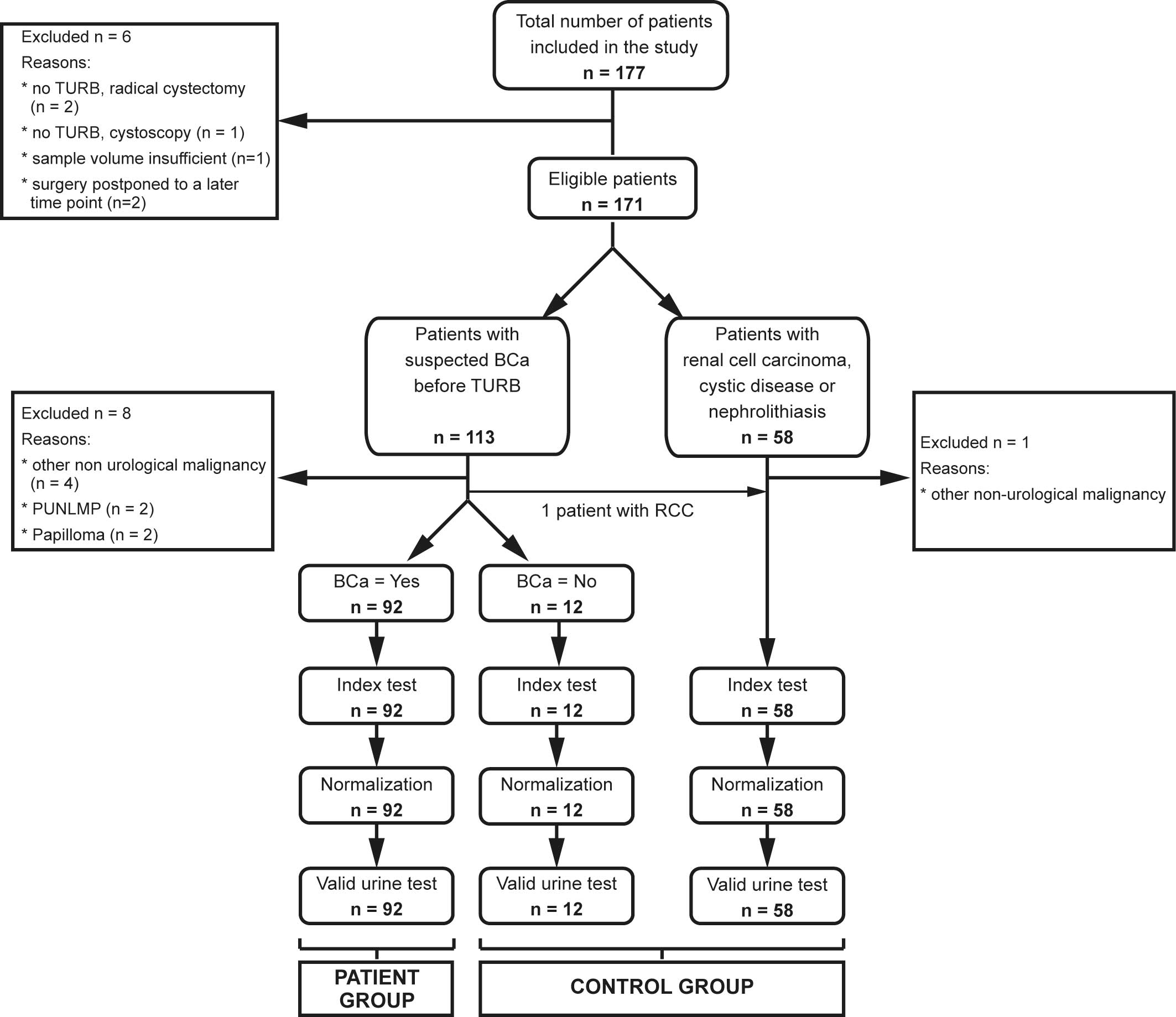
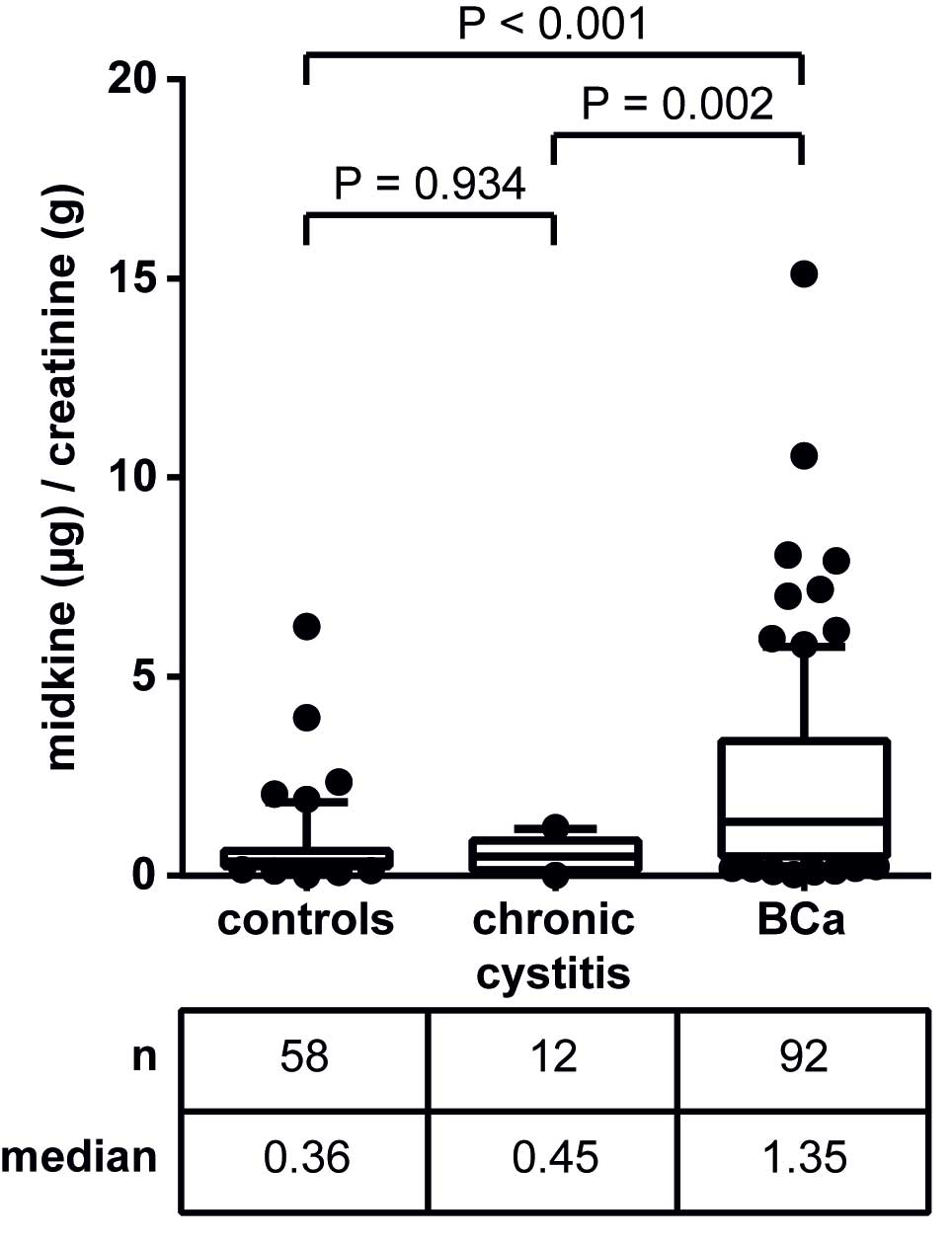
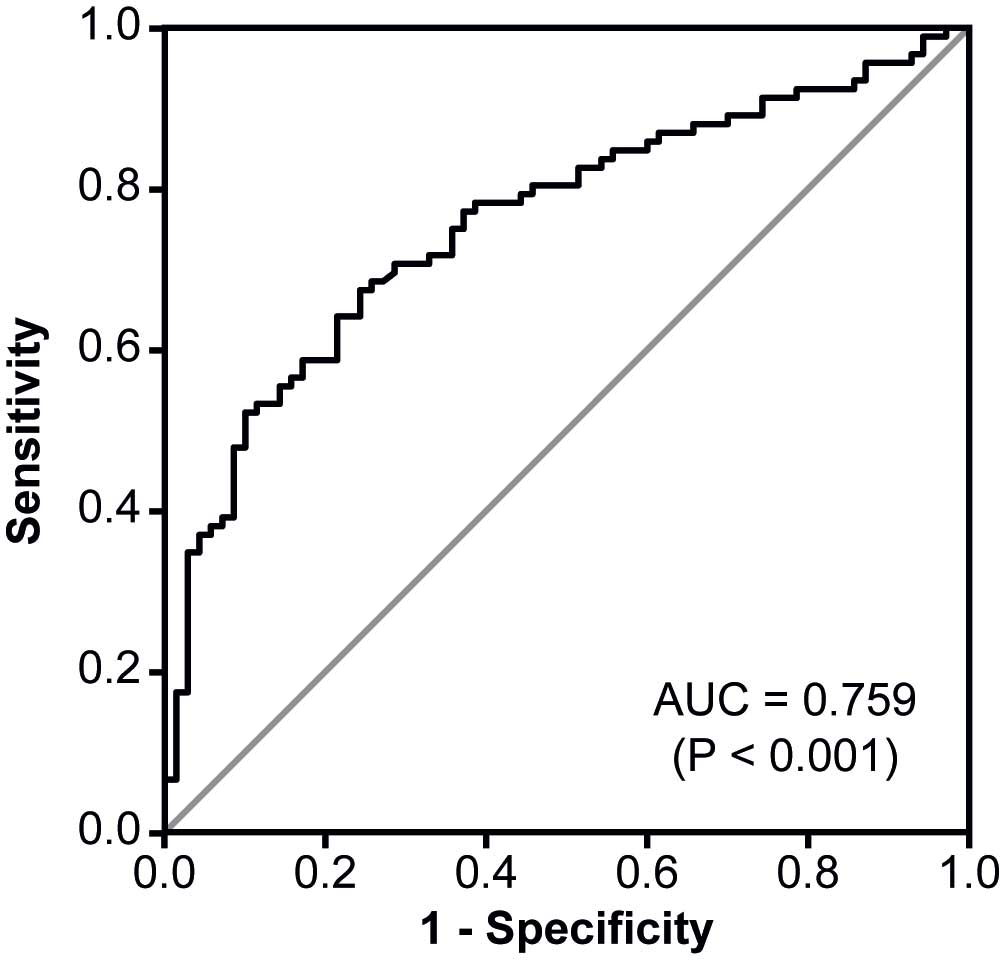
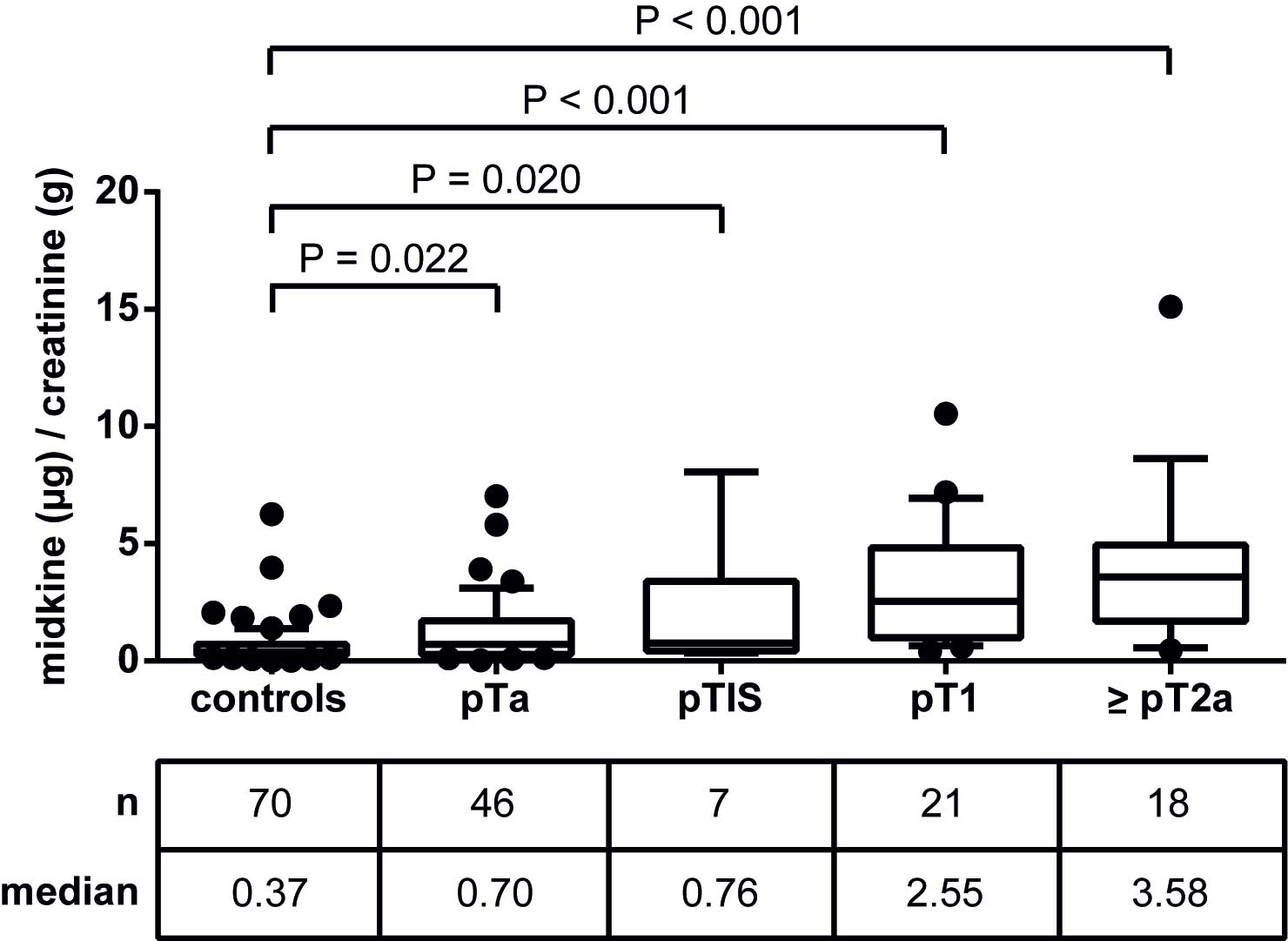
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Redorta J Palou and Rouprêt M: European Association of Urology: EAU
guidelines on non-muscle-invasive urothelial carcinoma of the
bladder: Update 2013. Eur Urol. 64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Rhijn BWG, Burger M, Lotan Y, Solsona
E, Stief CG, Sylvester RJ, Witjes JA and Zlotta AR: Recurrence and
Progression of disease in non-muscle-invasive bladder cancer: From
epidemiology to treatment strategy. Eur Urol. 56:430–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svatek RS, Hollenbeck BK, Holmäng S, Lee
R, Kim SP, Stenzl A and Lotan Y: The economics of bladder cancer:
Costs and considerations of caring for this disease. Eur Urol.
