Introduction
Aspirin, a nonsteroidal anti-inflammatory drug, is
used clinically as antipyretic, analgesic and anti-inflammatory
medicine. It has been indicated to reduce the risk of cancers,
including bladder cancer (1), breast
cancer (2), glioma (3), and particularly colorectal cancer
(4,5).
Although the considerable evidence demonstrating that aspirin
prevents cancer progression is compelling, the underlying molecular
mechanism remains enigmatic. Numerous molecular targets and
pathways have been implicated; however, the antitumor activity of
aspirin may be not attributed wholly to a single target or pathway
(6). It is likely that aspirin
influences several molecular pathways that cross each other.
Mammalian target of rapamycin (mTOR) is a 289-kDa
serine⁄threonine kinase which is highly expressed in the processes
of multiple types of tumors as the control center of cell growth,
differentiation, apoptosis and angiogenesis (7,8). In
mammalian cells, mTOR-dependent processes include regulating cell
growth by controlling mRNA translation, ribosome biogenesis,
autophagy and metabolism (9,10).
Angiogenesis, the formation of new blood vessels, is
required for tumor growth and metastasis. Several regulating
pathways have been involved in this process. Through
immunohistochemistry and western blot analysis, we observed that
hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial
growth factor-A (VEGF-A) are the downstream proteins of mTOR. Tumor
tissue is usually accompanied by hypoxia, which promotes HIF-1α
production. HIF-1α and its downstream target, VEGF-A, play critical
roles in tumor angiogenesis and represent an attractive
chemotherapeutic target (11–13). Ruan et al revealed that the
phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway as
‘the regulation center of angiogenesis’ could regulate the
expression of VEGF-A by hypoxia and HIF-1α, cancer genes, hormones,
growth factors and cytokines and other factors (14). Another study also revealed that
although mTOR activity was restrained in hypoxia, the cells still
mediated the production of HIF-1α through the mTOR pathway
(15). Under normoxia, improving the
activity of mTOR increases the expression of HIF-1α in tumor cells
(16).
Autophagy is an evolutionarily conserved process in
which cells recycle long-lived proteins and damaged organelles. It
involves the sequestration of cytoplasmic components within a
double membrane structure, called autophagosome, and subsequent
delivery to lysosomes for degradation (17,18). Atg1,
with its mammalian homologue UNC-51-like kinase-1 (ULK1), is a
conserved serine-threonine kinase that is required for autophagy
pathways, and its activity is regulated by the TOR kinase (19–21). Yeast
Atg8, and its mammalian homolog microtubule-associated protein 1
light chain 3 (LC3), are ubiquitin-like modifiers that are
localized on isolation membranes and play crucial roles in the
formation of autophagosomes. Yeast expresses a single Atg8 protein,
while mammals encode several isoforms, including three MAP1 light
chain 3 proteins [LC3A (two splice variants), B and C)] and four
γ-aminobutyrate receptor-associated proteins (22). mTOR exists in a phosphorylated form in
normal conditions and suppresses autophagy. However, when the
phosphorylated mTOR (p-mTOR) level is downregulated, as observed
during rapamycin treatment or nutrient starvation, cell autophagy
is induced (23).
Downregulation of the mTOR pathway due to treatment
with mTOR inhibitors suppresses tumor angiogenesis and enhances
autophagy (24,25). Aspirin inhibits mTOR signaling in
colorectal cancer and angiogenesis in murine sarcoma models
(6,26). Based on this evidence, experimental
studies using H22 hepatocarcinoma and S180 sarcoma
models were designed to investigate the underlying mechanisms of
the antitumor effects of aspirin.
Materials and methods
Materials
Aspirin, purchased from Qilu Pharmaceutical Co.,
Ltd. (Shandong, China), was dissolved from powder into drinking
water and stored at 4°C. Everolimus was purchased from Ruibio
(Sachsen, Germany) and an original concentration of 40 mM was
prepared with dimethyl sulfoxide (DMSO) as a stock solution at 4°C.
DMSO was obtained from Sigma-Aldrich (St. Louis, MO, USA). A
bicinchoninic acid (BCA) protein assay kit was obtained from Pierce
(Rockford, IL, USA). Polyvinylidene difluoride (PVDF) membranes
were from Pall Life Sciences (Ann Arbor, MI, USA). Western
blot-related reagents were purchased from the Shanghai Beyotime
Institute of Biotechnology, China.
Animal models
The animal experiments were approved by the
Institute of Medicine, Shandong Academy of Medical Sciences, China.
Forty male Kunming mice aged 5–6 weeks and weighing 18–22 g were
obtained from the Animal Experiment Center of Shandong University,
China. Under sterile conditions, ascites were extracted from
H22 ascitic mice which had been injected
intraperitoneally with H22 cells seven days previously.
Normal saline was added to adjust the cell concentration to
1×107/ml. Then 0.2 ml tumor cell suspension was
inoculated subcutaneously into the right flank of each mouse, and
the S180 sarcoma model was processed in the same way. The mice were
maintained under standard husbandry conditions and monitored for
body weight and water and food intake.
Drug treatment
Following tumor cell injection, the mice were
randomly divided into four groups with ten mice in each group when
the tumors were ~50–100 mm3. The four groups were as
follows: i) no treatment, used as control; ii) low-dose aspirin
(100 mg/kg); iii) high-dose aspirin (400 mg/kg); iv) everolimus
group (4 mg/kg). The control group took in purified water. Aspirin
with was administered by oral gavage once daily for 14 days.
Everolimus was administered by oral gavage at 4 mg/kg every day for
14 days. Tumor size was measured every other day using a digital
caliper, and tumor volume was calculated using the formula: V =
AxB2 / 2, where A and B are the largest diameter and the
perpendicular one, respectively. The body weight of the mice was
measured every day and the experiment lasted 3 weeks. At the end of
the experiment, blood was taken by removing the eyeball and the
tumors were dissected and weighed after euthanasia. The tumor
inhibitory rate was calculated using the formula: Inhibitory rate
(IR)=[average tumor weight of control group (g) - average tumor
weight of intervention group (g)] / average tumor weight of control
group (g) × 100%. Sections of each tumor were immediately placed in
4% paraformaldehyde for immunohistochemistry. The remainder of each
tumor was snap frozen for western blotting and stored at −70°C
until processing.
