Introduction
Multiple myeloma (MM) is a fatal hematological
cancer, which is characterized by clonal plasma cell proliferation
in the bone marrow, presence of osteolytic bone destruction causing
severe bone pain, pathological fractures and hypercalcaemia
(1). The global incidence of MM has
increased continuously in the last decade (2). Despite overall survival rates improving
significantly with recent therapeutic advancements, including
autologous stem cell transplantation, proteasome inhibitors and
immunomodulatory drugs, MM remains an incurable disease with a
median survival time of 4–5 years in adults, due to its resistance
to chemotherapeutic drugs (3,4). Therefore, the development of novel
alternative approaches to overcome drug resistance and improve
outcomes in MM is urgently required.
Flavonoids are widely distributed in plants and
possess a wide range of biological and pharmacological activities,
including anti-allergic, anti-inflammatory, antioxidant,
anti-microbial and anti-diarrheal activities (5,6). Previous
studies in vitro and in vivo have revealed that
flavonoids have anti-cancer effects in various types of cancer,
including human breast, lung and colorectal cancer, hepatocellular
carcinoma, osteosarcoma and glioma, by inducing apoptosis,
increasing chemotherapy sensitivity and suppressing metastasis
(7–9).
Apoptosis is the major mechanism for cancer cell
elimination. Previous studies have demonstrated that flavonoids
trigger apoptosis in numerous types of human cancer cells through
endoplasmic reticulum (ER) stress-dependent apoptotic pathways and
mitochondrial-mediated apoptotic pathways (10–12). A
recent study revealed that
7-{4-[Bis-(2-hydroxyethyl)-amino]-butoxy}
−5-hydroxy-8-methoxy-2-phenylchromen-4-one (V8), a novel flavonoid
that is synthesized from the natural product wogonin in two steps
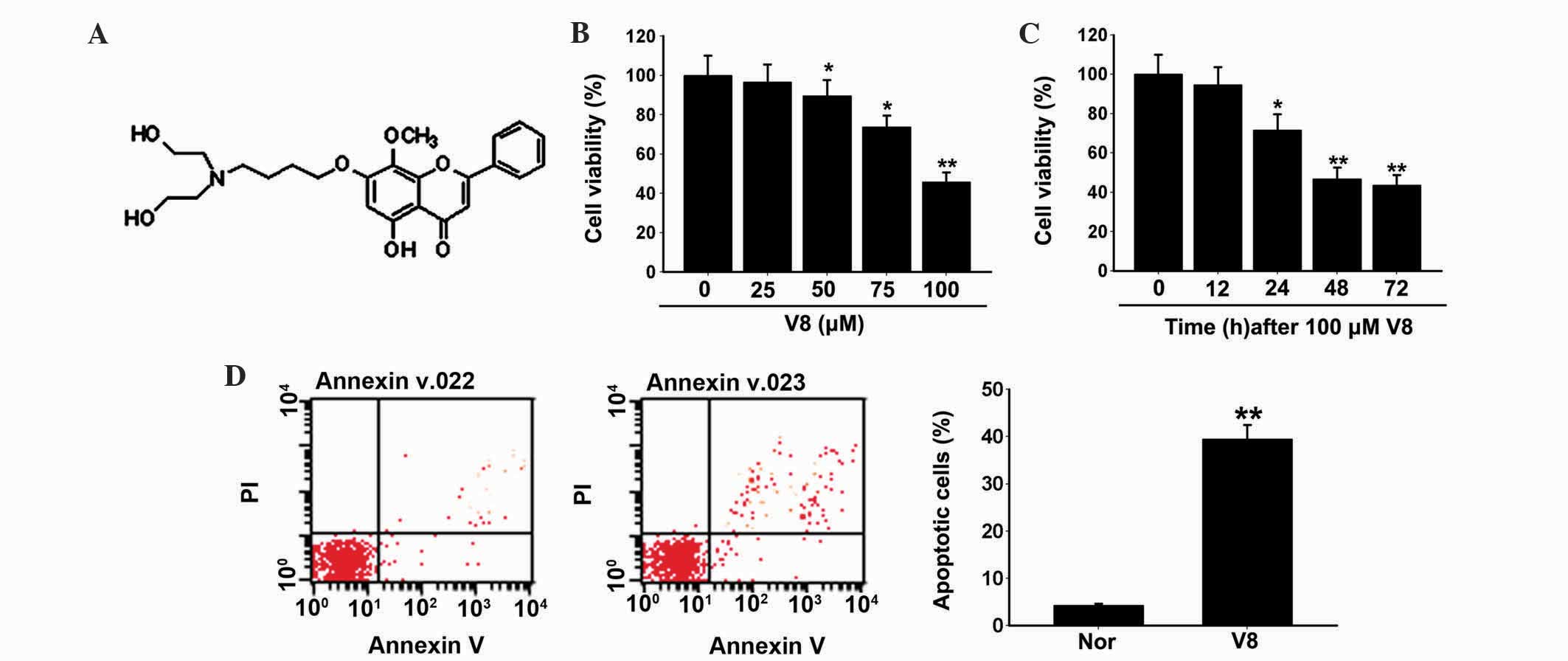
(Fig. 1A), induces apoptosis in
hepatocellular carcinoma cells through the reactive oxygen
species-mediated ER stress pathway (13). However, to the best of our knowledge,
no study has systematically investigated the cytotoxic effects and
mechanisms of V8 on MM cells. Therefore, the present study
investigated the cytotoxic potential of V8, and the mechanism by
which it acts, on MM cells by examining cell viability and
apoptosis signals in human MM RPMI 8226 cells. The present study
provides detailed information concerning the cytotoxic effects of
V8 on MM cells and offers a basic foundation for the clarification
of its toxicity mechanisms.
