Introduction
Every year there are 0.9 million new colorectal
cancer cases worldwide (1), which
accounts for 8.7% of all new cancer cases. In USA, colorectal
cancer has the second highest mortality rate after lung cancer, and
in China it ranks third.
Resectability of colorectal cancer is only indicated
in 35–48% of cases and the possibility of recurrence and metastasis
is ~55% (2). Chemotherapy is
typically the main treatment for terminal colorectal cancer.
Oxaliplatin is a third generation platinum-containing anticancer
drug after cis-platinum and carboplatin with less side
effects and better treatment effects (3). Gemcitabine (a vidarabine analogue) is a
cell cycle-specific drug with satisfactory effects on malignant
tumors of the digestive system (4).
Overexpression of high-mobility group box 1 (HMGB1) has been shown
in various cancers such as lung, colorectal and gastric tumors
(5). HMGB1 might be implicated in
tumor formation, tumor development, tumor infiltration and tumor
transfer and might influence the outcome of chemotherapy (5). HMGB1 inhibitors, such as the anti-HMGB1
antibody, soluble terminal receptor for advanced glycation
end-products (RAGE) and small interfering RNA (siRNA) can improve
the curative effect of chemotherapy through inhibition of HMGB1
expression (6). This study was
conducted to verify any possible impact that oxaliplatin combined
with gemcitabine might have on the expression of HMGB1 in terminal
colorectal cancer patients.
Patients and methods
Patients
From January 2014 to June 2015, 86 terminal
colorectal cancer patients were enrolled in this study. None of
these cases had received any type of cancer treatment prior to
their enrollment. The estimated survival time of the patients was
~12 months. After enrollment, the patients were treated with
oxaliplatin combined with gemcitabine and all patients completed
the course of treatment even those who suffered from severe side
effects. Karnofsky performance status scoring was >70 points;
hemoglobin level was ≥90 g/l, neutrophil differential count was
≥2.0×109/l, blood platelet count was
≥100×109/l; glutamic-pyruvic transaminase was within
normal range and no severe organ dysfunction was reported. The
patients included 50 males and 36 females aged from 48 to 73 years
(average age, 57.6±13.3 years). According to Dukes staging system,
57 patients were in stage C2 and 29 cases were in stage D, while
adenocarcinoma was the most common histologic type. The maximum
diameter of tumors ranged from 3.0 to 6.2 cm (average, 5.3±1.4 cm).
There were 66 cases with lymphatic metastasis, 13 cases with liver
metastasis and 7 cases with spread of cancer to the lung and other
parts of the body. This study was approved by the ethics committees
of the participating hospitals, and all patients and their family
members provided conformed consent.
Treatment method
We used the following doses of gemcitabine and
oxaliplatin: the gemcitabine dose was 1,000 mg/m2 ivgtt
in 100 ml normal saline for 30–60 min at days 1 and 8 of the cycle;
and oxaliplatin: 100 mg/m2 ivgtt for 2 h at day 2 of the
cycle. There were 21 days in each cycle and patients were treated
for 2 cycles under close observation. For severe stenosis of bowel
and adhesion, we performed minimally invasive palliative surgery
for resection and colorectal colonoscopy was conducted for tissue
sampling. All procedures were conducted following patient consent.
The follow-up visits continued for 18 months.
Observation index and testing
method
The total effective rate was analyzed based on WHO
tumor chemotherapy reference where complete remission (CR) implies
complete disappearance of the tumor, partial remission (PR) implies
that the tumor size was reduced at least by 50%, stable disease
(SD) implies that the tumor size remained unchanged and progressive
disease (PD) implies an increase in the size of the tumor by ≥25%.
CT scan examinations were conducted to measure the size of the
tumors. Immunohistochemistry was used to study the subcellular
localization of HMGB1 in the cancer tissues as well as the
para-carcinoma tissues (≥10 cm away from the tumor edge). RT-PCR
and western blotting were used to assess the mRNAand protein
expression level, respectively. Conventional method was used for
tissue preparation as follows. The samples were dewaxed using
xylene, washed with gradient alcohol, stained with hematoxylin,
dehydrated by ethyl alcohol, and the samples were sealed by neutral
resins followed by washing with phosphate-buffered saline (PBS). We
added induced antigen retrieval solution and goat serum blocking
reagent. The primary antibody was then added followed by washing
and addition of the secondary antibody (R&D Systems, Inc.,
Minneapolis, MN, USA). Samples were washed with PBS and
streptavidin peroxidase was added and then DAB color developing
agent was added after PBS washing and counterstaining was carried
out using hematoxylin. Intensity of staining (IHS) was calculated
using the following formula: IHS = A × B, where ‘A’ is the number
of positive cells and ‘B’ is the intensity of the color. Positive
expression was considered at IHS of 3.
