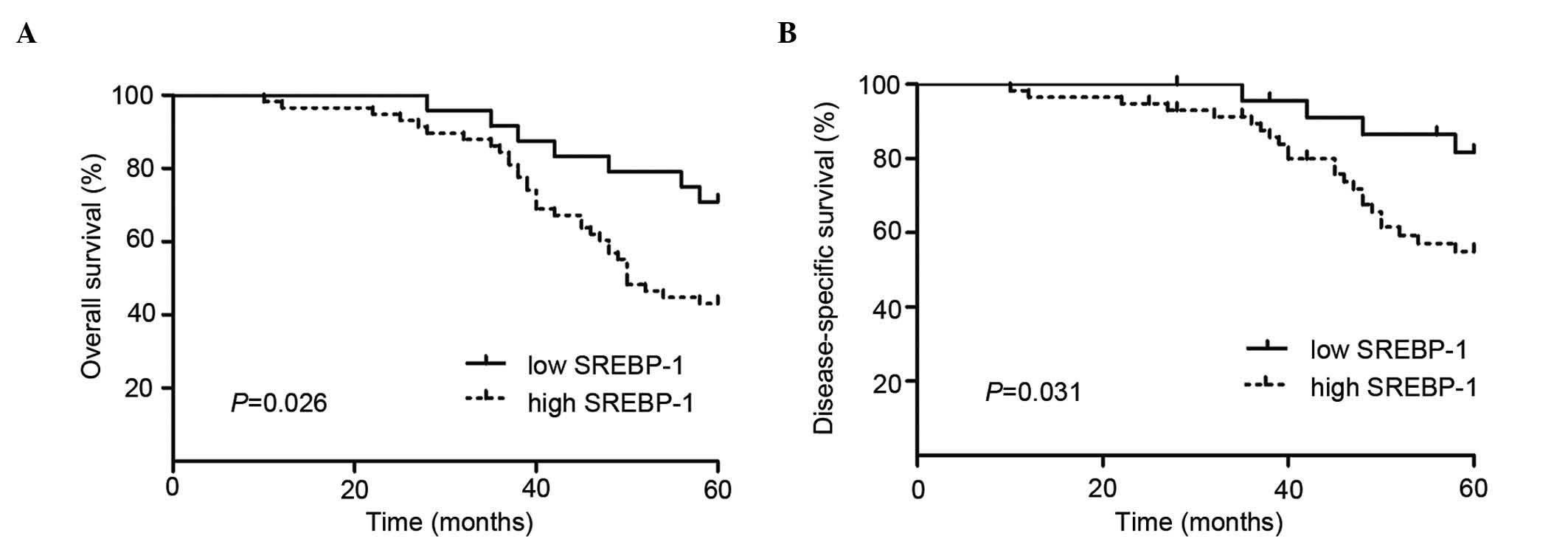
To determine the role of SREBP-1 in predicting the
prognosis of patients, Kaplan-Meier survival curves were
constructed using 5-year overall and disease-specific survival to
analyze cases with high and low SREBP-1 expression (n=82; follow-up
time, 60 months). The results indicate that high SREBP-1 staining
predicts a poor overall and disease-specific patient survival
(P<0.01, log-rank test; Fig. 2A and
B).
In addition, SREBP-1 was an independent prognostic
marker for both 5-year overall survival [hazard ratio, 2.976; 95%
confidence interval (CI), 1.109–7.987; P=0.030; Table II) and disease-specific survival
(hazard ratio, 2.327; 95% CI, 1.093–4.955; P=0.029; Table II) according to multivariate Cox
regression analysis. These results definitely confirmed that high
SREBP-1 expression is associated with poor prognosis, suggesting
that SREBP-1 may function as a prognostic marker for breast
cancer.
 | Table II.Multivariate Cox regression analysis
on 5-year overall and disease-specific survival of 82 breast cancer
patients. |
Table II.