Histology and
immunohistochemistry
Tumor tissue was treated by paraffin embedding after
being placed overnight in 4% paraformaldehyde. The paraffin blocks
within the tumor tissue were cut into 4-µm sections for
immunohistochemical staining. Sections were de-waxed in xylene,
then rehydrated through ethanol divided into different gradients
and rinsed by distilled water. Following treatment with 3% hydrogen
peroxide in absolute methanol to inactivate endogenous peroxidase
activity, sections were subjected to hot repair with citrate
antigen retrieval solution (pH 6.0) for 15 min to expose epitope,
and then cooled to room temperature. Sections were washed with
phosphate-buffered saline (PBS) three times, five minutes each
time, then the primary antibody was added and subsequently
incubated overnight at 4°C. The sections were incubated at room
temperature for 50 min and then washed again with PBS three times
and sequentially incubated with the secondary antibody. The tissue
was incubated at room temperature for 1 h, and then washed with PBS
and colored with 3,3′-diaminobenzidine for 2–10 min and finally
counterstained with hematoxylin.
Western blotting
The expression profiles for p-mTOR, HIF-1α, VEGF-A,
ULK1 and LC3A were determined by western blot analysis. The tumor
tissue stored at −70°C was sheared, ground, centrifugated and
boiled. The protein concentration was determined with the BCA kit
and then the samples were stored at −20°C. Equal quantities (20 µg)
of samples from different groups were loaded onto sodium dodecyl
sulphate-polyacrylamide electrophoresis gel and the resolved
proteins were electrotransferred to PVDF membranes. Membranes were
sealed with 5% skimmed milk in TBST (1 M Tris-buffered saline, pH
7.6; 5 M NaCl; 0.1% Tween-20) buffer for 2 h before adding primary
antibody, and incubated overnight at 4°C. Western blot analyses
were carried out using the appropriate antibody [p-mTOR, LC3A (Cell
Signaling Technology, Inc., Danvers, MA, USA); ULK1 (Abcam, Hong
Kong, China); HIF-1α, VEGF-A (Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China)]. The membranes were then developed
using the ECL Plus chemiluminescence detection system. The band
intensities were analyzed by ImageJ software (Wayne Rasband
National Institutes of Health, Bethesda, MD, USA) and normalized to
β-actin (Cell Signaling Technology, Inc.).
Statistical analysis
The descriptive statistics provided are the means ±
standard deviation. A one-way analysis of variance (ANOVA) test was
used to assess the effects of different doses for aspirin and
everolimus on tumor tissues. Data were analyzed using an ANOVA
pairwise comparison method (Bonferroni method) and the Spearman
rank correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of aspirin on H22
hepatoma tumor growth
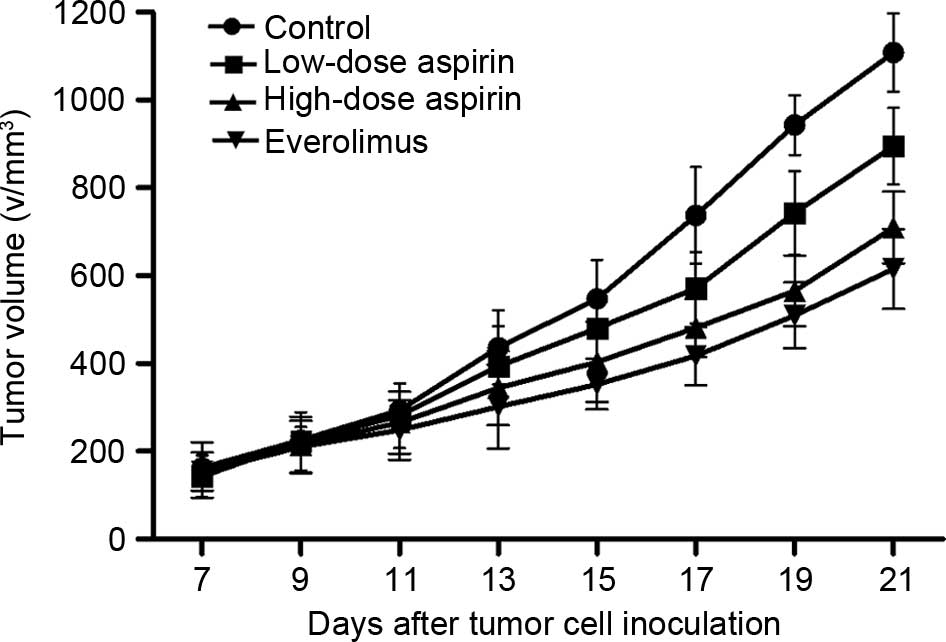
In the control and drug intervention groups, tumor
dimensions increased. Compared with the control group, the
everolimus group (4 mg/kg), high-dose aspirin group (400 mg/kg) and
low-dose aspirin (100 mg/kg) group markedly inhibited tumor growth
(Table I, Fig. 1). The inhibitory rates were 53.7, 36.7
and 21.1%, respectively (P<0.05 for each comparison between
treatment groups and the control group). The inhibitory effects
were more apparent in the high-dose aspirin group and the
everolimus group (P<0.01). Although the inhibitory rate of the
low-dose aspirin group was lower, the mice in this group were
generally in good condition, with a food and drink intake and
weight similar to those of mice in the high-dose aspirin group.
This suggests that aspirin may improve the living standard of
tumor-bearing mice.
 | Table I.Inhibitory effect of aspirin on
H22 hepatocarcinoma (mean ± standard deviation,
n=10). |
Table I.