RT-PCR. Conventional TRIzol reagent method was used
for RNA extraction and ultraviolet absorption spectroscopy method
was used to verify the concentration and purity. SuperScript™ III
Reverse Transcriptase kit (ABI, Invitrogen Life Technologies,
Carlsbad, CA, USA) was used for cDNA synthesis and Primer 3.0
online software was used to design the primers: HMGB1 (F),
5′-ATATGGCAAAAGCGGACAAG-3′ and HMGB1 (R),
5′-AGGCCAGGATGTTCTCCTTT-3′. The internal control was β-actin (F),
5′-CTCTGGCCGTACCACTGGC-3′, and β-actin (R), 5′-GTGAAGCTGTAGCCGCGC.
The reaction system used was the following: l µl of cDNA, 2 µl of
10X buffer, l µl of Mg2+, l µl assay and 0.2 µl
Taq and 14.8 µl of ddH2O. We used ABI 7500 qPCR
apparatus and the parameters were: 95°C for 2 min, 94°C for 20 sec,
60°C for 20 sec and 72°C for 30 sec for a total of 40 cycles.
Fluorescent quantitation PCR was used to monitor the expression of
HMGB1 mRNA, and Ct in all samples was tested using fold =
2−ΔΔCt to express the multiple proportion relation
between the target gene and internal control gene. Samples were
testing 3 times and the average values were recorded.
Western blot analysis. The HMGB1 antibody was
purchased from Abcam Company, the secondary antibody was obtained
from R&D Systems, Inc., and the β-actin antibody was purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Trans-blot was obtained from Bio-Rad (Berkeley, CA, USA). For
imaging, we used a gel image analysis system (UVP Co., San Gabriel,
CA, USA). Conventional RIPA lysate was used to extract total
protein, and the BCA protein quantification kit was purchased from