Multivariate Cox regression analysis
on 5-year overall and disease-specific survival of 82 breast cancer
patients.
|
| Overall
survival | Disease-specific
survival |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| SREBP-1 | 2.976 | 1.109–7.987 | 0.030 | 2.327 | 1.093–4.955 | 0.029 |
| Age | 0.993 | 0.741–1.352 | 0.836 | 0.854 | 0.651–1.223 | 0.885 |
| Tumor size | 2.731 | 1.945–3.786 | 0.003 | 2.912 | 1.883–4.147 | 0.001 |
| Lymph node
metastasis | 3.183 | 1.991–4.505 | 0.001 | 3.962 | 2.975–4.756 | <0.001 |
| Histology
grade | 1.842 | 1.185–2.846 | 0.023 | 1.954 | 1.512–2.513 | 0.032 |
SREBP-1 knockdown is established in
breast cancer cell lines
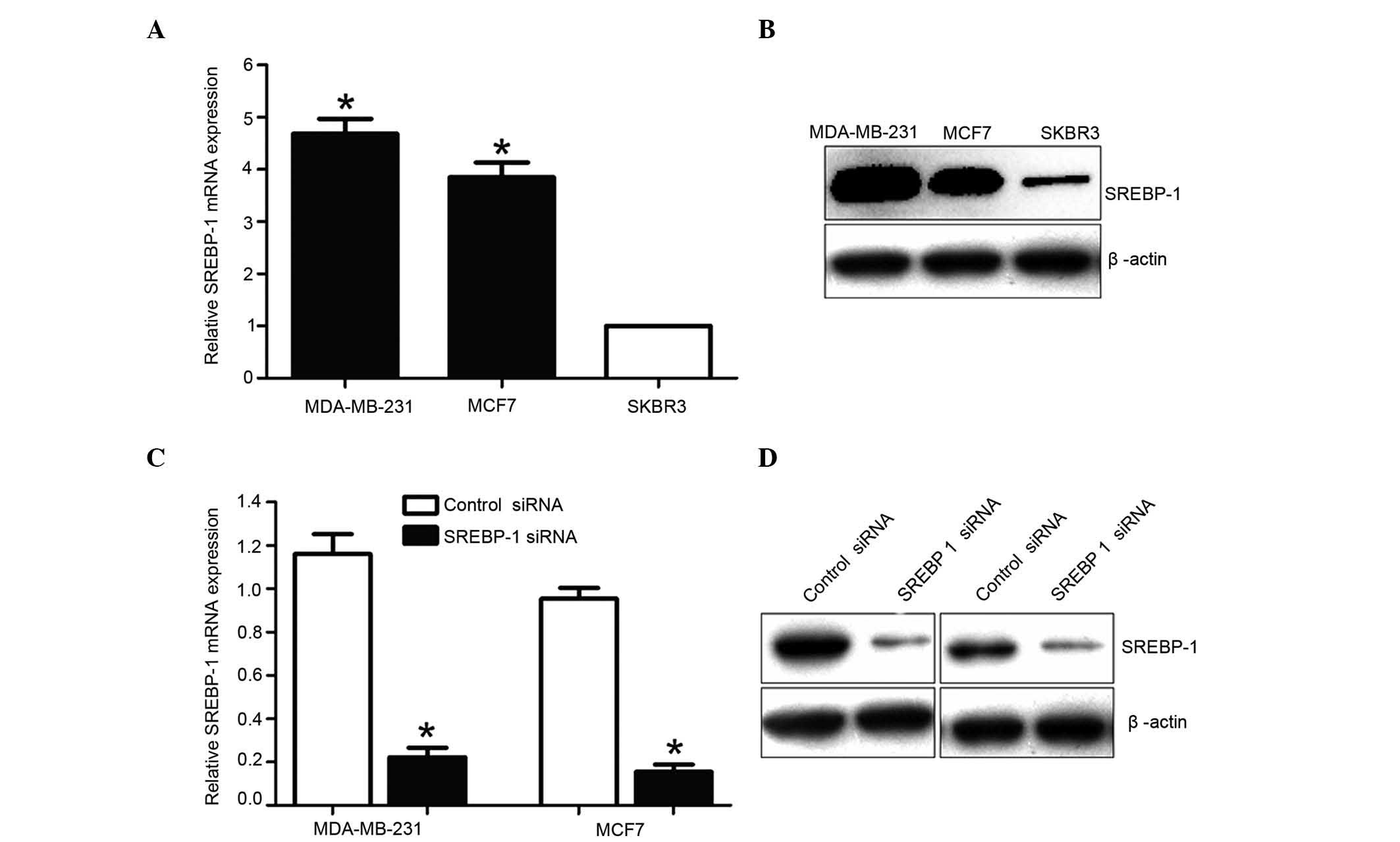
In order to identify the function of SREBP-1, the
SREBP-1 mRNA and protein levels were analyzed in three different
breast cancer cell lines. Consistent with the clinical results, the
expression levels of SREBP-1 were significantly higher in MCF7 and
MDA-MB-231 cells than in SKBR3 cells (Fig. 3A and B). Based on such difference in
