Introduction
Neuroendocrine tumors (NETs) include numerous
heterogeneous types of cancers with extremely varied biological
behaviors (1–3). The literature published on the subject
commonly divides NETs into two major classes, based on their
primary origin: Pancreatic neuroendocrine (islet cell) tumors and
gastrointestinal neuroendocrine tumors (carcinoids) (4–6). In
particular, gastrointestinal NETs are usually characterized by a
slow growth pattern, are commonly diagnosed in the advanced stages
of disease (5,7–12), and
present with liver metastases in 50–75% of cases (13–15).
The frequent occurrence of liver secondaries in
patients affected by gastrointestinal NETs is a clear sign of
clinical controversy. NETs represent a rare group of neoplastic
diseases; therefore, affected patients are recommended to be
transferred into larger, more experienced centers that can more
appropriately treat rare hepatic metastasis. Furthermore, although
metastatic malignancies were once commonly considered as a terminal
neoplastic stage, numerous therapeutic options have now been
introduced in order to improve the quantity and quality of life in
patients affected by rare liver metastasis (11,16–18).
However, the role of liver surgery for patients with
metastatic gastrointestinal NETs remains an argument of great
debate; the inert growth and long term natural history of
gastrointestinal NETs makes determining the real effectiveness of
the hepatic surgical approach on overall survival (OS) even more
challenging. In particular, in an analysis of 13,715 patients
conducted by Modlin et al (3),
synchronous distant metastases were already evident in the 12.9% of
patients with gastrointestinal NETs, and demonstrated a 5-year OS
rate of 67.2%. As a consequence, the widely promulgated benignity
of these neoplasms has been brought into question, so that the
current literature recognizes their malignant potential (2,19–22).
In patients with NETs, the occurrence of hepatic
secondaries is one of the most important prognostic factors for
survival (11,23–25).
Therefore, the present study aims to evaluate the impact of
surgery, including hepatic resection and orthotopic liver
transplantation (OLT), on the outcome of patients affected by
hepatic metastases from NETs, in terms of OS.
Materials and methods
Data collection
For this multicentric retrospective study, data was
collected on 26 patients, who underwent surgery for hepatic
metastases from NETs at the Departments of Surgery of ‘Santa Maria
della Misericordia’ University Hospital (Udine, Italy) and
‘Ospedali Riuniti Umberto I, G.M. Lancisi, G. Salesi’ University
Hospital (Ancona, Italy) between January 1990 and December 2012; of
which, 22 patients underwent hepatic resective surgery and 4
underwent OLT. In the present study, only patients treated with
surgical treatment were considered; as a result, none of the
included patients underwent intraoperative treatments associated
with surgical resection.
The present study was conducted in accordance with
the Declaration of Helsinki and following the dictates of the
general authorization to process personal data for scientific
research purposes by the Italian Data Protection Authority (Rome,
Italy). Furthermore, the present study was also approved by the
Internal Review Board of the Department of Experimental and
Clinical Medical Sciences, University of Udine (Udine, Italy).
Data collection took into consideration patients'
characteristics (age at diagnosis, presence of symptoms, bioumoral
markers and imaging findings), tumor characteristics (histotype,
stage, primary tumor and secondary localizations), and treatments
(hepatic resections or OLT). The main outcome considered in the
present study was the OS of the included patients.
Patient profiling
The imaging techniques used to diagnose and stage
patients affected by NETs included chest-abdominal-pelvic
computerized tomography (CT) scans, magnetic resonance imaging
(MRI), octreoscan and positron emission tomography- computed
tomography (PET/CT) with 111In-pentetreotide. CT or PET/CT scans
obtained by initial diagnosis were also used to determine the
percentage of diseased hepatic parenchyma. In addition, hepatic
parenchyma involvement was divided into lobar and bi-lobar, and the
major diameter of the greatest hepatic metastasis was also
registered.
Every patient was investigated for the presence of
symptoms, including tachycardia or flushing. Among the bioumoral
markers of NETs, the following were routinely preoperatively
tested: Urinary vanilmandelic acid dosage in the 24 h; urinary
catecolamine dosage; urinary 5-idrossi-indolo-acetic acid dosage;
seric cromogranin A; seric neuronal specific enolase; seric
insulin; seric glucagon; seric gastrin; seric vasoactive intestinal
peptide; and seric somatostatin. To complete the preoperative
assessment of patients, an electrocardiograph and endocrinological,
oncological and anaesthesiological evaluations were also
undertaken.
Patients with unresectable hepatic metastases were
candidates for OLT, and were required to satisfy the following
inclusion criteria: Histological confirmation of NET; diffuse
unresectable hepatic disease; substitution of ≤50% hepatic
parenchyma; stable disease during the preoperative period; absent
or stable extra-hepatic disease during the preoperative period; and
hepatic insufficiency following the hepatic resection of stable
disease (rescue OLT).
For the follow-up, bioumoral markers were tested at
3, 6 and 12 months, where appropriate. The instrumental follow-up
consisted of imaging repetition after 6 and 12 months from surgery.
All patients included in the present study were monitored for at
least 12 months following surgery. For patients that were monitored
for >12 months, bioumoral markers and imaging were then assessed
yearly for the first 5 years, or more frequently in case of
recurrence suspicion.