Pierce Biotechnology, Inc. (Rockford, IL, USA). The concentration
was assessed using a microplate reader. Proteins were separated
using SDS-PAGE, and the separated proteins were transferred and
blotted onto a polyvinylidene fluoride membrane. Next, the
membranes were blocked and chemiluminescence was performed.
Statistical analysis
We used the Statistical Package for Social Sciences
(SPSS, Inc., Chicago, IL, USA) for our statistical analyses. Data
are presented as mean ± standard deviation (SD) and proportions.
Continuous data are presented as mean ± SD. Categorical values were
evaluated using the Chi-square test. Survival was analyzed by
Kaplan-Meier method. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of the chemotherapeutic
effects
We identified 20 cases of CR, 37 cases of PR, 12
cases of SD and 17 cases of PD. The total effective rate (CR+PR)
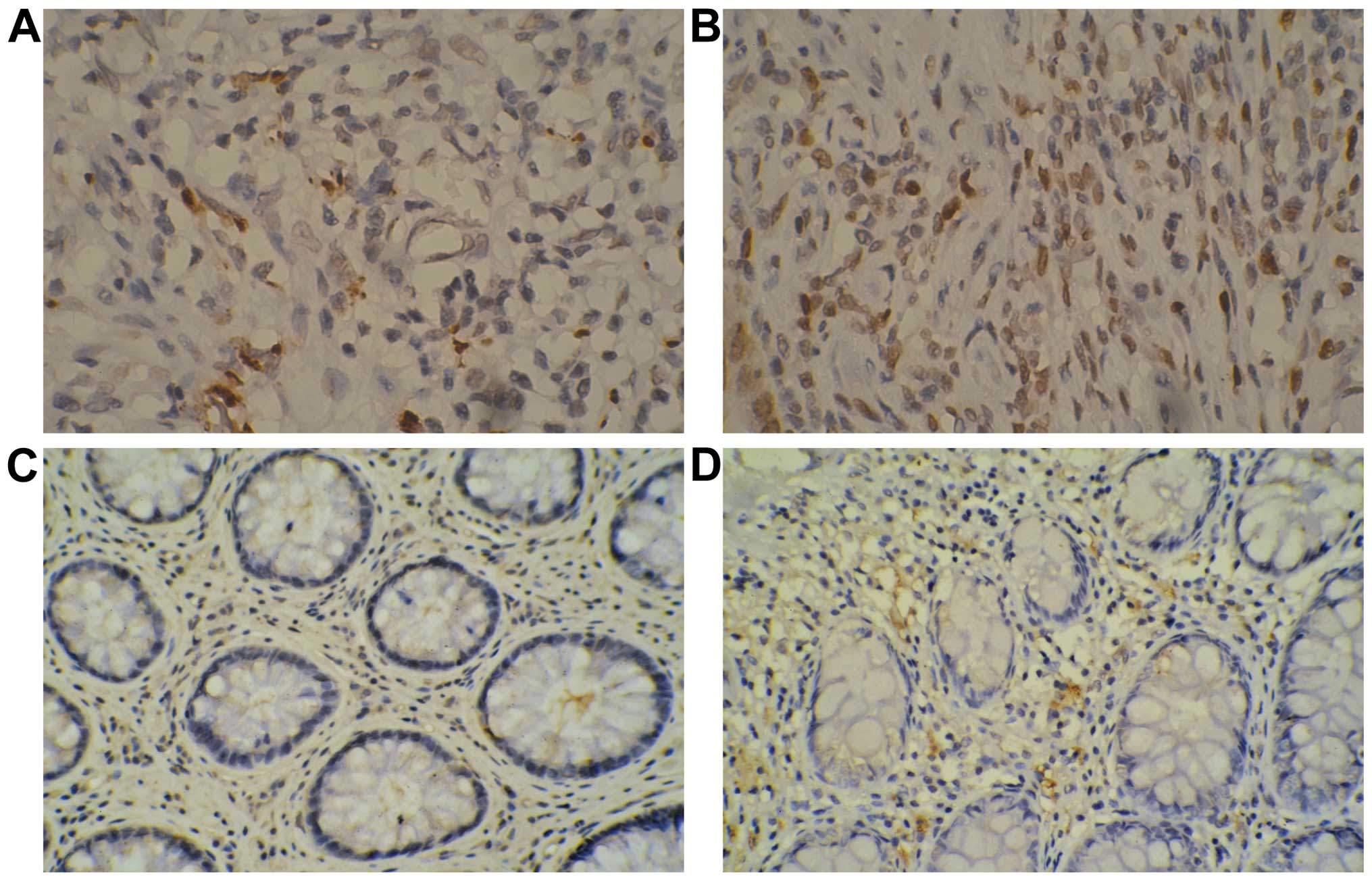
was 66.3%. Positive expression of HMGB1 protein was mainly
localized in the karyon (light yellow and dark yellow particles).
In the effective group, we observed 12 cases (21.1%) of positive
expression of HMGB1 in the cancer tissues while in the ineffective
group the number of positive cases was 12 (41.4%). HMGB1 expression
in the cancer tissues obtained from the effective group was
significantly lower than that in the ineffective group
(χ2=3.947, P=0.047). In the effective group, positive
expression of HMGB1 in the para-carcinoma tissues was detected in 5
cases (8.8%) while there were 3 cases in the ineffective group
(10.3%) and the difference was statistically significant
(χ2<0.001, P=1.000) (Fig.