Materials and methods
Reagents and antibodies
V8 [purity, >99.5%; obtained from Aiqing He,
Nantong University, Jiangsu, China (14)] was diluted in dimethyl sulfoxide
(DMSO) to 0.1 M and stored at −20°C. Cell counting kit-8 (CCK-8;
catalog no. CK04-3000T) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). FITC Annexin V Apoptosis
Detection kit (catalog no. 556570) was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Mouse monoclonal β-tubulin
(catalog no. sc-5274; 1:1,000 dilution) and mouse monoclonal
β-actin (catalog no. sc-47778; 1:1,000 dilution) primary antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA). Mouse monoclonal cytochrome c oxidase subunit IV
(COX4; catalog no. 4844; 1:1,000 dilution), rabbit monoclonal
cleaved caspase-12 (catalog no. 2202; 1:500 dilution), rabbit
monoclonal phosphorylated protein kinase RNA-like endoplasmic
reticulum kinase (p-PERK; catalog no. 4370S; 1:1,000 dilution),
rabbit monoclonal phosphorylated eukaryotic initiation factor 2α
(p-eIF2α; catalog no. 3597; 1:1,000 dilution) and rabbit monoclonal
activating transcription factor 4 (ATF4; catalog no. 11815; 1:1,000
dilution) antibodies were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Mouse monoclonal cytochrome c (cyto
c; catalog no. 13560; 1:800 dilution) antibody was purchased
from Santa Cruz Biotechnology, Inc. Goat anti-mouse IgG-conjugated
horseradish peroxidase (catalog no. sc-2004; 1:20,000 dilution) and
CY3-conjugated (catalog no. sc-2020; 1:5,000 dilution) secondary
antibodies were obtained from Santa Cruz Biotechnology, Inc.
Cell culture and cell viability
Human MM RPMI 8226 cell line was purchased from the
Chinese Academy of Sciences (Beijing, China) and maintained in
RPMI-1640 (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco®; Thermo
Fisher Scientific, Inc.), 2 mM glutamine and 100 U/ml
penicillin-streptomycin (Sigma-Aldrich) at 37°C in 5%
CO2. Following treatment with various concentrations of
V8 (0, 25, 50, 75 and 100 µM), cell viability was measured by
CCK-8. The absorbance was measured at 450 nm using a microplate
reader (Bio-Tek Instruments, Inc., Winooski, VT, USA).
Western blotting
Following treatment with various concentrations of
V8 (0, 25, 50, 75 and 100 µM), RPMI 8226 cells were harvested and
washed with ice-cold phosphate-buffered saline (PBS). Cell lysates
were prepared using radioimmunoprecipitation assay (RIPA) buffer
(Cell Signaling Technology, Inc.). The samples were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a
Mini Protean system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
on a 10% gel and transferred to a nitrocellulose membrane (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% skimmed
milk at room temperature for 2.5 h, the membranes were incubated
with primary antibodies against cleaved caspase-12, cyto c,
COX4, β-actin and β-tubulin at 4°C overnight. The samples were
visualized using goat anti-mouse IgG-conjugated horseradish
peroxidase antibody at room temperature for 2–3 h, with enhancement
from Pierce™ ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). Image J (1.49v; National Institutes of Health,
Bethesda, MD, USA) was used for western blotting analysis.
Flow cytometric analysis of
apoptosis
RPMI 8226 cells were cultured in 6-well plates for
48 h in the presence of 100 µM V8. The cells were washed twice with
cold PBS and then resuspended in 1X Binding Buffer at a
concentration of 1×106 cells/ml. In total, 100 µl of the
solution (1×105 cells) was transferred to a 5 ml culture
tube and 5 µl FITC Annexin V and 5 µl propidum iodide (PI) were
added. The cells were gently agitated and incubated for 15 min at
room temperature (25°C) in the dark. Following incubation, the
stained cells were diluted by addition of 400 µl 1X Binding Buffer.
Fluorescence was detected using FACSCalibur™ Flow Cytometer (BD
Biosciences) within 1 h and analyzed by FlowJo flow cytometric data
analysis software (FlowJo7.6, LLC, Ashland, OR, USA). PI and FITC
Annexin V positively stained cells were considered to be
apoptotic.
RNA isolation and quantification of
transcript levels
RNA was isolated from cells treated with 0, 25, 50,
75 and 100 µM V8 using TRIzol reagent (Invitrogen™; Thermo Fisher
Scientific, Inc.). Total RNA was converted to cDNA for quantitative
polymerase chain reaction (qPCR) using a High Capacity cDNA Reverse
Transcription kit (Applied Biosystems™; Thermo Fisher Scientific,
Inc.), according to the manufacturers protocol. DNase I (catalog
no. 18047019; 1:800 dilution; Invitrogen; Thermo Fisher Scientific,
Inc.) was used. qPCR was performed to detect the expression of
B-cell lymphoma 2 (Bcl-2), Bcl-2-like protein 4 (Bax), BH3
interacting domain death agonist (Bid), B-cell lymphoma-extra large
(Bcl-XL), glucose-regulated protein (GRP) 78, GRP94 and C/EBP
homologous protein (CHOP) using the SYBR Green PCR MasterMix and an
ABI 7500 Real-time PCR System (Applied Biosystems™; Thermo Fisher
Scientific, Inc.). The primers used in the qPCR were synthesized by
Sangon Biotech (Shanghai, China). The cycling conditions were as
follows: Stage 1, hold at 95°C for 120 sec; stage 2, 95°C for 15
sec and 60°C for 35 sec, for 40 cycles; and stage 3, dissociation.
Relative mRNA expression was determined by the 2−ΔΔCq
method (15) vs. glyceraldehyde
3-phosphate dehydrogenase. Sequences for PCR primers are listed in
Table I. The experiments were
repeated three times.
 | Table I.Primer sequences used in quantitative
polymerase chain reaction. |
Table I.
Primer sequences used in quantitative
polymerase chain reaction.
|
| Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| Bcl-2-like protein
4 |
TTTTGCTTCAGGGTTTCATC |
GACACTCGCTCAGCTTCTTG |
| BH3 interacting
domain death agonist |
GGTCAACAACGGTTCCAG |
CATCGTAGCCCTCCCACT |
| Bcl-2 |
CTGGGAGAACAGGGTACGATAA |
GGCTGGGAGGAGAAGATGC |
| Bcl-extra
large |
TGTGCGTGGAAAGCGTAG |
AGTGAGCCCAGCAGAACC |
| GRP78 |
CTCTGCCTCACCTCGCTCCA |
TCGCAATAGCAATGCCAATC |
| GRP94 |
CCACCTTCATCATCTACC |
ATGAGCCCTAACAGCAC |
| C/EBP homologous
protein |
ACCAGGAAACGGAAACAG |
TCACCATTCGGTCAATCA |
| Glyceraldehyde
3-phosphate dehydrogenase |
AAGGTCATCCCTGAGCTGAA |
TGCTGTAGCCAAATTCGTTG |
Caspase-3, −8 and −9 activity
assay
Activation of caspase-3, −8 and −9 was measured
using the Fluorometric Assay kit (Calbiochem®; EMD
Millipore), according to the manufacturer's protocol. In brief,
RPMI 8226 cells were treated with various concentrations of V8 (0,
25, 50, 75 and 100 µM) for 24 h. The treated cells were harvested
and washed with ice-cold PBS. Cell lysates were obtained by adding
100 µl RIPA buffer (Cell Signaling Technology, Inc.) for
1×105 cells. Subsequently, caspase inhibitors
(caspase-3, DEVD-CHO; caspase-8, z-IETD-FMK; caspase-9, z-LEHD-FMK)
were added to the cell lysates and incubated for 30 min. Reaction
buffer and fluorogenic peptide substrates (10 µl) (caspase-3,
Ac-DEVD-A MC; caspase-8, Ac-IETD-A MC; caspase-9, Ac-LEHD-A MC)
were added to the cell lysates, and incubated at 37°C in the dark
for 2 h. Lysate from RPMI 8226 cells treated with DMSO was used as
a control group. The activation of caspases in V8-treated RPMI 8226
cells was measured using the Infinite® 200 PRO
microplate reader (Tecan Group Ltd, Männedorf, Switzerland) at a
wavelength of 405 nm.