Statistical analysis
Statistical analysis was performed using R (version
3.0.1; www.r-project.org; The R Foundation for
Statistical Computing, Vienna, Austria). Distribution normality was
evaluated through the Kolmogorov-Smirnov test. In addition, the
following statistical tests were applied where appropriate:
Student's t-test, Wilcoxon test, one way analysis of variance and
Kruskal-Wallis test for continuous variables; chi-square test and
Fisher's exact test for categorical variables. To analyze the OS of
patients, Kaplan-Meier curves were drawn, and the differences
between various groups were tested using the Log-rank test.
Results
Description of the population
In total, 26 patients, whose characteristics are
exhibited in Table I, received
surgery for hepatic metastases from NETs. The mean age at the
diagnosis of hepatic metastases was 58.04±13.05 years, 59.73±12.78
years for patients that received hepatic resections and 48.75±11.84
years for those that underwent OLT. Among the patients that
received surgery, vasomotoric symptoms were reported in 4 cases
(15.4%). Primary tumors were located in the pancreas in 8 cases
(30.8%) and in the ileum in 6 cases (23.1%).
 | Table I.Description of the population
(n=26). |
Table I.
Description of the population
(n=26).
| Characteristic | No. of patients
(%) |
|---|
| Age at diagnosis,
yearsa | 58.04±13.05 |
| Length of
follow-up, monthsb | 24
(13–58) |
| Gender, male | 12 (46.2) |
| Symptoms associated
with carcinoid syndrome | 4
(15.4) |
| Presence of
synchronous liver metastases | 14 (53.8) |
| Presence of
extra-hepatic metastasesc | 8
(30.8) |
| Lymph
nodes | 5
(62.5) |
|
Bones | 3
(37.5) |
|
Lungs | 2
(25.0) |
|
Brain | 1
(12.5) |
|
Mediastinum | 1
(12.5) |
| Surgical treatment
for primary tumor in cases with synchronous liver metastasis
(n=14) |
|
|
Gastrectomy | 3
(21.4) |
| Ileal
resection | 3
(21.4) |
| Right
hemicolectomy | 3
(21.4) |
|
Resection of the rectum | 1 (7.1) |
| Partial
pancreatectomy | 5
(35.7) |
| Surgical treatment
of liver metastasesd |
|
| Hepatic
resections | 22 (84.6) |
|
Partial right
hepatectomy | 1 (4.5) |
|
Partial left
hepatectomy | 0 (0.0) |
|
Single hepatic
segmentectomy | 1 (4.5) |
|
Multiple hepatic
segmentectomies | 1 (4.5) |
|
Wedge
excision | 20 (90.9) |
|
Orthotopic liver
transplantation | 4
(15.4) |
| Number of hepatic
wedge resectionsb | 3 (2–6) |
| Maximum tumor
diameter, cme | 6 (3–8) |
| Post-surgical
complications | 3
(11.5) |
| Localization of the
primary tumor |
|
|
Stomach | 4
(15.4) |
|
Esophagus | 1 (3.8) |
|
Duodenum | 2 (7.7) |
|
Ileum | 6
(23.1) |
|
Pancreas | 8
(30.8) |
|
Colon | 3
(11.5) |
|
Lung | 1 (3.8) |
| Unknown
primary | 1 (3.8) |
In 53.8% of cases (14/26), hepatic metastases were
synchronous, 13 cases of which were surgically treated together
with the primary tumor. For these patients, the majority of cases
received a partial pancreasectomy (35.7%, 5/14), followed in terms
of prevalence by gastrectomy (21.4%, 3/14), right emicolectomy
(21.4%, 3/14) and ileal resection (14.3%, 2/14). In one case, an
ileal resection and liver transplantation were performed in two
different surgeries. Extra-hepatic metastases were present in 30.8%
of patients (8/26), with the most secondary cases occurring in the
lymph nodes (62.5%, 5/8) and bone (37.5%, 3/8).
Characteristics of hepatic
metastases
Hepatic metastases were synchronous in the 53.8% of
cases and metachronous in the 46.2% of cases. Metachronous hepatic
secondaries were diagnosed in 80% of cases within 4 years from the
primary tumor resection, at a median time interval of 13 months
(range, 6–40 months).
The median number of hepatic metastases was 3
[interquartile range (IQR), 2–6], ranging between 1–23. The hepatic
metastases were localized in a single hepatic segment in the 15.4%
of cases (4/26), in >1 segment within the same hepatic lobe in
the 30.8% of cases (8/26), and diffused to both lobes in the 53.8%
of cases (14/26). The final group mentioned includes all four
patients that underwent OLT.
Surgical treatment of hepatic
metastases
The majority of patients (84.6%, 22/26) underwent a
hepatic resection, which, in the majority of cases, was a wedge
metastasectomy (90%, 20/22) (Table
I). In 50% of cases, >3 resections were performed. The mean
diameter of the greatest resected metastasis was 6 cm (IQR, 3–8
cm).