66:253–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Talwar R, Sinha T, Karan SC, Doddamani D,
Sandhu A, Sethi GS, Srivastava A, Narang V, Agarwal A and Adhlakha
N: Voided urinary cytology in bladder cancer: Is it time to review
the indications? Urology. 70:267–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raitanen MP, Aine R, Rintala E, Kallio J,
Rajala P, Juusela H and Tammela TL: FinnBladder Group: Differences
between local and review urinary cytology in diagnosis of bladder
cancer. An interobserver multicenter analysis. Eur Urol.
41:284–289. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lokeshwar VB, Habuchi T, Grossman HB,
Murphy WM, Hautmann SH, Hemstreet GP III, Bono AV, Getzenberg RH,
Goebell P, Schmitz-Dräger BJ, et al: Bladder tumor markers beyond
cytology: International Consensus Panel on bladder tumor markers.
Urology. 66(6): Suppl 1. S35–S63. 2005. View Article : Google Scholar
|
|
8
|
van Rhijn BW, van der Poel HG and van der
Kwast TH: Urine markers for bladder cancer surveillance: A
systematic review. Eur Urol. 47:736–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vrooman OP and Witjes JA: Urinary markers
in bladder cancer. Eur Urol. 53:909–916. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xylinas E, Kluth LA, Rieken M, Karakiewicz
PI, Lotan Y and Shariat SF: Urine markers for detection and
surveillance of bladder cancer. Urol Oncol. 32:222–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith ZL and Guzzo TJ: Urinary markers for
bladder cancer. F1000Prime Reports. 5:212013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakamoto K and Kadomatsu K: Midkine in the
pathology of cancer, neural disease, and inflammation. Pathol Int.
62:445–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones DR: Measuring midkine: The utility
of midkine as a biomarker in cancer and other diseases. Br J
Pharmacol. 171:2925–2939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Velculescu VE, Madden SL, Zhang L, Lash
AE, Yu J, Rago C, Lal A, Wang CJ, Beaudry GA, Ciriello KM, et al:
Analysis of human transcriptomes. Nat Genet. 23:387–388. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ikematsu S, Okamoto K, Yoshida Y, Oda M,
Sugano-Nagano H, Ashida K, Kumai H, Kadomatsu K, Muramatsu H,
Muramatsu T and Sakuma S: High levels of urinary midkine in various
cancer patients. Biochem Biophys Res Commun. 306:329–332. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Brien T, Cranston D, Fuggle S, Bicknell
R and Harris AL: The angiogenic factor midkine is expressed in
bladder cancer, and overexpression correlates with a poor outcome
in patients with invasive cancers. Cancer Res. 56:2515–2518.