Inhibitory effect of aspirin on
H22 hepatocarcinoma (mean ± standard deviation,
n=10).
| Group | Tumor weight
(g) | IR (%) |
|---|
| Control | 1.47±0.21 | – |
| Low-dose
aspirin |
1.16±0.19a | 21.1 |
| High-dose
aspirin |
0.93±0.14b | 36.7 |
| Everolimus |
0.68±0.12b | 53.7 |
Expression of p-mTOR, HIF-1α, VEGF-A,
ULK1 and LC3A in H22 hepatocarcinoma tumors
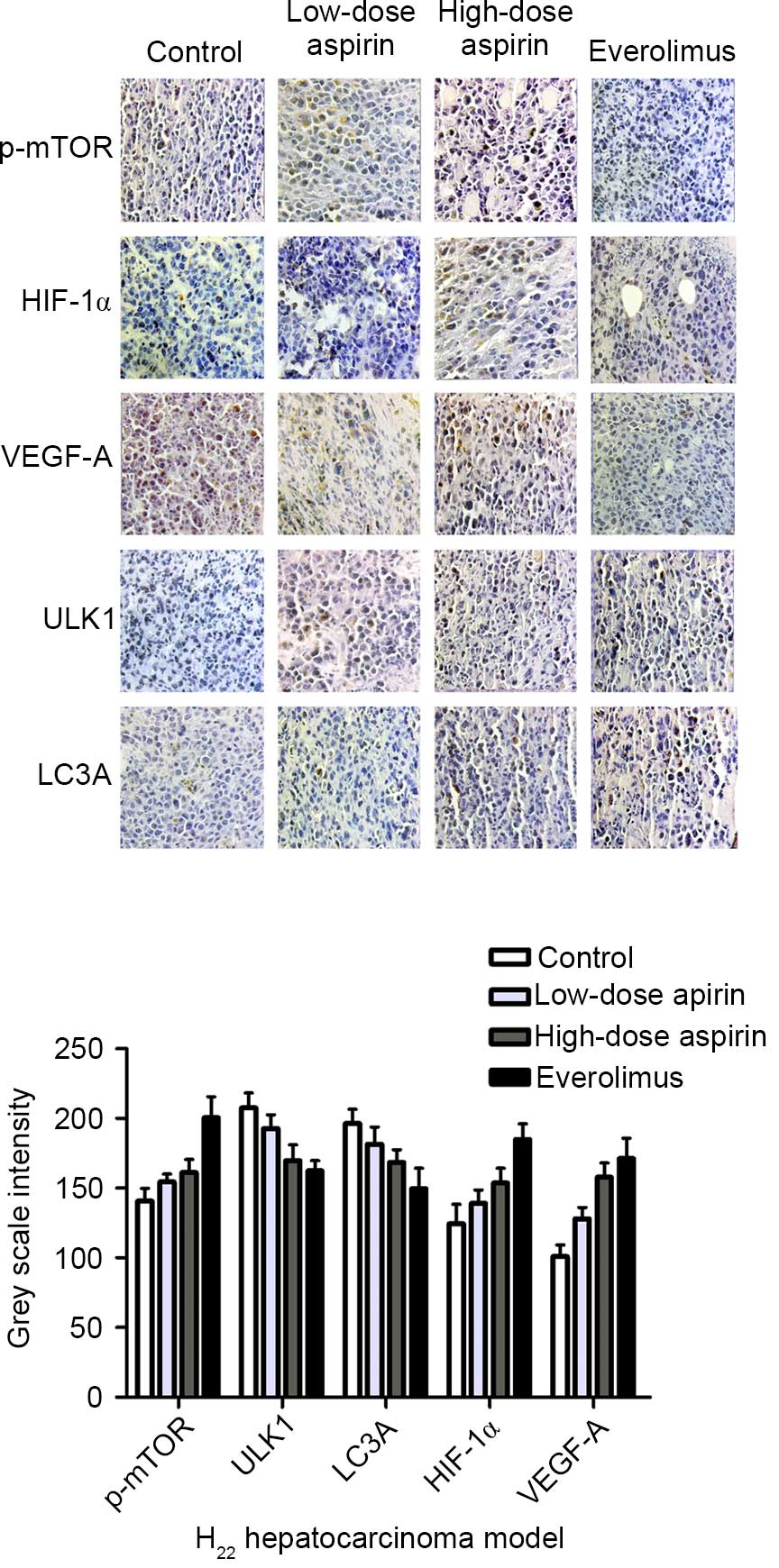
Based on the immunohistochemical staining results,
p-mTOR, ULK1 and VEGF-A were expressed in the cytoplasm of tumor
cells. HIF-1α was expressed in the nucleus and cytoplasm of tumor
cells. LC3A was expressed in the cytoplasm or membrane. Cells
positive for p-mTOR, HIF-1α, VEGF-A, ULK1 and LC3A were stained
brown (Fig. 2). The expression of
p-mTOR, HIF-1α and VEGF-A in the control group was markedly higher
than that in the treatment groups. The expression of ULK1 and LC3A
in the treatment groups was higher than that in the control group,
particularly in the everolimus and high-dose aspirin groups. Gray
scale intensity variants of p-mTOR, HIF-1α, VEGF-A, ULK1 and LC3A
immunoreactivity were evaluated by Leica Qwin V3 software (Leica
Microsystems GmbH, Wetzlar, Germany). Five positive regions
selected randomly from each section were analyzed at an original
magnification of ×400. An inverse correlation was observed between
the gray scale intensity and the protein expression: the higher the
gray scale intensity, the weaker the protein expression, and vice
versa (Fig. 2). In each comparison,
there was a significant difference (P<0.05). In addition, the
high-dose aspirin and everolimus groups were significantly
different compared with the control group (P<0.01). p-mTOR,
HIF-1α and VEGF-A expression was positively correlated (r=0.788,
P<0.01; r=0.776, P<0.01); and HIF-1α and VEGF-A expression
was positively correlated (r=0.796, P<0.01). p-mTOR, ULK1 and
LC3A expression was negatively correlated (r=−0.804, P<0.01;
r=−0.703, P<0.01); ULK1 and LC3A expression were positively
correlated (r=0.734, P<0.01).