Complete hepatic disease resection was reached in
the 84.6% of patients (22/26), whereas residual hepatic disease was
classified as microscopic in 3 cases (11.5%) and macroscopic in one
single case (3.8%). For patients that received typical (lobectomy,
segmentectomy or pluri-segmentectomy) and atypical hepatic
resections (wedge resection or metastasectomy), complete hepatic
disease resection was reached in 66.7% (2/3) and 84.2% (16/19) of
cases, respectively. No residual disease was left in all four
transplanted patients. For patients with hepatic complete disease
resections, the 5-year OS rate was 58.4% (95% CI, 38.0–90.0%),
while the 5-year OS rate for patients with microscopic or
macroscopic hepatic residual disease the was 0% (P=0.0003).
The mean length of surgery was 4.94±0.83 h, the mean
recovery length was 10.60±4.67 days, and 71% of patients required a
recovery in the intensive care unit in the immediate post-operative
time. Post-operative complications affected 11.5% of patients
(3/26), including intra-abdominal bleeding, intra-abdominal
sero-hematic collections, pleural effusion and pancreatic fistula,
which for only 1/3 cases required a surgical re-intervention.
Another patient should be included among surgical complications, a
woman that experienced a dramatic post-operative diffuse hepatic
necrosis required an urgent rescue OLT; however, this case has been
considered among the 4 cases of OLT and resulted in no
complications following transplantation. In only 1 case of OLT
(right lobe living related liver transplantation, already presented
as a case report), liver failure was registered at the 1 year
follow-up, due to intrahepatic multiple arterio-portal fistulas
(26). The patient later succumbed
while waiting for a rescue re-transplantation, and at the time of
liver failure this patient was free of disease.
No significant differences were identified between
patients that received hepatic resections and those that underwent
OLTs (Table II). Furthermore,
considering the differences between people that survived and passed
away, only an increased prevalence of extra-hepatic metastases were
observed in patients that had passed away compared with patients
that remained alive at 5 years subsequent to surgery (P=0.064).
 | Table II.Description of the population in
patients treated with hepatic resection (n=22) or OLT (n=4). |
Table II.
Description of the population in
patients treated with hepatic resection (n=22) or OLT (n=4).
| Characteristic | Hepatic resections,
no. of patients (%) | OLT, no. of
patients (%) | P-value |
|---|
| Age at diagnosis,