Preparation of cytosolic extracts and
mitochondria isolation
Mitochondria Isolation Kit for Cultured Cells
(Thermo Fisher Scientific, Inc.) was used for cyto c
analysis, according to the manufacturer's protocol. Briefly, RPMI
8226 cells (2×107) treated with 0, 25, 50, 75 and 100 µM
V8 were washed with ice-cold PBS and resuspended in 800 µl reagent
A. Following incubation on ice for 2 min, 10 µl reagent B was added
and incubated on the ice for 5 min and vortexed every minute.
Subsequently, 800 µl reagent C was added and centrifuged in a
microcentrifuge at 700 × g for 10 min at 4°C to collect the
supernatant. The supernatant was transferred to a new tube and
subjected to centrifugation at 12,000 × g for 15 min at 4°C. The
supernatant was collected as cytosolic extracts and the
mitochondria were washed with reagent C and centrifuged at 12,000 ×
g for 10 min at 4°C for the analysis of cyto c. Distribution
of cyto c in cytosolic extracts and isolated mitochondria
was determined by western blotting and normalized to β-tubulin and
COX4, respectively.
Immunofluorescent staining
RPMI 8226 cells were plated onto coverslips the day
prior to treatment with V8. Following exposure to 75 µM V8 for 48
h, the cells were fixed with 4% paraformaldehyde for 30 min,
permeabilized with PBS with Tween 20 (0.5%) for 10 min and
incubated with primary cleaved caspase-12 antibodies at 4°C
overnight, followed by CY3-conjugated secondary antibody staining
at room temperature for 2 h. Following washing, the cells were
stained with 4′,6-diamidino-2-phenylindole (1 mmol/l;
Sigma-Aldrich) for 5 min. The cells were observed using a
fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean from 3 or 4 independent experiments and analyzed using
Student's t-test. Statistical analysis was performed using
SigmaPlot software version 10.0 (Systat Software, Inc., San Jose,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
V8 induces apoptosis of RPMI 8226
cells
To investigate the effect of V8 on human MM cell
growth, RPMI 8226 cells were treated with various concentrations of
V8 (0, 25, 50, 75 and 100 µM) and cell viability and apoptosis were
evaluated. Inhibition of cell viability was observed following
treatment with V8 in a dose- and time-dependent manner (Fig. 1B and C; P<0.05). Following a 48 h
exposure to V8 at 100 µM, RPMI 8226 cells exhibited typical
apoptotic alterations, including cell shrinkage and loss of normal
nuclear architecture (date not shown). To elucidate these
observations more definitively, FITC Annexin-V/PI staining was
performed. The percentage of Annexin-V labeled apoptotic cells was
significantly upregulated following treatment with V8 compared with
the control (Fig. 1D; P<0.05).
Caspase pathway was activated in RPMI
8226 cells following treatment with V8
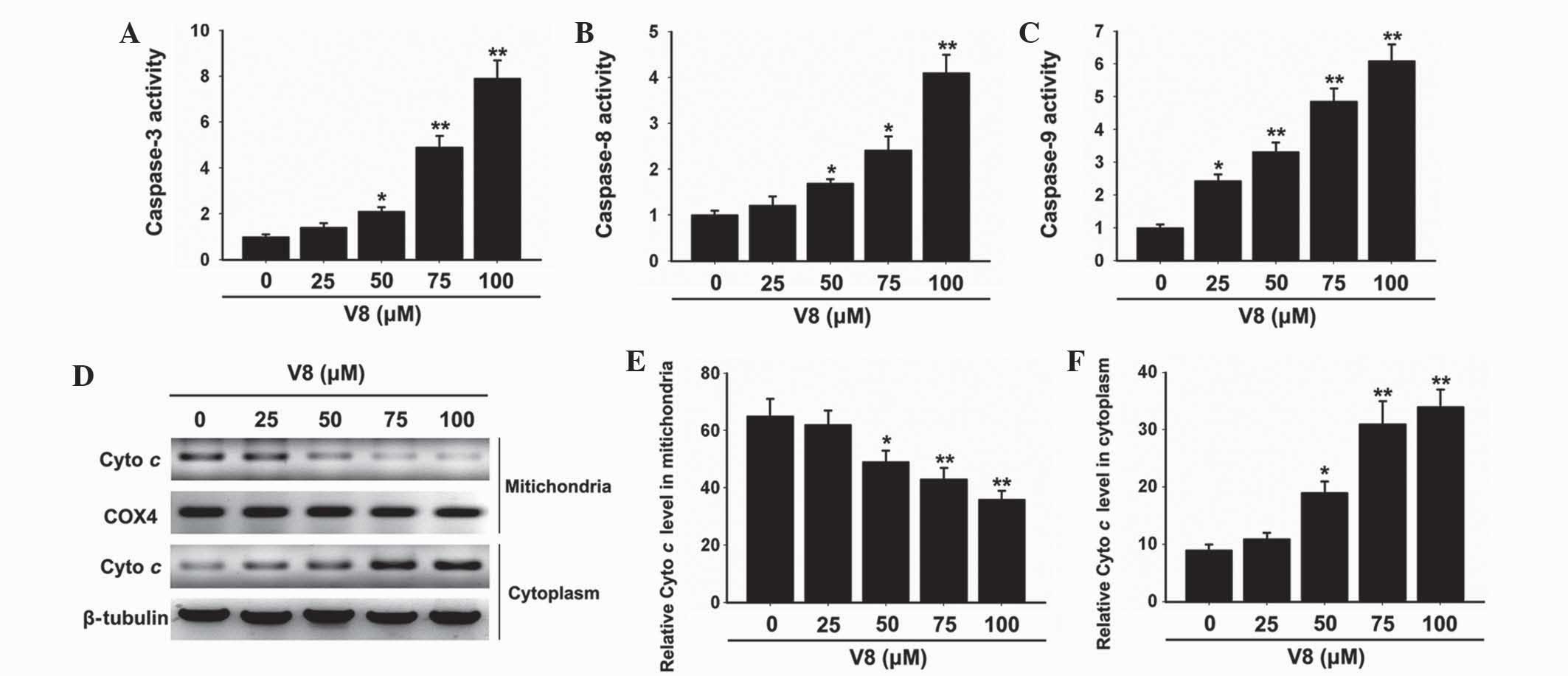
To determine the possible mechanism of action in
which V8 induces apoptosis of RPMI 8226 cells, alterations in the
expression of critical apoptosis-associated factors were evaluated.