Effect of aspirin treatment on p-mTOR,
HIF-1α, VEGF-A, ULK1 and LC3A protein expression as assessed by
western blot analysis in H22 hepatocarcinoma tumors
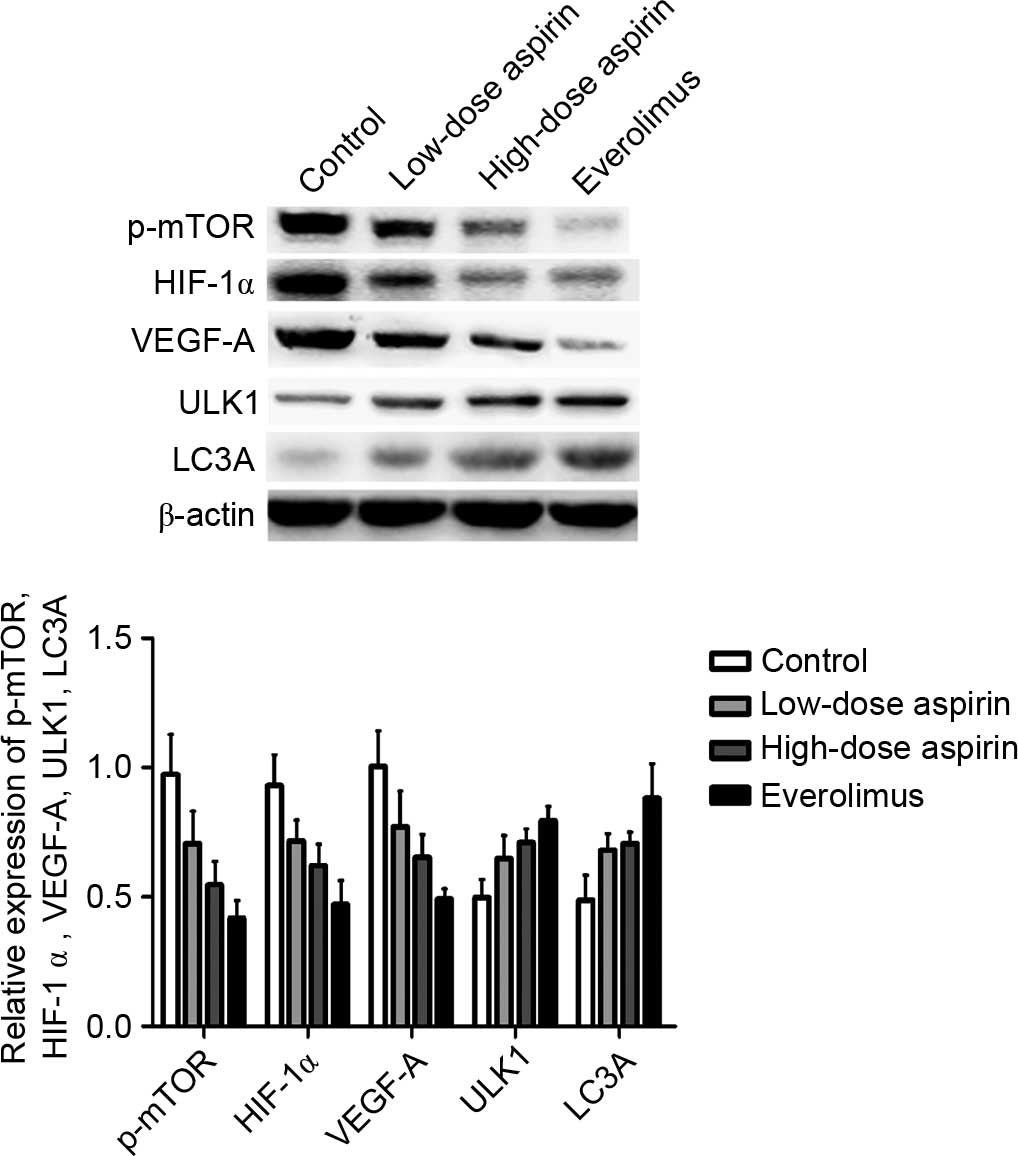
P-mTOR, HIF-1α, VEGF-A, ULK1 and LC3A expression was
normalized to β-actin expression by band intensity. As shown in
Fig. 3, the expression of p-mTOR,
HIF-1α and VEGF-A decreased in the low-dose and high-dose aspirin
and everolimus groups, but the expression of ULK1 and LC3A
increased when compared with the control group in the
H22 hepatocarcinoma tissue. Band intensities were
analyzed using ImageJ software. The expression demonstrated a
dose-dependent trend in the high-dose and low-dose aspirin groups.
In addition, aspirin at each concentration demonstrated a
significant difference among groups (P<0.05). Furthermore,
p-mTOR, HIF-1α and VEGF-A expression was positively correlated
(r=0.845, P<0.01; r=0.802, P<0.01); HIF-1α and VEGF-A
expression was positively correlated (r=0.856, P<0.01); p-mTOR,
ULK1 and LC3A expression was negatively correlated (r=−0.528,
P<0.05; r=−0.636, P<0.01); and ULK1 and LC3A expression was
positively correlated (r=0.779, P<0.01). P-mTOR expression also
demonstrated a dose-dependent trend in the high-dose and low-dose
aspirin groups. In addition, aspirin at each concentration was
significantly different between groups (P<0.05).