1996.PubMed/NCBI
|
|
17
|
Shimwell NJ, Bryan RT, Wei W, James ND,
Cheng KK, Zeegers MP, Johnson PJ, Martin A and Ward DG: Combined
proteome and transcriptome analyses for the discovery of urinary
biomarkers for urothelial carcinoma. Br J Cancer. 108:1854–1861.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soukup V, Kalousová M, Capoun O, Sobotka
R, Breyl Z, Pešl M, Zima T and Hanuš T: Panel of urinary diagnostic
markers for non-invasive detection of primary and recurrent
urothelial urinary bladder carcinoma. Urol Int. 95:56–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sobin LH, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell;
Hoboken, NJ: pp. 262–265. 2009
|
|
20
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: World Health Organization Classification of
TumoursPathology and Genetics of Tumours of the Urinary System and
Male Genital Organs. IARC Press; Lyon: pp. 87–157. 2004
|
|
21
|
Mostofi FK, Sobin LH and Torlonie H:
Histological Typing of Urinary Bladder Tumours. 10. World Health
Organization; Geneva: pp. 171973
|
|
22
|
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis
CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC and Lijmer
JG: Standards for Reporting of Diagnostic Accuracy Group: The STARD
statement for reporting studies of diagnostic accuracy: Explanation
and elaboration. The Standards for Reporting of Diagnostic Accuracy
Group. Croat Med J. 44:639–650. 2003.PubMed/NCBI
|
|
23
|
Papanicolaou GN and Marshall VF: Urine
sediment smears as a diagnostic procedure in cancers of the urinary
tract. Science. 101:519–520. 1945. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Youden WJ: Index for rating diagnostic
tests. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lalkhen AG and McCluskey A: Clinical
tests: Sensitivity and specificity. Contin Educ Anaesth Crit Care
Pain. 8:221–223. 2008. View Article : Google Scholar
|
|
26
|
Todenhöfer T, Hennenlotter J, Witstruk M,
Gakis G, Aufderklamm S, Kuehs U, Stenzl A and Schwentner C:
Influence of renal excretory function on the performance of urine
based markers to detect bladder cancer. J Urol. 187:68–73. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konishi N, Nakamura M, Nakaoka S, Hiasa Y,
Cho M, Uemura H, Hirao Y, Muramatsu T and Kadomatsu K:
Immunohistochemical analysis of midkine expression in human
prostate carcinoma. Oncology. 57:253–257. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Davies B, Chen J, Modugno F, Weissfeld J,
Landsittel D, Dhir R, Nelson J and Getzenberg RH: Contribution of
the prostate limits the usefulness of survivin for the detection of
bladder cancer. J Urol. 174:1767–1770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boman H, Hedelin H and Holmäng S: Four
bladder tumor markers have a disappointingly low sensitivity for
small size and low grade recurrence. J Urol. 167:80–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lotan Y and Roehrborn CG: Sensitivity and
specificity of commonly available bladder tumor markers versus
cytology: Results of a comprehensive literature review and
meta-analyses. Urology. 61:109–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, He DL, Ma Q, Wan XY, Zhu GD, Li L,
Luo Y, He H and Yang L: Comparison of seven screening methods in
the diagnosis of bladder cancer. Chin Med J (Engl). 119:1763–1771.
2006.PubMed/NCBI
|
|
32
|
Holyoake A, O'Sullivan P, Pollock R, Best
T, Watanabe J, Kajita Y, Matsui Y, Ito M, Nishiyama H, Kerr N, et
al: Development of a multiplex RNA urine test for the detection and
stratification of transitional cell carcinoma of the bladder. Clin
Cancer Res. 14:742–749. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikematsu S, Nakagawara A, Nakamura Y,
Sakuma S, Wakai K, Muramatsu T and Kadomatsu K: Correlation of
elevated level of blood midkine with poor prognostic factors of
human neuroblastomas. Br J Cancer. 88:1522–1526. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sánchez-Carbayo M, Urrutia M, González de
Buitrago JM and Navajo JA: Evaluation of two new urinary tumor
markers: Bladder tumor fibronectin and cytokeratin 18 for the
diagnosis of bladder cancer. Clin Cancer Res. 6:3585–3594.