Caspase protease activation was assessed to determine the
involvement in the cell death response. Notably, the activity of
caspase-3, −8 and −9 was clearly elevated in cells treated with V8
(Fig. 2A-C; P<0.05). Subsequently,
the effects of V8 on the release of mitochondrial cyto c
into the cytosol of cells was evaluated. Western blot analysis
revealed that the level of cyto c was decreased in
mitochondria and increased in the cytoplasm with increasing
concentrations of V8, indicating that V8 promotes the release of
mitochondrial cyto c into the cytosol (Fig. 2D-F; P<0.05).
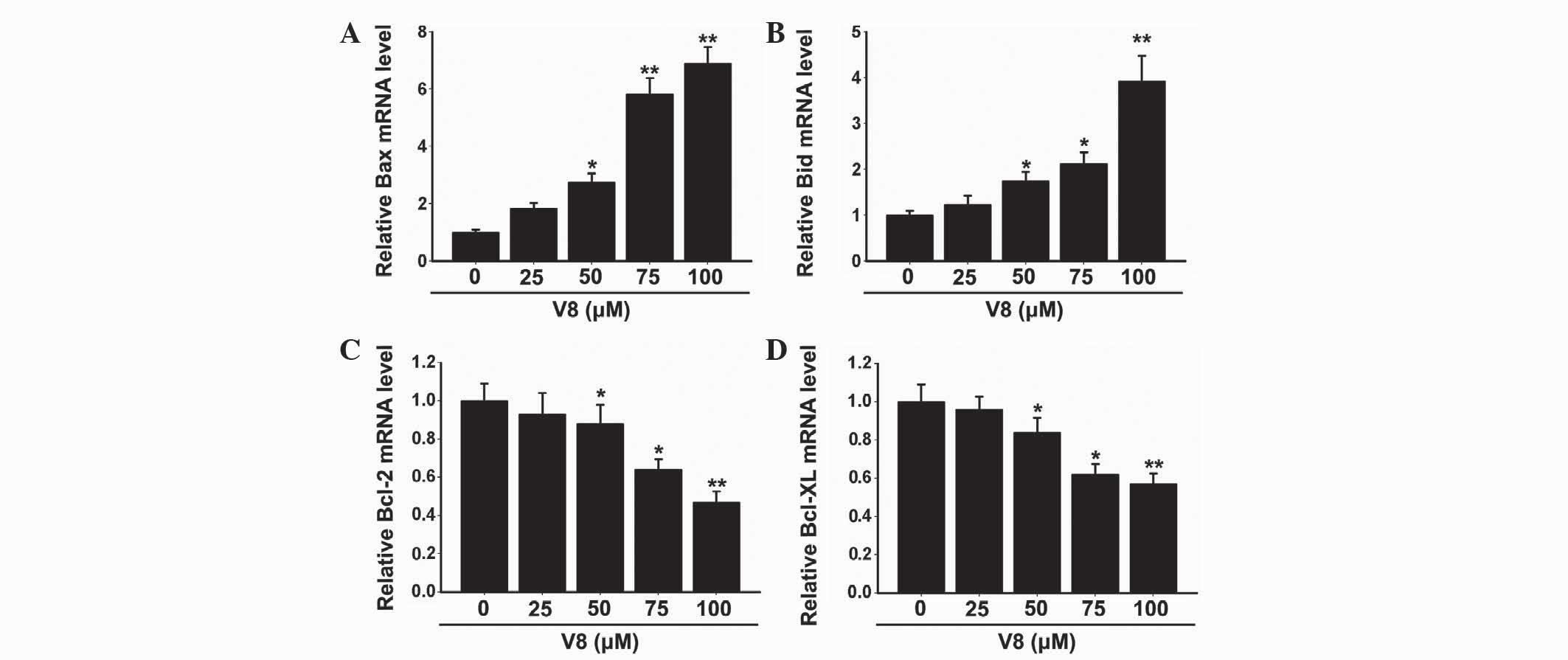
Expression of apoptosis factors was
altered following V8 treatment
To further investigate the molecular basis of the
apoptosis induced by V8 in RPMI 8226 cells, the expression of
apoptosis-associated factors were evaluated. The Bcl-2 family, also
known as fundamental death regulatory proteins, are key regulators
in mitochondrial outer membrane permeabilization (16). As shown in Fig. 3, the mRNA expression of Bax and Bid
was clearly downregulated (P<0.05), while the mRNA expression of
Bcl-2 and Bcl-XL was significantly upregulated (P<0.05)
following treatment with 50, 75 and 100 µM V8 for 48 h. There was
no alteration in the expression of Bax, Bid, Bcl-2 and Bcl-XL with
low concentrations of V8 (P>0.05). The alterations observed in
Bcl-2 family member expression was consistent with the cellular
apoptosis induced by V8.
ER stress response was activated in
RPMI 8226 cells following treatment with V8
ER stress has emerged as a key instigator of the
intrinsic apoptotic pathway (17). To
investigate whether the apoptosis of RPMI 8226 cells, induced by
V8, was associated with ER stress, components of the ER stress
pathway were evaluated. Notably, a significant upregulation in the
ER stress response elements GRP78, GPR94 and CHOP was observed
following treatment with 50, 75 and 100 µM V8 (Fig. 4A-C; P<0.05). In addition, cleaved
caspase-12 was significantly increased, as shown by western
blotting (Fig. 4D; P<0.05) and
immunofluorescence (Fig. 4D and
E).