Effect of aspirin on S180 sarcoma
tumor growth
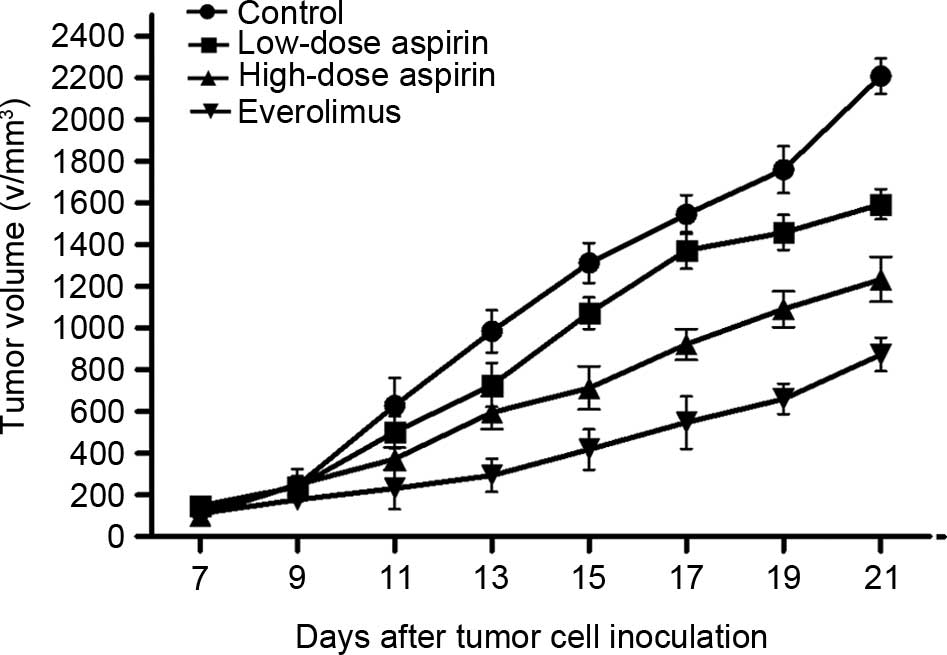
The experimental conditions and the processing
method of the S180 sarcoma model was the same as that used for
H22 tumors. Data were analyzed using an ANOVA pairwise
comparison method (Bonferroni method). The average tumor dimension
of each group was larger and the inhibitory rates were 48.7, 35.6
and 23.4%, respectively (Table II;
Fig. 4; P<0.05 for each comparison
between treatment groups and the control group). In the low-dose
aspirin group and high-dose aspirin group, the mice were in a good
general condition, with normal diet and mental state.
 | Table II.Inhibitory effect of aspirin on S180
sarcoma (mean ± standard deviation, n=10). |
Table II.
Inhibitory effect of aspirin on S180
sarcoma (mean ± standard deviation, n=10).
| Group | Tumor weight
(g) | IR (%) |
|---|
| Control | 2.05±0.33 | – |
| Low-dose
aspirin |
1.57±0.38a | 23.4 |
| High-dose
aspirin |
1.32±0.27a | 35.6 |
| Everolimus |
1.06±0.22a | 48.7 |
Expression of p-mTOR, HIF-1α, VEGF-A,
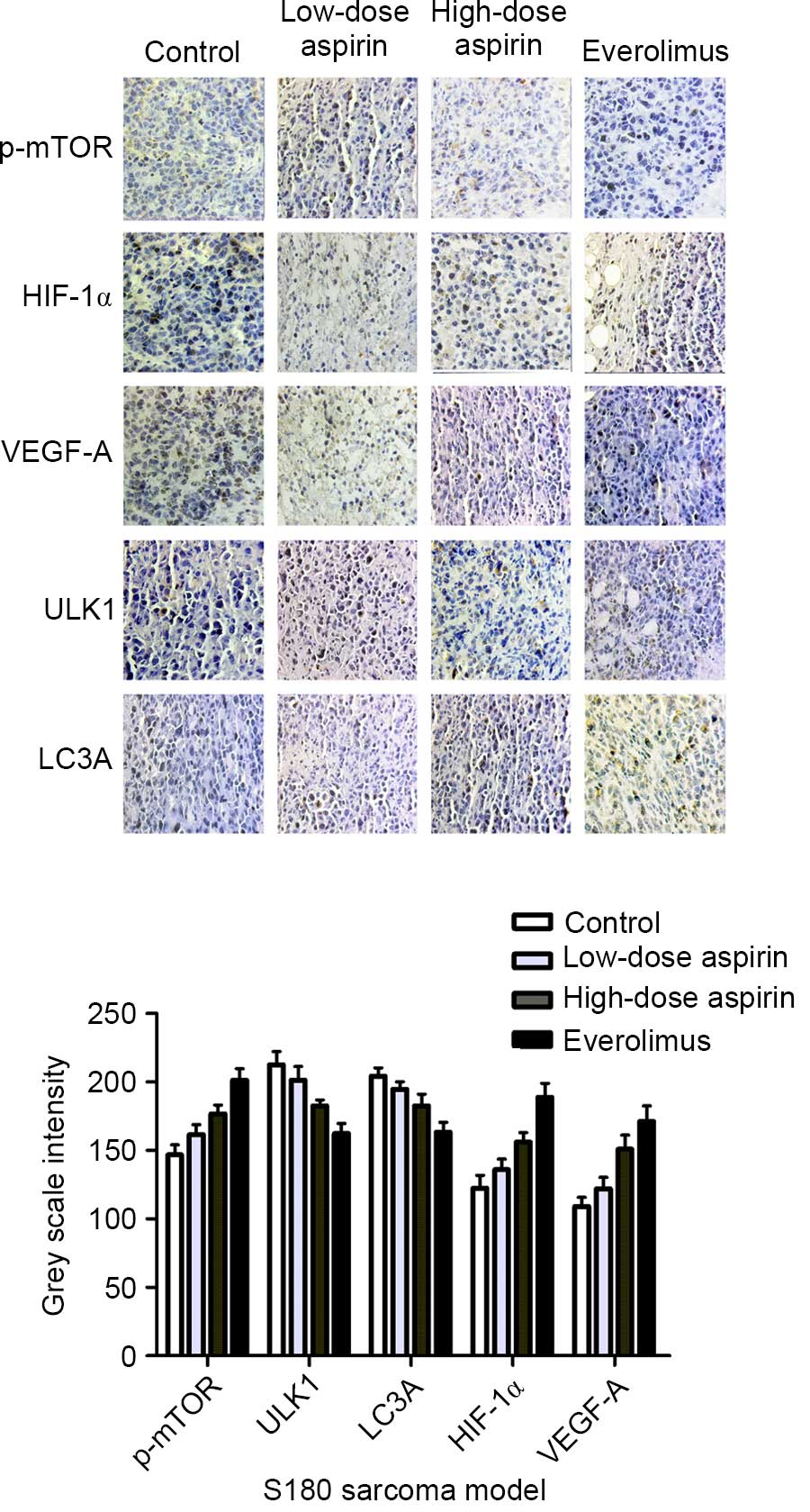
ULK1 and LC3A in S180 sarcoma tumors
Cells positive for p-mTOR, HIF-1α, VEGF-A, ULK1 and
LC3A were stained brown (Fig. 5). The
expression of p-mTOR, HIF-1α and VEGF-A in the control group was
markedly higher than that in the treatment groups, but the
expression comparison of ULK1, LC3A was the opposite. In each
comparison, there was a significant difference (P<0.05). In
addition, aspirin at high concentration and everolimus were
significantly different compared with the control group
(P<0.01). As shown in Fig. 5,
p-mTOR, HIF-1α and VEGF-A expression was positively correlated
(r=0.911, P<0.01; r=0.887 P<0.01); HIF-1α and VEGF-A
expression was positively correlated (r=0.884, P<0.01); p-mTOR,
ULK1 and LC3A expression was negatively correlated (r=−0.86,
P<0.01; r=−0.856, P<0.01); and ULK1 and LC3A expression was
positively correlated (r=0.836, P<0.01).