SREBP-1 expression, MCF7 and MDA-MB-231 cells were selected, and
SREBP-1 was knocked down by transfecting specific SREBP-1 siRNA
into these cells. The RT-qPCR and western blotting results
demonstrated that SREBP-1 was markedly downregulated by SREBP-1
siRNA in these cell lines (Fig. 3C and
D).
Effect of SREBP-1 knockdown on the
invasion and migration of breast cancer cells
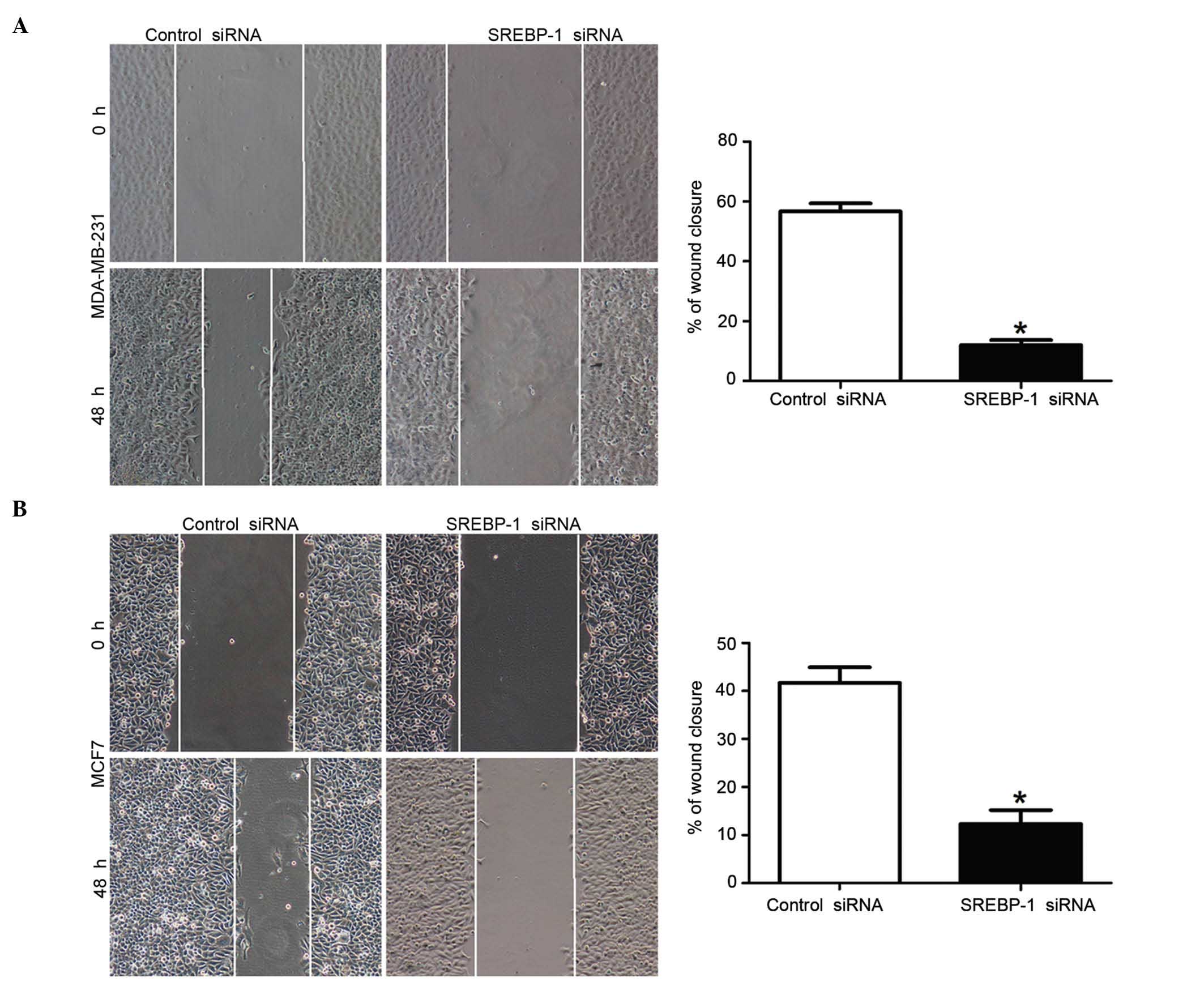
To elucidate effect of SREBP-1 knockdown on breast
cancer cells migration and invasion, mechanical scrape wound
healing and transwell assays were performed. The wound healing
assay revealed that the loss of SREBP-1 expression could
significantly reduce the migration rate in both MDA-MB-231 and MCF7
breast cancer cells (P<0.01; Fig. 4A
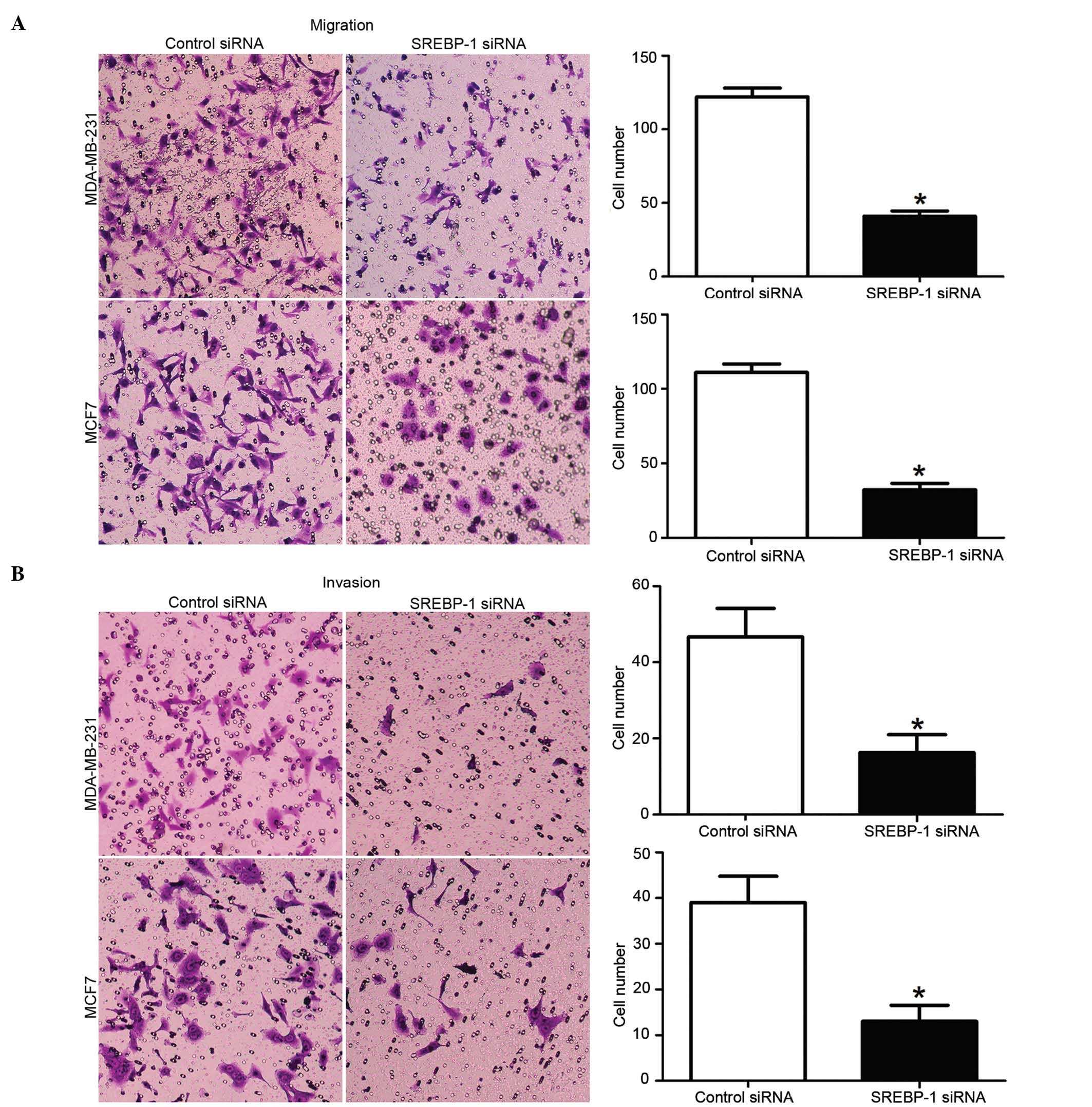
and B, respectively). Consistently, the transwell assay also
confirmed that the cell migration rate was significantly decreased
by SREBP-1 knockdown in these cells (P<0.01; Fig. 5A). Furthermore, compared with those in