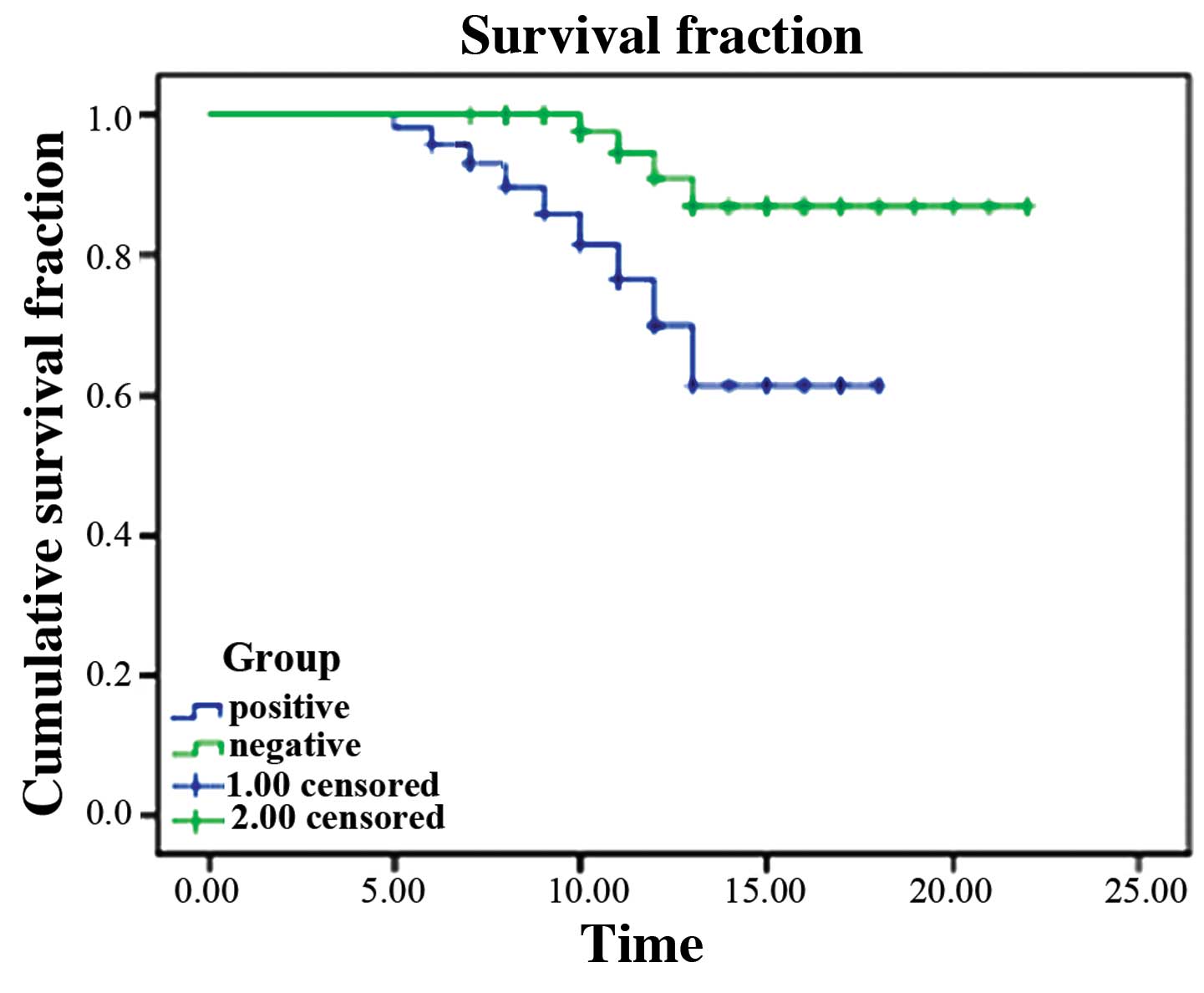
1). Survival time in the HMGB1-positive cases was obviously
shorter than that in the negative cases (log-rank test
χ2=65.384, P<0.001; Fig.
2).