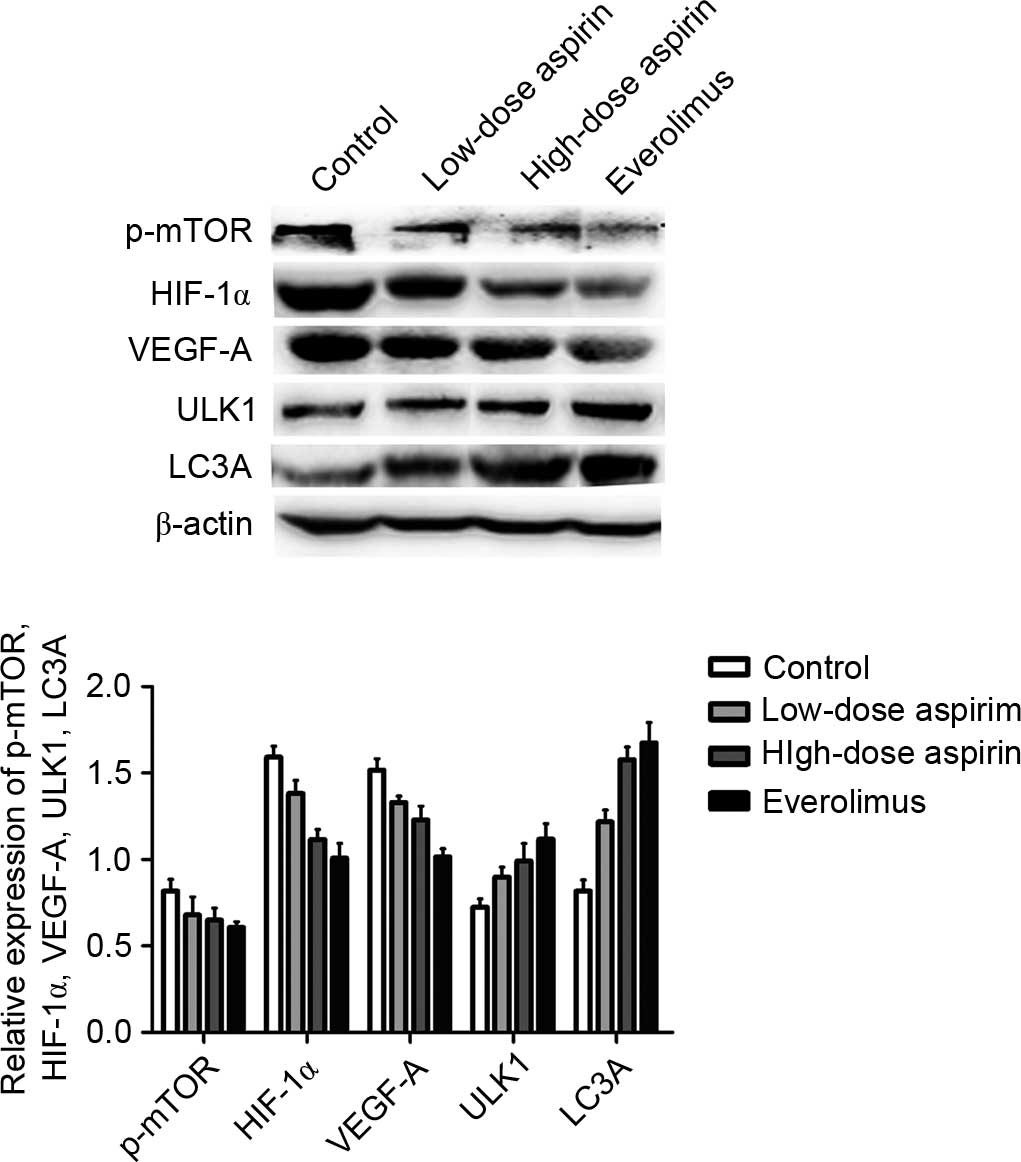
Effect of aspirin treatment on p-mTOR,
HIF-1α, VEGF-A, ULK1 and LC3A protein expression as assessed by
western blot analysis in S180 sarcoma tumors
P-mTOR, HIF-1α, VEGF-A, ULK1 and LC3A expression was
normalized to β-actin expression by band intensity. As shown in
Fig. 6, the expression of p-mTOR,
HIF-1α and VEGF-A decreased in the treatment groups, but the
expression of ULK1 and LC3A increased when compared with the
control group in the S180 sarcoma tissue as with the H22
hepatocarcinoma model. Band intensities were analyzed by ImageJ
software, and aspirin at each concentration demonstrated a
significant difference between groups (P<0.05). In the related
comparison, p-mTOR, HIF-1α and VEGF-A expression was positively
correlated (r=0.648, P<0.01; r=0.66, P<0.01); HIF-1α and
VEGF-A expression was positively correlated (r=0.907, P<0.01);
p-mTOR, ULK1 and LC3A expression was negatively correlated
(r=−0.621, P<0.05; r=−0.705, P<0.01); and ULK1 and LC3A
expression was positively correlated (r=0.823, P<0.01). The
tendency was similar to that observed in the H22
hepatocarcinoma model.