yearsa | 59.73±12.78 | 48.75±11.84 |
0.161 |
| Length of
follow-up, monthsb | 26
(10–58) | 16
(14–33) |
0.670 |
| Gender, male | 12 (54.5) | 0 (0.0) |
0.100 |
| Symptoms associated
with carcinoid syndrome | 2 (9.1) | 2
(50.0) |
0.099 |
| Presence of
synchronous liver metastases | 12 (54.5) | 2
(50.0) |
0.867 |
| Presence of
extra-hepatic metastasesc | 6
(27.3) | 2
(50.0) |
0.563 |
| Lymph
nodes | 4
(66.7) | 1
(50.0) |
1.000 |
|
Bones | 2
(33.4) | 1
(50.0) |
0.673 |
|
Lungs | 2
(33.4) | 0 (0.0) |
0.346 |
|
Brain | 1
(16.7) | 0 (0.0) |
0.537 |
|
Mediastinum | 1
(16.7) | 0 (0.0) |
0.537 |
| Post-surgical
complications | 2 (9.1) | 1
(25.0) |
0.408 |
| Localization of the
primary tumor |
|
|
|
|
Stomach | 4
(18.2) | 0 (0.0) |
0.354 |
|
Esophagus | 1 (4.5) | 0 (0.0) |
0.664 |
|
Duodenum | 2 (9.1) | 0 (0.0) |
0.530 |
|
Ileum | 3
(13.6) | 3
(75.0) | <0.050 |
|
Pancreas | 7
(31.8) | 1
(25.0) |
0.786 |
|
Colon | 3
(13.6) | 0 (0.0) |
0.432 |
|
Lung | 1 (4.5) | 0 (0.0) |
0.664 |
| Unknown
primary | 1 (4.5) | 0 (0.0) |
0.664 |
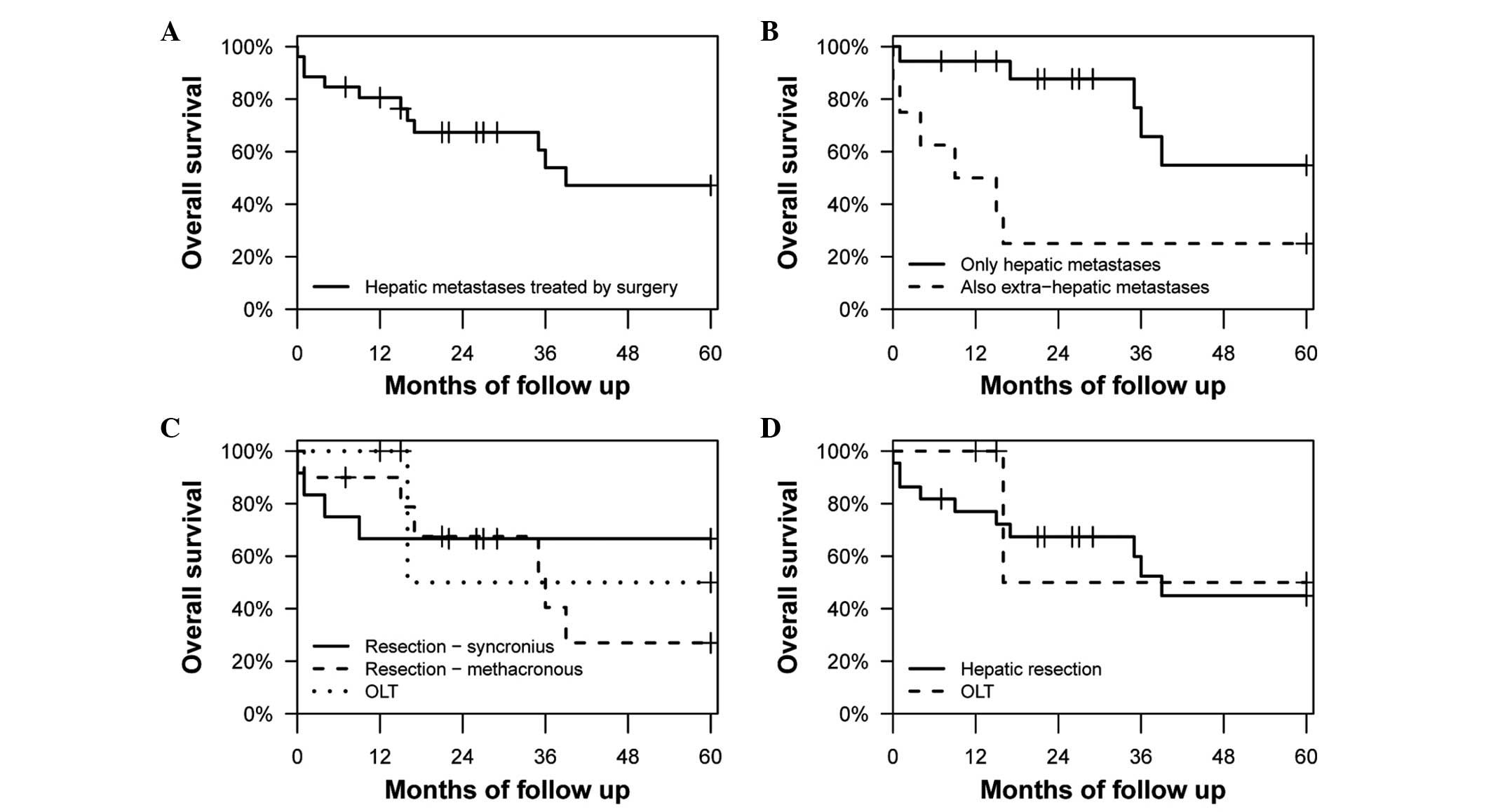
The OS rate at 5 years was 47.2% (95% CI,
28.8–77.1%) (Fig. 1A). Fig. 1B shows the OS rates of patients with
and without extra-hepatic metastases at 5 years [25.0% (95% CI,
7.5–83.0%) and 54.8% (95% CI, 31.1–96.7%), respectively], which
were found to be significantly different (P=0.012).
In Fig. 1C and D, the
OS rates were compared between patients that received OLTs and
hepatic resections, with hepatic resections subdivided into
synchronous and metachronous ones in Fig.
1C, and no significant differences were observed in either
case. In Fig. 1D the OS rates of
patients that underwent hepatic resections and OLTs were 44.9% (95%
CI, 26.0–77.7%) and 50% (95% CI, 12.5–100.0%) at 5 years,
respectively (P=0.651).
Table III evaluates
the differences between patients with synchronous and metachronous
hepatic metastases, and Table IV
compares the patients with or without extra-hepatic metastases.
Also in these cases, no significant differences have been
highlighted. Recurrences affected 57.7% of the patients studied. In
particular, among patients with a recurrence, 66.7% experienced a
hepatic recurrence and a single case was successfully re-resected,
whereas 33.3% of the patients had an extra-hepatic recurrence for
which a palliative systemic treatment was undertaken.
 | Table III.Description of the population in
patients with metachronous (n=12) or synchronous (n=14) liver
metastases. |
Table III.
Description of the population in
patients with metachronous (n=12) or synchronous (n=14) liver
metastases.