the control groups, the number of invaded cells in the SREBP-1
siRNA group was significantly reduced (P<0.01; Fig. 5B). These data, therefore, indicate
that SREBP-1 has an impact on the migration and invasion of breast
cancer cells.
Discussion
Numerous types of cancer exhibit increased de
novo lipogenesis irrespective of the extracellular lipid
availability (20). Exacerbated
lipogenesis has been demonstrated to be one of main characteristics
of cancer (19). The maintenance of
intracellular lipid homeostasis depends on the balance between
lipid biosynthesis and degradation (21). The upregulated lipogenesis in tumor
cells is reflected by a marked increase in lipogenic enzymes, which
is partly due to the transcriptional activation mediated by SREBP-1
in cancer cells (6,21). Consistent with the fundamental role of
SREBP-1 in regulating multiple types of cancer, elevated SREBP-1
expression had a great impact on cancer progression and metastasis
(22,23). However, the impact of SREBP-1 on the
progression of breast cancer remains largely unknown, and the
underlying mechanisms require to be elucidated.
The present study demonstrated that both the mRNA
and protein levels of SREBP-1 were significantly overexpressed in
breast cancer tissues compared with para-cancer tissues. In
addition, upregulated SREBP-1 expression was highly correlated with
tumor differentiation, TNM stage and lymph node metastasis.
Kaplan-Meier analysis revealed that breast cancer patients with
SREBP-1 positive expression had a poorer survival following surgery
than those without SREBP-1 expression. These findings are
consistent with previous data reporting that the level of SREBP-1
is associated with the tumorigenesis and prognosis of cancer
(24). Additionally, the Cox
proportional hazards model employed in the present study revealed
that SREBP-1 was an independent factor for predicting the 5-year
survival of patients. Therefore, these results demonstrate that
SREBP-1 is a critical molecule for prognosis determination in
breast cancer.