2000.PubMed/NCBI
|
|
35
|
Abd El-Hakim TF, El-Shafie MK, Abdou AG,
Azmy RM, El-Naidany SS and El-Din MO Badr: Value of urinary
survivin as a diagnostic marker in bladder cancer. Anal Quant
Cytopathol Histpathol. 36:121–127. 2014.PubMed/NCBI
|
|
36
|
Konety BR: Molecular markers in bladder
cancer: A critical appraisal. Urol Oncol. 24:326–337. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yafi FA, Brimo F, Steinberg J, Aprikian
AG, Tanguay S and Kassouf W: Prospective analysis of sensitivity
and specificity of urinary cytology and other urinary biomarkers
for bladder cancer. Urol Oncol. 33:e25–e31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye F, Wang L, Castillo-Martin M, McBride
R, Galsky MD, Zhu J, Boffetta P, Zhang DY and Cordon-Cardo C:
Biomarkers for bladder cancer management: Present and future. Am J
Clin Exp Urol. 2:1–14. 2014.PubMed/NCBI
|
|
39
|
Raab SS, Grzybicki DM, Vrbin CM and
Geisinger KR: Urine cytology discrepancies-Frequency, causes, and
outcomes. Am J Clin Pathol. 127:946–953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reid MD, Osunkoya AO, Siddiqui MT and
Looney SW: Accuracy of grading of urothelial carcinoma on urine
cytology: An analysis of interobserver and intraobserver agreement.
Int J Clin Exp Pathol. 5:882–891. 2012.PubMed/NCBI
|
|
41
|
Sullivan PS, Chan JB, Levin MR and Rao JY:
Urine cytology and adjunct markers for detection and surveillance
of bladder cancer. Am J Transl Res. 2:412–440. 2010.PubMed/NCBI
|
|
42
|
Ku JH, Godoy G, Amiel GE and Lerner SP:
Urine survivin as a diagnostic biomarker for bladder cancer: A
systematic review. BJU Int. 110:630–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Schultz IJ, Witjes JA, Swinkels DW and de
Kok JB: Bladder cancer diagnosis and recurrence prognosis:
Comparison of markers with emphasis on survivin. Clin Chim Acta.
368:20–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Srivastava AK, Singh PK, Srivastava K,
Singh D, Dalela D, Rath SK, Goel MM and Bhatt ML Brahma: Diagnostic
role of survivin in urinary bladder cancer. Asian Pac J Cancer
Prev. 14:81–85. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vriesema JL, Poucki MH, Kiemeney LA and
Witjes JA: Patient opinion of urinary tests versus flexible
urethrocystoscopy in follow-up examination for superficial bladder
cancer: A utility analysis. Urology. 56:793–797. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yossepowitch O, Herr HW and Donat SM: Use
of urinary biomarkers for bladder cancer surveillance: Patient
perspectives. J Urol. 177:1277–1282. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Todenhöfer T, Hennenlotter J, Esser M,
Mohrhardt S, Tews V, Aufderklamm S, Gakis G, Kuehs U, Stenzl A and
Schwentner C: Combined application of cytology and molecular urine
markers to improve the detection of urothelial carcinoma. Cancer
cytopathology. 121:252–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Todenhöfer T, Hennenlotter J, Guttenberg
P, Mohrhardt S, Kuehs U, Esser M, Aufderklamm S, Bier S, Harland N,
Rausch S, et al: Prognostic relevance of positive urine markers in
patients with negative cystoscopy during surveillance of bladder
cancer. BMC Cancer. 15:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eissa S, Salem AM, Zohny SF and Hegazy MG:
The diagnostic efficacy of urinary TGF-beta1 and VEGF in bladder
cancer: Comparison with voided urine cytology. Cancer Biomark.
3:275–285. 2007.PubMed/NCBI
|
|
50
|
Lokeshwar VB, Obek C, Pham HT, Wei D,
Young MJ, Duncan RC, Soloway MS and Block NL: Urinary hyaluronic
acid and hyaluronidase: Markers for bladder cancer detection and
evaluation of grade. J Urol. 163:348–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schmidt J, Propping C, Siow WY,
Lohse-Fischer A, Toma M, Baldauf-Twelker A, Hakenberg OW, Wirth MP
and Fuessel S: Diagnostic and prognostic value of bladder
cancer-related transcript markers in urine. J Cancer Res Clin
Oncol. 142:401–414. 2016. View Article : Google Scholar : PubMed/NCBI
|