| Characteristic | Metachronous
metastases, no. of patients (%) | Synchronous
metastases, no. of patients (%) | P-value |
|---|
| Age at diagnosis,
yearsa | 60.50±11.56 | 53.07±15.43 | 0.174 |
| Length of
follow-up, monthsb | 28
(15–46) | 24
(10–56) | 0.758 |
| Gender, male | 7
(58.3) | 5
(35.7) | 0.249 |
| Symptoms associated
with carcinoid syndrome | 0 (0.0) | 4
(28.6) | 0.100 |
| Presence of
extra-hepatic metastasesc | 4
(33.3) | 4
(28.6) | 0.793 |
| Lymph
nodes | 2
(50.0) | 3
(75.0) | 1.000 |
|
Bones | 1
(25.0) | 2
(50.0) | 0.465 |
|
Lungs | 0 (0.0) | 2
(50.0) | 0.102 |
|
Brain | 1
(25.0) | 0 (0.0) | 0.285 |
|
Mediastinum | 1
(25.0) | 0 (0.0) | 0.285 |
| Surgical treatment
of liver metastasesd |
|
|
|
| Hepatic
resections | 10 (83.3) | 12 (85.7) | 0.867 |
|
Partial right
hepatectomy | 1
(10.0) | 0 (0.0) | 0.262 |
|
Partial left
hepatectomy | 0 (0.0) | 0 (0.0) | 1.000 |
|
Single hepatic
segmentectomy | 1
(10.0) | 0 (0.0) | 0.262 |
|
Multiple hepatic
segmentectomies | 0 (0.0) | 1 (8.3) | 0.350 |
|
Wedge
excision | 9
(90.0) | 11 (91.7) | 0.892 |
|
Orthotopic liver
transplantation | 2
(16.7) | 2
(14.3) | 0.867 |
| Maximum tumor
diameter, cme | 6 (4–8) | 6 (3–8) | 1.000 |
| Post-surgical
complications | 0 (0.0) | 3
(21.4) | 0.225 |
| Localization of the
primary tumor |
|
|
|
|
Stomach | 2
(16.7) | 2
(14.3) | 0.867 |
|
Esophagus | 0 (0.0) | 1 (7.1) | 0.345 |
|
Duodenum | 1 (8.3) | 1 (7.1) | 0.910 |
|
Ileum | 2
(16.7) | 4
(28.6) | 0.473 |
|
Pancreas | 4
(33.3) | 4
(28.6) | 0.973 |
|
Colon | 1 (8.3) | 2
(14.3) | 0.636 |
|
Lung | 1 (8.3) | 0 (0.0) | 0.271 |
| Unknown
primary | 1 (8.3) | 0 (0.0) | 0.271 |
 | Table IV.Differences between patients with
hepatic metastases only (n=18) and patients with hepatic and
extra-hepatic metastases (n=8). |
Table IV.
Differences between patients with
hepatic metastases only (n=18) and patients with hepatic and
extra-hepatic metastases (n=8).
| Characteristic | Hepatic metastases
only, no. of patients (%) | Hepatic and
extra-hepatic metastases, no. of patients (%) | P-value |
|---|
| Age at diagnosis,
yearsa | 56.39±15.2 | 56.75±11.91 | 0.949 |
| Length of
follow-up, monthsb | 28
(18–58) | 12 (3–28) | 0.071 |
| Gender, male | 8
(44.4) | 4
(50.0) | 0.793 |
| Symptoms associated
with carcinoid syndrome | 2
(11.1) | 2
(25.0) | 0.365 |
| Post-surgical
complications | 2
(11.1) | 1
(12.5) | 0.919 |
| Presence of
synchronous liver metastases | 10 (55.6) | 4
(50.0) | 0.793 |
| Surgical treatment
of liver metastasesc |
|
|
|
| Hepatic
resections | 16 (88.9) | 6
(75.0) | 0.365 |
|
Partial right
hepatectomy | 0 (0.0) | 1
(16.7) | 0.095 |
|
Partial left
hepatectomy | 0 (0.0) | 0 (0.0) | 1.000 |
|
Single hepatic
segmentectomy | 1 (6.2) | 0 (0.0) | 0.531 |
|
Multiple hepatic
segmentectomies | 1 (6.2) | 0 (0.0) | 0.531 |
|
Wedge
excision | 14 (87.5) | 6
(100.0) | 0.364 |
|
Orthotopic liver
transplantation | 2
(11.1) | 2
(25.0) | 0.365 |
| Maximum tumor
diameter, cmd | 6
(2–10) | 6 (4–7) | 1.000 |
| Localization of the
primary tumor |
|
|
|
|
Stomach | 3
(16.7) | 1
(12.5) | 0.786 |
|
Esophagus | 0 (0.0) | 1
(12.5) | 0.126 |
|
Duodenum | 2
(11.1) | 0 (0.0) | 0.326 |
|
Ileum | 4
(22.2) | 2
(25.0) | 0.877 |
|
Pancreas | 6
(33.3) | 2
(25.0) | 0.671 |
|
Colon | 2
(11.1) | 1
(12.5) | 0.919 |
|
Lung | 1 (5.6) | 0 (0.0) | 0.497 |
| Unknown
primary | 0 (0.0) | 1
(12.5) | 0.126 |
Discussion
Hepatic metastases were synchronous in 53.8% of
cases and metachronous in 46.2% of cases. The median number of
resected hepatic metastases was 3. Complete resection of hepatic
disease was achieved in 84.6% of cases. Recurrences were observed
in 57.7% of patients, of which 66.7% were intra-hepatic and 33.3%
extra-hepatic. Post-operative complications affected 11.5% of
patients, but required re-intervention in a single case. The OS
rates of patients that underwent hepatic resections and OLTs were
44.9% (95% CI, 26.0–77.7%) and 50.0% (95% CI, 12.5–100.0%) at 5
years following surgery, respectively.
There is currently no accordance regarding the best
therapeutic management of patients with non-resectable liver
metastases from NETs; however, numerous non-surgical treatments
have been developed in order to provide a chance of survival. The
treatments that may be employed, which are currently considered as
palliative options, include biotherapy with somatostatin analogs,
peptide-mediated radioreceptor therapy, transarterial
chemoembolisation, selective intra-arterial radiotherapy and novel
molecular target-directed therapy (6,27,28).
Currently, for cases of resectable liver metastases
from NETs, the current literature demonstrates surgery to be the
most efficient approach (4,17,18,23,24,29–32).