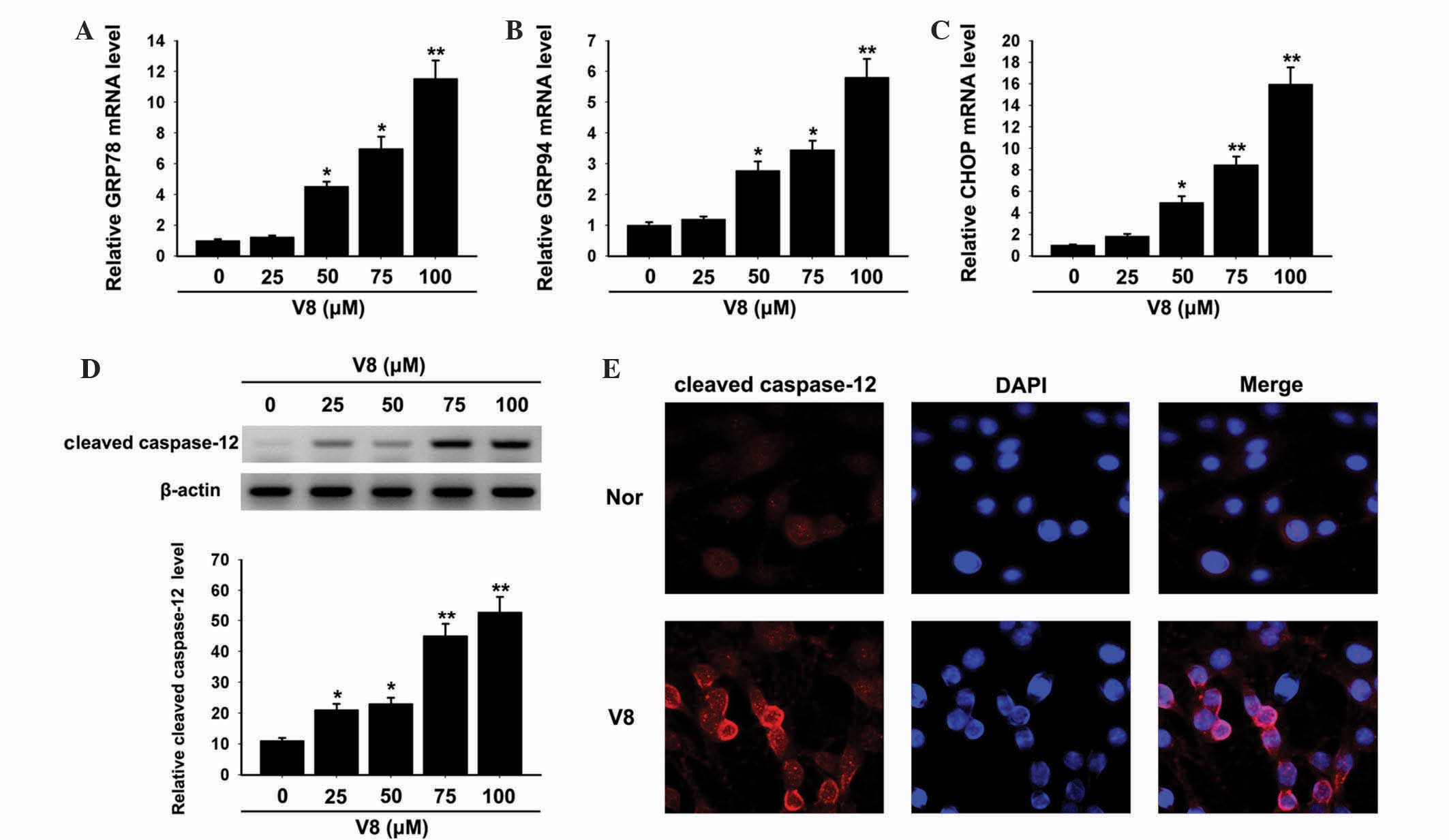 | Figure 4.ER stress response was activated by
V8. Relative mRNA expression of (A) GRP78, (B) GRP94 and (C) CHOP
in human multiple myeloma RPMI 8226 cells was detected following
treatment with various concentrations of V8 (25, 50, 75 and 100 µM)
for 48 h by quantitative polymerase chain reaction. (D) Alteration
in the expression of cleaved caspase-12 following V8 treatment was
determined by western blotting. The bar chart demonstrates the
level of cleaved caspase-12 relative to glyceraldehyde 3-phosphate
dehydrogenase by densitometry. (E) Cleaved caspase-12 (red) and
DAPI (blue) immunofluorescence double stain was performed to detect
the level and distribution of cleaved caspase-12 in RPMI 8226 cells
treated with 75 µM V8. Scale bar, 20 µm. Data are presented as the
mean ± standard error of the mean of three independent experiments.