Discussion
The function of the mTOR signaling pathway as a
target of cancer therapy is being actively pursued (27). A study revealed that tumor recurrence
was accompanied by strong abnormal expression of rapamycin
molecular targets, implying that excessive activation of mTOR
signaling pathways was also a significant feature of tumor
progression (28,29). Rapamycin and its analogs, including
everolimus, which was approved for use as an immunosuppressive
agent in transplant patients, have been tested actively in clinical
trials for the past few years and have demonstrated preliminary
promise of efficacy in several tumor types (10,30). In
the present study, we focused primarily on tumor angiogenesis and
autophagy effects of drug treatments related to mTOR.
Previous studies have revealed that HIF-1α
expression is necessary for tumor growth in certain tumor cell
lines, including hepatomas. Decreased expression of HIF-1α is
associated with slower cell growth and tumor angiogenesis (12,31).
HIF-1α and VEGF-A expression are strongly associated with cancer
progression and angiogenesis (12–14). In
our study, the control group demonstrated marked expression of
p-mTOR, which is the activated form of mTOR and its downstream
protein, HIF-1α and VEGF-A, while the high and low-dose aspirin and
everolimus groups effectively inhibited the expression of p-mTOR,
HIF-1α and VEGF-A. To identify and characterize whether aspirin
inhibited the overexpression of HIF-1α and VEGF-A by decreasing
p-mTOR expression, we analyzed the expression of p-mTOR, HIF-1α and
VEGF-A by immunohistochemistry and western blotting. The results
from the models revealed that p-mTOR, HIF-1α and VEGF-A expression
was positively correlated and decreased in a aspirin dose-dependent
manner.
ULK1 as a initiatory protein is regulated by mTOR in
the process of autophagy (25). LC3
is a commonly used autophagy marker; its processed form resides in
cytoplasm and it could not form without ULK1 (32). mTOR negatively regulates autophagy,
and therefore we assessed aspirin's effects on autophagy by
immunohistochemistry and western blotting. The results revealed
that the expression of p-mTOR decreased in the aspirin groups but
ULK1 and LC3A did the opposite. Aspirin may induce autophagy, as
evidenced by the increased LC3A. However, in cancer therapy, the
role of autophagy is paradoxical. In certain studies, autophagy
appeared to function as a protective mechanism against cellular
stress (33,34). However, the induction of autophagy
still played a pivotal role in cell death induced by radiations or
reagents in other studies (35–37). In
the present study, there was an increase in autophagy protein
expression and there was clear tissue necrosis. This results
suggests that aspirin induces autophagy, leading to an inhibition
in tutor growth. This study might form a basis for future studies
into the anticancer effects of aspirin.
The inhibitory rates induced by aspirin were 36.7
and 21.1% in the murine hepatocarcinoma model (Table I and Fig.
1), and 35.6 and 23.4% in the sarcoma model (Table II and Fig.
4). We also concluded that aspirin reduces tumor growth rates
significantly (Figs. 1 and 4).
In the present study, we observed that the levels
HIF-1α and VEGF-A, the downstream proteins of mTOR associated with
angiogenesis, decreased in the murine hepatocarcinoma and sarcoma
models compared with levels in the non-intervention groups.
However, the autophagy-related proteins ULK1 and LC3A were induced,
as shown in Figs. 2, 3, 5 and
6. p-mTOR, HIF-1α and VEGF-A
expression was positively correlated, and p-mTOR, ULK1 and LC3A
expression was negatively correlated. We have demonstrated that
aspirin may inhibit mTOR signaling associated with
anti-angiogenesis and promoting autophagy on the protein expression
level. We intend to continue with further experiments on the
genetic level. Our study has significant clinical reference value
and may potentially lead to therapeutic treatment options for
hepatoma or sarcoma and other types of cancer.
Acknowledgements
This study was supported by funding from the
National Natural Science Foundation of China (nos. 81073102,
30873408, 81403150 and 81303077).
Glossary
Abbreviations
Abbreviations:
|
mTOR
|
mammalian target of rapamycin
|
|
p-mTOR
|
phosphorylated mammalian target of
rapamycin
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
VEGF-A
|
vascular endothelial growth
factor-A
|
|
ULK1
|
UNC-51-like kinase-1
|
|
LC3A
|
microtubule-associated protein 1 light
chain 3A
|
References
|
1
|
Gee JR, Jarrard DF, Bruskewitz RC, Moon
TD, Hedican SP, Leverson GE, Nakada SY and Messing EM: Reduced
bladder cancer recurrence rate with cardioprotective aspirin after
intravesical bacille Calmette-Guérin. BJU Int. 103:736–739. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alfonso LF, Srivenugopal KS, Arumugam TV,
Abbruscato TJ, Weidanz JA and Bhat GJ: Aspirin inhibits
camptothecin-induced p21CIP1 levels and potentiates apoptosis in
human breast cancer cells. Int J Oncol. 34:597–608. 2009.PubMed/NCBI
|
|
3
|
Iwama T: NSAIDs and colorectal cancer
prevention. J Gastroenterol. 44(Suppl 19): S72–S76. 2009.