In addition, a potentially curative resection of liver secondaries
may be undertaken in 13.7–24.5% of patients with metastatic NETs
(33–35). However, although cytoreductive
procedures are recognized to have a crucial role among patients
with hormonal symptoms, who can consequently have a great benefit
from the reduction of secreting mass and a relief from symptoms,
the role of such aggressive surgery in case of asymptomatic disease
remains a subject of debate. In fact, the majority of patients with
liver metastases from NETs recur after hepatic resection, with
recurrence rates up to 70–94% at 5 years (7,23,25,36–39), and
the role of repeated operations remains ill-defined (37).
The survival impact of hepatic resection is
challenging to assess for several reasons. First, the patient
selection criteria for hepatic surgery differed across centers, and
the completeness of the resection was not determined clearly in
several studies. Second, numerous studies reporting the outcome of
surgical management of liver metastases from NETs focused solely on
resection rather than combined approaches, such as resection and
ablation, and the results of liver resection or other therapies
were often not determined separately. Third, as prospective
randomized data of surgical resection in metastatic neuroendocrine
tumors are lacking, recommendations have to rely on recently
published retrospective series. Fourth, the majority of studies
provide an analysis of pooled data from a mixed group of NETs,
which are of foregut, midgut and hindgut origin.
In the literature, the 5-year OS rate of patients
that have received surgery for hepatic metastases from NETs ranges
between 67–93% (29); for cases of
surgery with palliative intent, the rate is 64%, and for cases that
received conservative non-surgical treatments the rate varies
between 18–52% (29). The 5-year OS
rates of the population examined in the present study were 44.9 and
50.0% for hepatic resections and OLTs, respectively, and therefore
lower compared with the data presented in the previous literature
(29). However, this finding is
probably a consequence of the option for patients that are affected
by metachronous metastases to receive palliative resections at the
Santa Maria della Misericordia’ and ‘Ospedali Riuniti Umberto I, GM
Lancisi, G Salesi’ university hospitals. Furthermore, taking into
consideration only patients affected by synchronous metastases, the
5-year OS rate was >60%. Moreover, if the rescue OLT is
excluded, the 5-year OS rate of transplanted patients was 100%.
Finally, the present study showed that, for the differences between
hepatic resections and OLT, the results of the surgeries were
encouraging and comparable in terms of survival to those of Coppa
et al (35). In addition, in a
recent publication in Europe, the 5-year OS following OLT was found
to be 52% (40), which is in
accordance with the present results. In the same study, which
focused on OLT for liver metastases of NET, the authors found
certain predictors of poor outcome, including hepatomegaly, age
>45 years and any amount of resection concurrent with OLT
(40). By considering these poor
outcome predictors as exclusion criteria, it was possible to
achieve a 5-year OS of 60–80% (40).
However, with these strict criteria certain patients that could
benefit from OLT would be excluded (40). Furthermore, in other studies,
lengthening the diagnosis-to-OLT time interval had a positive
effect or had a no negative effect on the outcome of OLT (40,41). In
addition, contradictory statements remain to exist on the
indications of OLT with regards to the diagnosis-to-OLT interval in
liver metastases of NETs (40,41).
In accordance with the natural history and high
recurrence rates of the disease, hepatic metastases from NETs could
indicate that subclinical disease is already present, and
therefore, in the opinion of certain authors, aggressive liver
resection cannot be considered curative (15,37,42). In a
study conducted by Saxena et al (30), the majority of patients with hepatic
metastases from NETs experienced treatment failure after receiving
a liver resection. In particular, 57 patients (79%) developed
disease progression at a median time of 23 months, and the liver
accounted for the most common site for the progression of disease
(69%) (30). In another
multi-institutional study on 339 patients, Mayo et al
(37) demonstrated that the majority
of liver metastases from NETs originated as carcinoid tumors (53%)
and, at 5 years subsequent to surgery, the recurrence rate was 94%.
In the same study, according with the multivariate analysis,
synchronous disease, non-functional NET hormonal status and
extrahepatic disease were the most important predictive factors for
worse survival (37).
The current literature suggests that surgical
resection of hepatic neuroendocrine neoplasms may be associated
with favorable outcomes; however, clinical and oncological
variables to distinguish the patient cohorts that would benefit
most from such aggressive therapy have not been clearly identified.
Patients with an increased number of hepatic tumors tend to be
managed without surgical resection and patients with synchronous
disease are more likely to be treated without surgery (29).
In a previous meta-analysis, an increase in the
5-year OS rate was also observed in patients affected by hepatic
metastases from NETs that underwent hepatic surgery (29); however, randomized clinical trials are
necessary to more adequately evaluate the effect of surgery on
survival of this group of patients.
Liver metastases are frequently encountered in
patients with gastrointestinal NETs and are an important factor in
the prognosis of the patient. For patients with resectable hepatic
disease, the majority of the authors recommend the use of liver
resection, as this treatment most likely offers the best long-term
outcome. However, the number of patients that can be considered as
candidates for hepatic resection is very restricted, and the
recurrence of disease following surgery is a common occurrence.
Since no randomized clinical trial has provided
meaningful information regarding the sustained advantages of
hepatic resection, no certain conclusion on the impact of this
aggressive approach can be achieved. Therefore, further studies
comparing liver resection alone or in combination with other
therapy are recommended to be undertaken. In addition, an accurate
evaluation of novel clinical and biological parameters may be
useful to improve the identification of patients that may better
benefit from hepatic surgical therapy.