*P<0.05, **P<0.01 vs. untreated cells. V8,
7-{4-[Bis-(2-hydroxyethyl)-amino]-butoxy}-5-hydroxy-8-methoxy-2-phenylchromen-4-one;
GRP, glucose-regulated protein; CHOP, C/EBP homologous protein;
DAPI, 4′,6-diamidino-2-phenylindole; nor, untreated RPMI 8226
cells. |
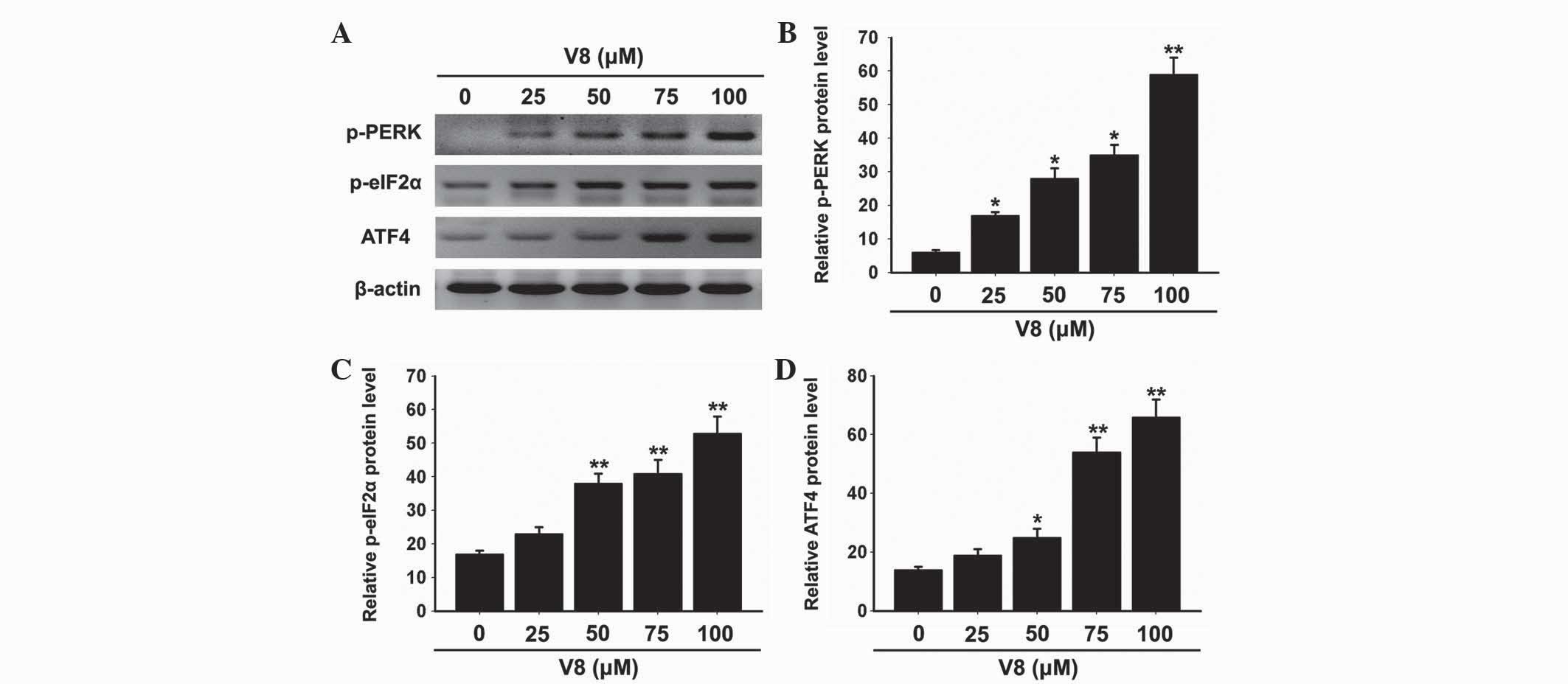
The unfolded protein response (UPR) is activated to
prevent further translational loading of the ER in response to ER
stress. PERK activates itself by oligomerization and
autophosphorylation of the free luminal domain and causes
translational attenuation by directly phosphorylating eIF2α and
activating certain transcription factors (18). Further investigation was performed by
the present study to determine whether V8-induced apoptosis was
associated with the UPR and PERK-eIF2α-ATF4 signaling pathway.
Notably, phosphorylation of PERK and eIF2α was increased by V8
treatment (Fig. 5A-C; P<0.05). In
addition, the expression of ATF4 was promoted by V8 treatment
(Fig. 5A and D; P<0.05). The
activation of the PERK-eIF2α-ATF4 signaling pathway was consistent
with the increasing levels of GPR78, GPR94 and CHOP. These results
suggest that the PERK-eIF2α-ATF4 signaling pathway and ER stress
response are involved in V8 induced apoptosis of RPMI 8226
cells.
Discussion
Traditional Chinese medicines, including Curcuma
longa, C. phaeocaulis and C. wenyujin, which are
well known herbal medicines, have been proposed to have cytotoxic
and antitumor properties with lower toxicity and fewer side effects
compared with traditional chemotherapeutic agents (19). Due to the extensive anti-inflammatory,
anti-angiogenesis and anti-microbial biological activity reported
for the chemical constituents of certain plants, including in
Alzheimer's disease (20), studies
have been performed to understand the function and mechanism of
flavonoids in cancer cells (21,22).
Various chemical constituents of Chinese medicine have been
considered as a novel source of anti-cancer drugs; however, the
molecular mechanisms of their actions remain largely unknown. The
present study demonstrated that the novel compound V8, derived from
natural wogonin, induces apoptosis and ER stress in human MM RPMI
8226 cells in a dose- and time-dependent manner.
Apoptosis has been widely accepted as an important
mechanism that contributes to cell death and survival, and is a
major treatment modality to kill cancer cells (23). It is well-established that caspase-3,
a member of the caspase family enzymes, is the key effector caspase
that executes apoptosis, and is activated by initiators, including
caspase-8 and −9, through mitochondrial-mediated pathways in
response to various stimulation (10). In the current study, the activity of
caspase-3, −8 and −9 was greatly enhanced by V8 treatment,
suggesting that V8-induced apoptosis is associated with the
activation of the caspase cascade.
To understand the molecular basis of V8-induced
apoptosis in RPMI 8226 cells, leakage of cyto c from
mitochondria to the cytosol, which is regarded as a preceding event
for the activation of caspase cascades (11), was evaluated. The present study
demonstrated that the level of cyto c was increased in
mitochondria and decreased in the cytoplasm indicating that the
release of mitochondrial cyto c was promoted by V8
treatment. Cyto c release is mediated and tightly regulated
by the Bcl-2 family of proteins, which consists of pro- and
anti-apoptotic proteins (24). In
previous studies, dimerization of Bax and Bcl-2 homologous
antagonist/killer (Bak) induced cyto c release from
mitochondria, while anti-apoptotic Bcl-2 family members functioned
as dominant negative inhibitors by binding and inhibiting Bax and
Bak (25,26). In the present study, the upregulation
of Bax and Bid and the downregulation of Bcl-2 and Bcl-XL were
observed following V8 treatment, which induced clear cyto c
leakage to the cytosol in RPMI 8226 cells.
According to the present data, it is clearly
conceivable that the alteration of Bcl-2 family members in RPMI
8226 cells initiates the mitochondrial-initiated events leading to
cyto c release and activation of the caspase cascade.
Previous studies have demonstrated that activation of caspase-12
occurs prior to the activation of executioner caspase-3 in the
apoptosis of various cells associated with ER-stress (17,18,27).
Consistent with the results of previous studies (11), the protein level of cleaved caspase-12
was increased following the same concentration of V8 stimulation in
the present study. Caspase-12 is localized to the cytosolic
interface of the ER once it is cleaved and activated, which renders
it vulnerable to ER stress, leading to further activation of the
caspase cascade (28,29). Therefore, the present study considered
the possibility that ER stress may be activated by V8.