View Article : Google Scholar
|
|
4
|
Din FV, Theodoratou E, Farrington SM,
Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME,
Campbell H and Dunlop MG: Effect of aspirin and NSAIDs on risk and
survival from colorectal cancer. Gut. 59:1670–1679. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rothwell PM, Wilson M, Elwin CE, Norrving
B, Algra A, Warlow CP and Meade TW: Long-term effect of aspirin on
colorectal cancer incidence and mortality: 20-year follow-up of
five randomised trials. Lancet. 376:1741–1750. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activatedprotein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
from growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alayev A and Holz MK: mTOR signaling for
biological control and cancer. J Cell Physiol. 228:1658–1664. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim
B, Soliman GA, Carriere A, Roux PP, Ballif BA and Fingar DC:
Regulation of mTOR complex l (mTORCI) by raptor Ser863 and
multisite phosphorylation. J Biol Chem. 285:80–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carbajo-Pescador S, Ordoñez R, Benet M,
Jover R, GarcíaPalomo A, Mauriz JL and González-Gallego J:
Inhibition of VEGF expression through blockade of Hif1α and STAT3
signalling mediates the anti-angiogenic effect of melatonin in
HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie SR, Wang Y, Liu CW, Luo K and Cai YQ:
Liquiritigenin inhibits serum-induced HIF-1α and VEGF expression
via the AKT/mTOR-p70S6K signalling pathway in HeLa cells. Phytother
Res. 26:1133–1141. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruan GX and Kazlauskas A: Axl is essential
for VEGF-A-dependent activation of PI3K/Akt. EMBO J. 31:1692–1703.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wouters BG and Koritzinsky M: Hypoxia
signalling through mTOR and the unfolded protein response in
cancer. Nat Rev Cancer. 8:851–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sengupta S, Peterson TR and Sabatini DM:
Regulation of the mTOR complex 1 pathway by nutrients, growth
factors, and stress. Mol Cell. 40:310–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jain K, Paranandi KS, Sridharan S and Basu
A: Autophagy in breast cancer and its implications for therapy. Am
J Cancer Res. 3:251–265. 2013.PubMed/NCBI
|
|
18
|
Morselli E, Galluzzi L, Kepp O, Vicencio
JM, Criollo A, Maiuri MC and Kroemer G: Anti- and pro-tumor
functions of autophagy. Biochim Biophys Acta. 1793:1524–1532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chan EY, Longatti A, McKnight NC and Tooze
SA: Kinase-inactivated ULK proteins inhibit autophagy via their
conserved C-terminal domains using an Atg13-independent mechanism.
Mol Cell Biol. 29:157–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hosokawa N, Sasaki T, Iemura S, Natsume T,
Hara T and Mizushima N: Atg101, a novel mammalian autophagy protein
interacting with Atg13. Autophagy. 5:973–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamada Y, Yoshino K, Kondo C, Kawamata T,
Oshiro N, Yonezawa K and Ohsumi Y: Tor directly controls the Atg1
kinase complex to regulate autophagy. Mol Cell Biol. 30:1049–1058.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behrends C, Sowa ME, Gygi SP and Harper
JW: Network organization of the human autophagy system. Nature.
466:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wirawan E, Berghe T Vanden, Lippens S,
Agostinis P and Vandenabeele P: Autophagy: for better or for worse.
Cell Res. 22:43–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moretti L, Yang ES, Kim KW and Lu B:
Autophagy signaling in cancer and its potential as novel target to
improve anticancer therapy. Drug Resist Updat. 10:135–143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang X, Wang Z, Wang Z, Zhang Y, Jia Q,
Wu L and Zhang W: Impact of acetylsalicylic acid on tumor
angiogenesis and lymphangiogenesis through inhibition of VEGF
signaling in a murine sarcoma model. Oncol Rep. 29:1907–1913.
2013.PubMed/NCBI
|
|
27
|
Dazert E and Hall MN: mTOR signaling in
disease. Curr Opin Cell Biol. 23:744–755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López-Knowles E, O'Toole SA, McNeil CM,
Millar EK, Qiu MR, Crea P, Daly RJ, Musgrove EA and Sutherland RL:
PI3K pathway activation in breast cancer is associated with the
basal-like phenotype and cancer-specific mortality. Int J Cancer.
126:1121–1131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hydbring P and Larsson LG: Cdk2: a key
regulator of the senescence control function of Myc. Aging (Albany
NY). 2:244–250. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cavazzoni A, Bonelli MA, Fumarola C, La
Monica S, Airoud K, Bertoni R, Alfieri RR, Galetti M, Tramonti S,
Galvani E, et al: Overcoming acquired resistance to letrozole by
targeting the PI3K/AKT/mTOR pathway in breast cancer cell clones.
Cancer Lett. 323:77–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang FZ, Peng-Jiao, Yang NN, Chuang-Yuan,
Zhao YL, Liu QQ, Fei HR and Zhang JG: PF-04691502 triggers cell
cycle arrest, apoptosis and inhibits the angiogenesis in
hepatocellular carcinoma cells. Toxicol Lett. 220:150–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaachouay H, Ohneseit P, Toulany M,
Kehlbach R, Multhoff G and Rodemann HP: Autophagy contributes to
resistance of tumor cells to ionizing radiation. Radiother Oncol.
99:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
O'Donovan TR, O'Sullivan GC and McKenna
SL: Induction of autophagy by drug-resistant esophageal cancer
cells promotes their survival and recovery following treatment with
chemotherapeutics. Autophagy. 7:509–524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anbalagan S, Pires IM, Blick C, Hill MA,
Ferguson DJ, Chan DA and Hammond EM: Radiosensitization of renal
cell carcinoma in vitro through the induction of autophagy.
Radiother Oncol. 103:388–393. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hao J, Pei Y, Ji G, Li W, Feng S and Qiu
S: Autophagy is induced by 3β-Osuccinyl-lupeol (LD9-4) in A549
cells via up-regulation of Beclin 1 and down-regulation mTOR
pathway. Eur J Pharmacol. 670:29–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He Z, Mangala LS, Theriot CA, Rohde LH, Wu
H and Zhang Y: Cell killing and radiosensitizing effects of
atorvastatin in PC3 prostate cancer cells. J Radiat Res.
53:225–233. 2012. View Article : Google Scholar : PubMed/NCBI
|