OLT appears to be safe and effective in the
treatment of selected patients and demonstrates a survival that is
comparable with patients treated by hepatic resection; however,
additional randomized clinical trials are also required on this
subject.
Glossary
Abbreviations
Abbreviations:
|
NET
|
neuroendocrine tumor
|
|
OLT
|
orthotopic liver transplantation
|
References
|
1
|
Schimmack S, Svejda B, Lawrence B, Kidd M
and Modlin IM: The diversity and commonalities of
gastroenteropancreatic neuroendocrine tumors. Langenbecks Arch
Surg. 396:273–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence B, Gustafsson BI, Chan A, Svejda
B, Kidd M and Modlin IM: The epidemiology of gastroenteropancreatic
neuroendocrine tumors. Endocrinol Metab Clin North Am. 401–18.
(vii)2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Modlin IM, Lye KD and Kidd M: A 5-decade
analysis of 13,715 carcinoid tumors. Cancer. 97:934–959. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oberg K and Castellano D: Current
knowledge on diagnosis and staging of neuroendocrine tumors. Cancer
Metastasis Rev. 30(Suppl 1): 3–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Auernhammer CJ and Göke B: Therapeutic
strategies for advanced neuroendocrine carcinomas of jejunum/ileum
and pancreatic origin. Gut. 60:1009–1021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eriksson B New drugs in neuroendocrine
tumors, . Rising of new therapeutic philosophies? Curr Opin Oncol.
22:381–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho CS, Labow DM, Tang L, Klimstra DS,
Loeffler AG, Leverson GE, Fong Y, Jarnagin WR, D'Angelica MI, Weber
SM, et al: Histologic grade is correlated with outcome after
resection of hepatic neuroendocrine neoplasms. Cancer. 113:126–134.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nikou GC, Lygidakis NJ, Toubanakis C,
Pavlatos S, Tseleni-Balafouta S, Giannatou E, Mallas E and
Safioleas M: Current diagnosis and treatment of gastrointestinal
carcinoids in a series of 101 patients: The significance of serum
chromogranin-A, somatostatin receptor scintigraphy and somatostatin
analogues. Hepatogastroenterology. 52:731–741. 2005.PubMed/NCBI
|
|
9
|
Mazzaferro V, Pulvirenti A and Coppa J:
Neuroendocrine tumors metastatic to the liver: How to select
patients for liver transplantation? J Hepatol. 47:460–466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Norton JA: Endocrine tumours of the
gastrointestinal tract. Surgical treatment of neuroendocrine
metastases. Best Pract Res Clin Gastroenterol. 19:577–583. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hellman P, Lundström T, Ohrvall U,
Eriksson B, Skogseid B, Oberg K, Janson E Tiensuu and Akerström G:
Effect of surgery on the outcome of midgut carcinoid disease with
lymph node and liver metastases. World J Surg. 26:991–997. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Janson ET, Holmberg L, Stridsberg M,
Eriksson B, Theodorsson E, Wilander E and Oberg K: Carcinoid
tumors: Analysis of prognostic factors and survival in 301 patients
from a referral center. Ann Oncol. 8:685–690. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nykjaer KM, Grønbaek H, Nielsen DT,
Christiansen P and Astrup LB: Description of patients with midgut
carcinoid tumours: Clinical database from a Danish centre. In Vivo.
21:679–684. 2007.PubMed/NCBI
|
|
14
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Fave G Delle, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomez D, Malik HZ, Al-Mukthar A, Menon KV,
Toogood GJ, Lodge JP and Prasad KR: Hepatic resection for
metastatic gastrointestinal and pancreatic neuroendocrine tumours:
Outcome and prognostic predictors. HPB (Oxford). 9:345–351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frilling A, Sotiropoulos GC, Li J,
Kornasiewicz O and Plöckinger U: Multimodal management of
neuroendocrine liver metastases. HPB (Oxford). 12:361–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landry CS, Scoggins CR, McMasters KM and
Martin RC: Management of hepatic metastasis of gastrointestinal
carcinoid tumors. J Surg Oncol. 97:253–258. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Musunuru S, Chen H, Rajpal S, Stephani N,
McDermott JC, Holen K, Rikkers LF and Weber SM: Metastatic
neuroendocrine hepatic tumors: Resection improves survival. Arch
Surg. 141:1000–1004; discussion 1005. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Turaga KK and Kvols LK: Recent progress in
the understanding, diagnosis, and treatment of
gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin.
61:113–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klimstra DS, Modlin IR, Coppola D, Lloyd
RV and Suster S: The pathologic classification of neuroendocrine
tumors: A review of nomenclature, grading, and staging systems.
Pancreas. 39:707–712. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soga J: The term ‘carcinoid’ is a
misnomer: The evidence based on local invasion. J Exp Clin Cancer
Res. 28:152009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rorstad O: Prognostic indicators for
carcinoid neuroendocrine tumors of the gastrointestinal tract. J
Surg Oncol. 89:151–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sarmiento JM, Heywood G, Rubin J, Ilstrup
DM, Nagorney DM and Que FG: Surgical treatment of neuroendocrine
metastases to the liver: A plea for resection to increase survival.