The ER has numerous general functions, including the
folding of protein molecules, transport of synthesized proteins in
vesicles to the Golgi apparatus, posttranslational modifications,
lipid and steroid synthesis and calcium signaling. Various
conditions, such as ischemia, hypoxia, heat shock, gene mutation
and elevated protein synthesis, may impair ER function and result
in ER stress, a state in which protein folding slows, leading to an
increase in unfolded proteins. This type of stress is characterized
by the upregulation of ER chaperones, including GRP78 and GRP94. It
is widely known that excessive and prolonged ER stress triggers the
cell apoptotic signaling pathway (30). The current study demonstrated that V8
treatment resulted in upregulation of the ER stress response
proteins GRP78, GRP94 and CHOP in RPMI 8226 cells. The
transcription factor CHOP is also activated in ER stress and causes
downregulation of the anti-apoptotic mitochondrial protein Bcl-2
(31). In addition, phosphorylation
of PERK and eIF2α were enhanced, and the expression of ATF4 was
clearly induced by V8 treatment in the present study. In response
to ER stress, PERK and other protein kinases initiate the UPR,
which is tightly associated with the regulation of programmed cell
death (32). A study by Rouschop
et al (33) indicated that the
UPR of the PERK-eIF2α-ATF4 pathway was a potent stimulator of
autophagy and apoptosis in response to ER. The present results
suggest that V8-induced apoptosis is mediated by ER stress
associated with the PERK-eIF2α-ATF4 cascade, which further induced
apoptotic signals downstream.
In conclusion, on the basis of the present results,
the present study considers the possibility that V8 activates the
PERK-eIF2α-ATF4-CHOP axis of ER stress signaling, and that
increasing the expression of PERK, eIF2α, ATF4 and CHOP promotes
the expression of Bcl-2 and Bcl-XL in mitochondria. Consequently,
the activation of the mitochondrial apoptosis pathway induces the
upregulation of Bax/Bid, which affects mitochondrial outer membrane
permeabilization and promotes the release of cyto c into
cytoplasm. This increases the activity of caspase-9, −8 and −3,
ultimately leading to cell apoptosis (Fig. 6). Overall, V8-induced apoptosis and ER
stress in human MM RPMI 8226 cells is associated with the
PERK-eIF2α-ATF4 signaling pathway. This suggests that the small
molecule V8 may target the ER stress response, and therefore may
possess great pharmaceutical value to improve the treatment
efficacy of MM.
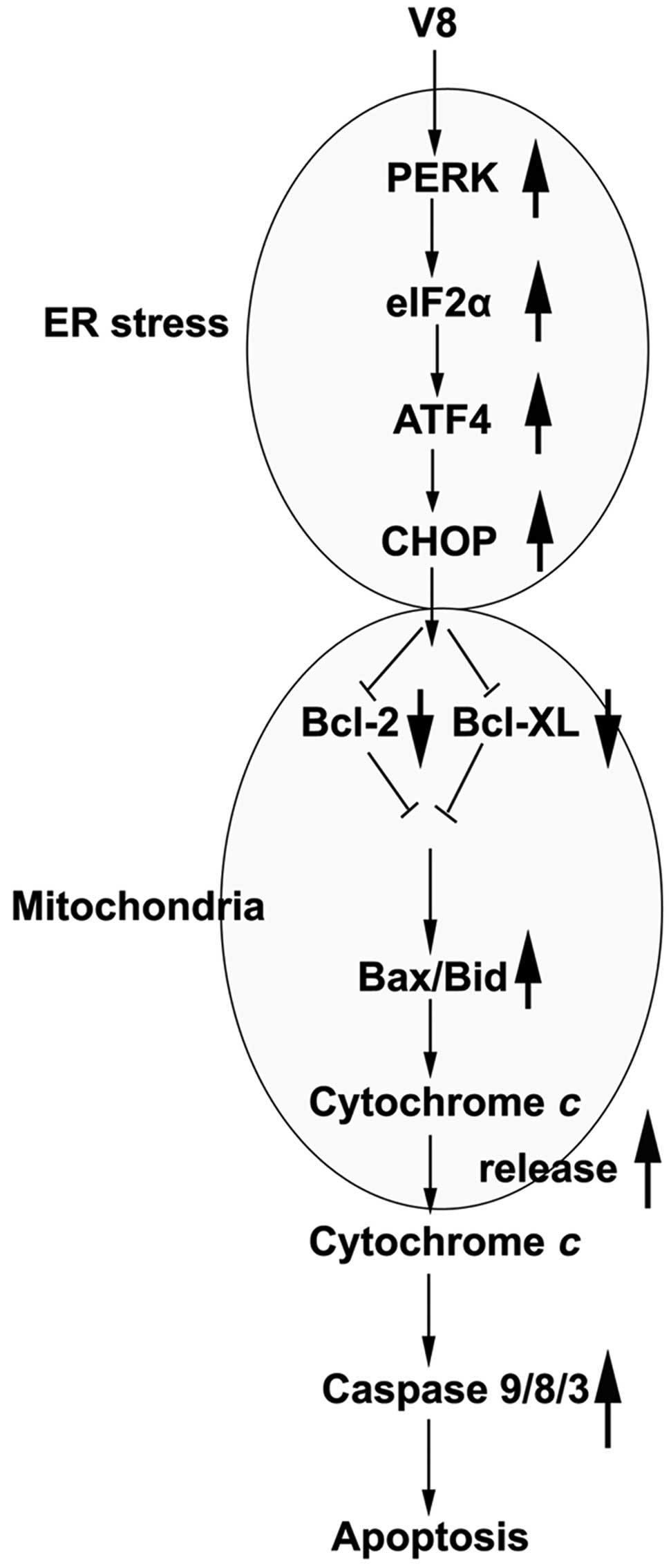 | Figure 6.Proposed mechanism by which V8 induces
apoptotic cell death in human multiple myeloma RPMI 8226 cells. V8
activates the PERK-eIF2α-ATF4-CHOP axis of ER stress signaling.
Increasing the expression of PERK, eIF2α, ATF4 and CHOP promotes
the expression of Bcl-2 and Bcl-XL in mitochondria. Activation of
the mitochondrial apoptosis pathway induces the upregulation of
Bax/Bid, and the release of cytochrome c into cytoplasm.