To investigate the pathological function of SREBP-1
in breast cancer, several biophysiological experiments were further
conducted in SREBP-1-silenced cell lines, which were established by
being transfected with specific siRNA targeting SREBP-1. The
results revealed that SREBP-1 knockdown in breast cancer cells
restrained cell migration and invasion, which are the cytological
fundament of tumor metastasis. Previous studies have reported that
the downregulation of SREBP-1 induces a decrease in the expression
of several enzymes in the fatty acids signaling pathways, including
acetyl-CoA carboxylase, fatty-acid synthase and stearoyl-CoA
desaturase 1, which are involved in lipid metabolism, lipogenesis,
proliferation, apoptosis and survival in breast cancer (25,26).
Through approaches of either siRNA or small molecule inhibitors,
depletion of the expression and activity of these genes suppressed
tumor cell proliferation and growth (27,28).
Therefore, SREBP-1 may be important in tumorigenesis via these
molecular signaling pathways.
In conclusion, the present study found that SREBP-1
is upregulated in breast cancer tissues and cells, and that its
elevated expression is associated with poor prognostic features.
The in vitro studies indicated that SREBP-1-knockdown
inhibits breast cancer cell migration and invasion. Therefore,
SREBP-1 has the potential to be a valuable diagnostic and
prognostic biomarker for breast cancer.
Acknowledgements
The present study was supported by Nanjing Medical
University Affiliated Wuxi Second Hospital (Wuxi, China; grant no.
2014K-12).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giordano SH, Buzdar AU, Smith TL, Kau SW,
Yang Y and Hortobagyi GN: Is breast cancer survival improving?
Cancer. 100:44–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurvitz SA, Hu Y, O'Brien N and Finn RS:
Current approaches and future directions in the treatment of
HER2-positive breast cancer. Cancer Treat Rev. 39:219–229. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almendro V, Kim HJ, Cheng YK, Gönen M,
Itzkovitz S, Argani P, van Oudenaarden A, Sukumar S, Michor F and
Polyak K: Genetic and phenotypic diversity in breast tumor
metastases. Cancer Res. 74:1338–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J and Gallo KA: MLK3 regulates
paxillin phosphorylation in chemokine-mediated breast cancer cell
migration and invasion to drive metastasis. Cancer Res.
72:4130–4140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nature
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuhajda FP: Fatty-acid synthase and human
cancer: New perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puig T, Vázquez-Martin A, Relat J, Pétriz
J, Menéndez JA, Porta R, Casals G, Marrero PF, Haro D, Brunet J and
Colomer R: Fatty acid metabolism in breast cancer cells:
Differential inhibitory effects of epigallocatechin gallate (EGCG)
and C75. Breast Cancer Res Treat. 109:471–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakakuki M, Shimano H, Inoue N, Tamura M,
Matsuzaka T, Nakagawa Y, Yahagi N, Toyoshima H, Sato R and Yamada
N: A transcription factor of lipid synthesis, sterol regulatory
element-binding protein (SREBP)-1a causes G(1) cell-cycle arrest
after accumulation of cyclin-dependent kinase (cdk) inhibitors.
FEBS J. 274:4440–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taskinen MR: Diabetic dyslipidaemia: From
basic research to clinical practice. Diabetologia. 46:733–749.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruiz R, Jideonwo V, Ahn M, Surendran S,
Tagliabracci VS, Hou Y, Gamble A, Kerner J, Irimia-Dominguez JM,
Puchowicz MA, et al: Sterol regulatory element-binding protein-1
(SREBP-1) is required to regulate glycogen synthesis and
gluconeogenic gene expression in mouse liver. J Biol Chem.
289:5510–5517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo D, Prins RM, Dang J, Kuga D, Iwanami
A, Soto H, Lin KY, Huang TT, Akhavan D, Hock MB, et al: EGFR
signaling through an Akt-SREBP-1-dependent, rapamycin-resistant
pathway sensitizes glioblastomas to antilipogenic therapy. Sci
Signal. 2:ra822009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Porstmann T, Griffiths B, Chung YL,
Delpuech O, Griffiths JR, Downward J and Schulze A: PKB/Akt induces
transcription of enzymes involved in cholesterol and fatty acid
biosynthesis via activation of SREBP. Oncogene. 24:6465–6481.