J Am Coll Surg. 197:29–37. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Norton JA, Warren RS, Kelly MG, Zuraek MB
and Jensen RT: Aggressive surgery for metastatic liver
neuroendocrine tumors. Surgery. 134:1057–1063; discussion
1063–1065. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chamberlain RS, Canes D, Brown KT, Saltz
L, Jarnagin W, Fong Y and Blumgart LH: Hepatic neuroendocrine
metastases: Does intervention alter outcomes? J Am Coll Surg. 190.
432–445. 2000.
|
|
26
|
Adani GL, Baccarani U, Risaliti A,
Sainz-Barriga M, Lorenzin D and Bresadola F: Right lobe living
related graft loss due to intrahepatic multiple arterio-portal
fistulas. Transplant Proc. 36:2733–2735. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strosberg JR, Cheema A and Kvols LK: A
review of systemic and liver-directed therapies for metastatic
neuroendocrine tumors of the gastroenteropancreatic tract. Cancer
Control. 18:127–137. 2011.PubMed/NCBI
|
|
28
|
Srirajaskanthan R, Toumpanakis C, Meyer T
and Caplin ME: Review article: Future therapies for management of
metastatic gastroenteropancreatic neuroendocrine tumours. Aliment
Pharmacol Ther. 29:1143–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bacchetti S, Bertozzi S, Londero AP, Uzzau
A and Pasqual EM: Surgical treatment and survival in patients with
liver metastases from neuroendocrine tumors: A meta-analysis of
observational studies. Int J Hepatol. 2013:2350402013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saxena A, Chua TC, Sarkar A, Chu F, Liauw
W, Zhao J and Morris DL: Progression and survival results after
radical hepatic metastasectomy of indolent advanced neuroendocrine
neoplasms (NENs) supports an aggressive surgical approach. Surgery.
149:209–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonaccorsi-Riani E, Apestegui C,
Jouret-Mourin A, Sempoux C, Goffette P, Ciccarelli O, Borbath I,
Hubert C, Gigot JF, Hassoun Z and Lerut J: Liver transplantation
and neuroendocrine tumors: Lessons from a single centre experience
and from the literature review. Transpl Int. 23:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sutton R, Doran HE, Williams EMI, Vora J,
Vinjamuri S, Evans J, Campbell F, Raraty MG, Ghaneh P, Hartley M,
et al: Surgery for midgut carcinoid. Endocr Relat Cancer.
10:469–481. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glazer ES, Tseng JF, Al-Refaie W,
Solorzano CC, Liu P, Willborn KA, Abdalla EK, Vauthey JN and Curley
SA: Long-term survival after surgical management of neuroendocrine
hepatic metastases. HPB (Oxford). 12:427–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Frilling A, Rogiers X, Malagó M, Liedke
OM, Kaun M and Broelsch CE: Treatment of liver metastases in
patients with neuroendocrine tumors. Langenbecks Arch Surg.
383:62–70. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coppa J, Pulvirenti A, Schiavo M, Romito
R, Collini P, Di Bartolomeo M, Fabbri A, Regalia E and Mazzaferro
V: Resection versus transplantation for liver metastases from
neuroendocrine tumors. Transplant Proc. 33:1537–1539. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jaeck D, Oussoultzoglou E, Bachellier P,
Lemarque P, Weber JC, Nakano H and Wolf P: Hepatic metastases of
gastroenteropancreatic neuroendocrine tumors: Safe hepatic surgery.
World J Surg. 25:689–692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mayo SC, de Jong MC, Pulitano C, Clary BM,
Reddy SK, Gamblin TC, Celinksi SA, Kooby DA, Staley CA, Stokes JB,
et al: Surgical management of hepatic neuroendocrine tumor
metastasis: Results from an international multi-institutional
analysis. Ann Surg Oncol. 17:3129–3136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yao KA, Talamonti MS, Nemcek A, Angelos P,
Chrisman H, Skarda J, Benson AB, Rao S and Joehl RJ: Indications
and results of liver resection and hepatic chemoembolization for
metastatic gastrointestinal neuroendocrine tumors. Surgery.
130:677–682; discussion 682–685. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nave H, Mössinger E, Feist H, Lang H and
Raab H: Surgery as primary treatment in patients with liver
metastases from carcinoid tumors: A retrospective, unicentric study
over 13 years. Surgery. 129:170–175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Le Treut YP, Grégoire E, Klempnauer J,
Belghiti J, Jouve E, Lerut J, Castaing D, Soubrane O, Boillot O,
Mantion G, et al: Liver transplantation for neuroendocrine tumors
in Europe-results and trends in patient selection: A 213-case
European liver transplant registry study. Ann Surg. 257:807–815.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gedaly R, Daily MF, Davenport D, McHugh
PP, Koch A, Angulo P and Hundley JC: Liver transplantation for the
treatment of liver metastases from neuroendocrine tumors: An
analysis of the UNOS database. Arch Surg. 146:953–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sartori P, Mussi C, Angelini C, Crippa S,
Caprotti R and Uggeri F: Palliative management strategies of
advanced gastrointestinal carcinoid neoplasms. Langenbecks Arch
Surg. 390:391–396. 2005. View Article : Google Scholar : PubMed/NCBI
|