This increases the activity of caspase-9/8/3 and leads to cell
apoptosis. V8, 8,
7-{4-[Bis-(2-hydroxyethyl)-amino]-butoxy}-5-hydroxy-8-methoxy-2-phenylchromen-4-one;
ER, endoplasmic reticulum; PERK, protein kinase RNA-like
endoplasmic reticulum kinase; eIF2α, eukaryotic initiation factor
2α; ATF4, activating transcription factor 4; CHOP, C/EBP homologous
protein; Bcl, B-cell lymphoma; Bcl-XL, Bcl-extra large; Bax,
Bcl-2-like protein 4; Bid, BH3 interacting domain death
agonist. |
References
|
1
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sergentanis TN, Kastritis E, Terpos E,
Dimopoulos MA and Psaltopoulou T: Cytogenetics and survival of
multiple myeloma: Isolated and combined effects. Clin Lymphoma
Myeloma Leuk. 16:335–340. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colson K: Treatment-related symptom
management in patients with multiple myeloma: A review. Support
Care Cancer. 23:1431–1445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aslan E, Guler C and Adem S: In vitro
effects of some flavonoids and phenolic acids on human pyruvate
kinase isoenzyme M2. J Enzyme Inhib Med Chem. 31:314–317. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan R, Saif AQ, Quradha MM, Ali J, Rauf A
and Khan A: Antioxidant, antimicrobial and urease inhibiting
activities of methanolic extracts from Cyphostemma digitatum stem
and roots. Nat Prod Res. 30:486–488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pirouzpanah MB, Sabzichi M, Pirouzpanah S,
Chavoshi H and Samadi N: Silibilin-induces apoptosis in breast
cancer cells by modulating p53, p21, Bak and Bcl-xl Pathways. Asian
Pac J Cancer Prev. 16:2087–2092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Han A, Chen E, Singh RK,
Chichester CO, Moore RG, Singh AP and Vorsa N: The cranberry
flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and
cell cycle arrest and increase cisplatin sensitivity in ovarian
cancer cells. Int J Oncol. 46:1924–1934. 2015.PubMed/NCBI
|
|
9
|
Hung JY, Chang WA, Tsai YM, Hsu YL, Chiang
HH, Chou SH, Huang MS and Kuo PL: Tricetin, a dietary flavonoid,
suppresses benzo(a)pyrene-induced human non-small cell lung cancer
bone metastasis. Int J Oncol. 46:1985–1993. 2015.PubMed/NCBI
|
|
10
|
Ge W, Yin Q and Xian H: Wogonin induced
mitochondrial dysfunction and endoplasmic reticulum stress in human
malignant neuroblastoma cells via IRE1α-dependent pathway. J Mol
Neurosci. 56:652–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falank C, Fairfield H and Reagan MR:
Signaling interplay between bone marrow adipose tissue and multiple
myeloma cells. Front Endocrinol (Lausanne). 7:672016.PubMed/NCBI
|
|
12
|
Rengarajan T and Yaacob NS: The flavonoid
fisetin as an anticancer agent targeting the growth signaling
pathways. Eur J Pharmacol. Jul 1–2016.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Zhao L, Li X, Wang Y, Yao J, Wang
H, Li F, Li Z and Guo Q: V8, a newly synthetic flavonoid, induces
apoptosis through ROS-mediated ER stress pathway in hepatocellular
carcinoma. Arch Toxicol. 88:97–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He A, Ji R, Shao J, He C, Jin M and Xu Y:
TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-kappaB activation
contribute to the anti-inflammatory effect of V8 in LPS-induced
human cervical cancer SiHa cells. Inflammation. 39:172–181. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Gratz J, Amour C, Nshama R, Walongo
T, Maro A, Mduma E, Platts-Mills J, Boisen N, Nataro J, et al:
Optimization of quantitative PCR methods for enteropathogen
detection. PLoS One. 11:e01581992016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhola PD and Letai A: Mitochondria -
Judges and executioners of cell death sentences. Molecular cell.
61:695–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bittremieux M, Parys JB, Pinton P and
Bultynck G: ER functions of oncogenes and tumor suppressors:
Modulators of intracellular Ca(2+) signaling. Biochim Biophys Acta.
1863:1364–1378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lagace TA and Ridgway ND: The role of
phospholipids in the biological activity and structure of the
endoplasmic reticulum. Biochim Biophys Acta. 1833:2499–2510. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Volgyi K, Juhasz G, Kovacs Z and Penke B:
Dysfunction of endoplasmic reticulum (ER) and mitochondria (MT) in
Alzheimer's disease: The role of the ER-MT cross-talk. Curr
Alzheimer Res. 12:655–672. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lall RK, Adhami VM and Mukhtar H: Dietary
flavonoid fisetin for cancer prevention and treatment. Mol Nutr
Food Res. 60:1396–1405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devi KP, Rajavel T, Habtemariam S, Nabavi
SF and Nabavi SM: Molecular mechanisms underlying anticancer
effects of myricetin. Life Sci. 142:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kelloff GJ, Crowell JA, Steele VE, Lubet
RA, Malone WA, Boone CW, Kopelovich L, Hawk ET, Lieberman R,
Lawrence JA, et al: Progress in cancer chemoprevention: Development
of diet-derived chemopreventive agents. J Nutr. 130(Suppl 2S):
S467–S471. 2000.
|
|
24
|
Kurokawa M and Kornbluth S: Caspases and
kinases in a death grip. Cell. 138:838–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suen DF, Norris KL and Youle RJ:
Mitochondrial dynamics and apoptosis. Genes Dev. 22:1577–1590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moorwood C and Barton ER: Caspase-12
ablation preserves muscle function in the mdx mouse. Hum Mol Genet.
23:5325–5341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Wang Z and Nowicki MJ: Caspase-12
mediates carbon tetrachloride-induced hepatocyte apoptosis in mice.
World J Gastroenterol. 20:18189–18198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakagawa T, Zhu H, Morishima N, Li E, Xu
J, Yankner BA and Yuan J: Caspase-12 mediates
endoplasmic-reticulum-specific apoptosis and cytotoxicity by
amyloid-beta. Nature. 403:98–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Zhang T, Li L and Wang J: Induction
of apoptosis by hypertension via endoplasmic reticulum stress.
Kidney Blood Press Res. 40:41–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang YS, Shen Q and Li J: Traditional
Chinese medicine targeting apoptotic mechanisms for esophageal
cancer therapy. Acta Pharmacol Sin. 37:295–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang Q, Li F, Shi K, Wu P, An J, Yang Y
and Xu C: Involvement of p38 in signal switching from autophagy to
apoptosis via the PERK/eIF2α/ATF4 axis in selenite-treated NB4
cells. Cell Death Dis. 5:e12702014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rouschop KM, van den Beucken T, Dubois L,
Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W,
Voncken JW, et al: The unfolded protein response protects human
tumor cells during hypoxia through regulation of the autophagy
genes MAP1LC3B and ATG5. J Clin Invest. 120:127–141. 2010.
View Article : Google Scholar : PubMed/NCBI
|