2005.PubMed/NCBI
|
|
14
|
Yecies JL, Zhang HH, Menon S, Liu S,
Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS,
Lee CH and Manning BD: Akt stimulates hepatic SREBP1c and
lipogenesis through parallel mTORC1-dependent and independent
pathways. Cell Metab. 14:21–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie LY, Lu QT, Li WH, Yang N, Dongol S,
Zhang X and Jiang J: Sterol regulatory element-binding protein 1 is
required for ovarian tumor growth. Oncology Rep. 30:1346–1354.
2013.
|
|
16
|
Guo D, Bell EH and Chakravarti A: Lipid
metabolism emerges as a promising target for malignant glioma
therapy. CNS Oncol. 2:289–299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li W, Tai Y, Zhou J, Gu W, Bai Z, Zhou T,
Zhong Z, McCue PA, Sang N, Ji JY, et al: Repression of endometrial
tumor growth by targeting SREBP1 and lipogenesis. Cell Cycle.
11:2348–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang WC, Li X, Liu J, Lin J and Chung LW:
Activation of androgen receptor, lipogenesis, and oxidative stress
converged by SREBP-1 is responsible for regulating growth and
progression of prostate cancer cells. Mol Cancer Res. 10:133–142.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyachi K, Sawada Y, Shida Y, Sugawara A
and Hisatomi H: Lipogenic gene expression profile in patients with
gastric cancer. Mol Clin Oncol. 1:825–827. 2013.PubMed/NCBI
|
|
20
|
Mashima T, Seimiya H and Tsuruo T: De novo
fatty-acid synthesis and related pathways as molecular targets for
cancer therapy. Br J Cancer. 100:1369–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ameer F, Scandiuzzi L, Hasnain S,
Kalbacher H and Zaidi N: De novo lipogenesis in health and disease.
Metabolism. 63:895–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calvisi DF, Wang C, Ho C, Ladu S, Lee SA,
Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al:
Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling,
promotes development of human hepatocellular carcinoma.
Gastroenterology. 140:1071–1083. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamashita T, Honda M, Takatori H, Nishino
R, Minato H, Takamura H, Ohta T and Kaneko S: Activation of
lipogenic pathway correlates with cell proliferation and poor
prognosis in hepatocellular carcinoma. J Hepatol. 50:100–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swinnen JV, Heemers H, van de Sande T, de
Schrijver E, Brusselmans K, Heyns W and Verhoeven G: Androgens,
lipogenesis and prostate cancer. J Steroid Biochem Mol Biol.
92:273–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song HJ, Sneddon AA, Heys SD and Wahle KW:
Regulation of fatty acid synthase (FAS) and apoptosis in
estrogen-receptor positive and negative breast cancer cells by
conjugated linoleic acids. Prostaglandins Leukot Essent Fatty
Acids. 87:197–203. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee JS, Sul JY, Park JB, Lee MS, Cha EY,
Song IS, Kim JR and Chang ES: Fatty acid synthase inhibition by
amentoflavone suppresses HER2/neu (erbB2) oncogene in SKBR3 human
breast cancer cells. Phytother Res. 27:713–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roongta UV, Pabalan JG, Wang X, Ryseck RP,
Fargnoli J, Henley BJ, Yang WP, Zhu J, Madireddi MT, Lawrence RM,
et al: Cancer cell dependence on unsaturated fatty acids implicates
stearoyl-CoA desaturase as a target for cancer therapy. Mol Cancer
Res. 9:1551–1561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tong L and Harwood HJ Jr: Acetyl-coenzyme
A carboxylases: Versatile targets for drug discovery. J Cell
Biochem. 99:1476–1488. 2006. View Article : Google Scholar : PubMed/NCBI
|