Comparison of mRNA and protein
expression levels
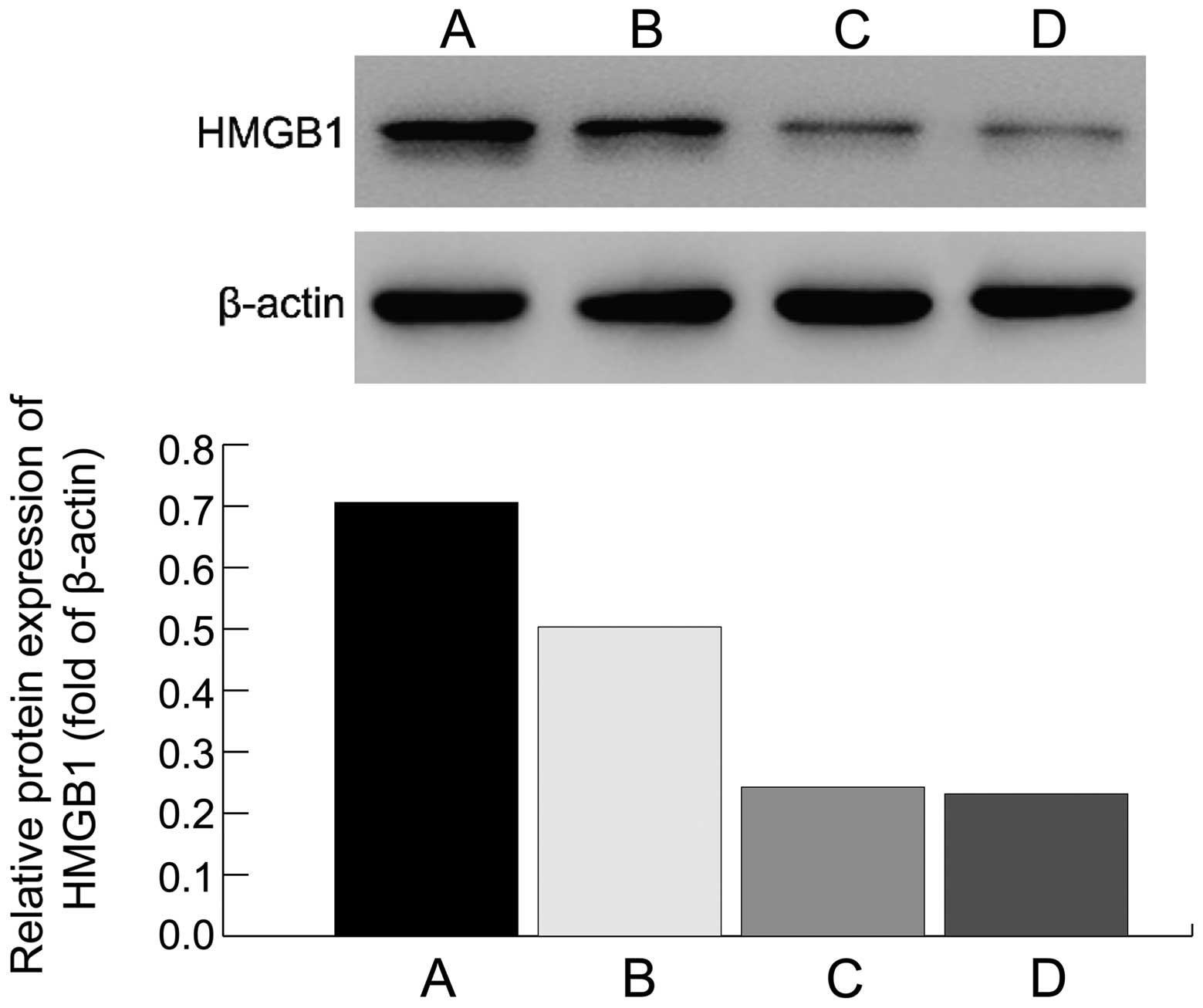
In the effective group, the HMGB1 mRNA expression
level was obviously lower than that in the ineffective group
(0.16±0.02 vs. 0.44±0.03, t=8.246, P<0.001), and also, the
protein expression level was lower in the effective group than that
in the ineffective group; differences were statistically
significant (P<0.05; Fig. 3).
Discussion
Non-histones are highly conserved proteins and major
components of chromosomes. Non-histone proteins can be divided into
three families: HMGA, HMGB and HMGN. The HMGB1 gene is located on
chromosome 13q12 and HMGB1 protein is mainly located inside the
cell nucleus attached to DNA and is involved in gene transfer,
recombination, repair, construction and stabilization of
nucleosomes (7). Extracellular HMGB1
can combine with RAGE and the Toll-like receptor. Elevated levels
of RAGE have been shown to be associated with poor prognosis in
many types of tumors (8). Following
engagement with extracellular HMGB1, RAGE internalizes and promotes
the survival and migration of cancer cells through activation of
nuclear factor (NF)-κB. This can also induce intracellular signal
transduction and mediate an inflammatory response, cell
proliferation, differentiation and metastasis (8). In colorectal cancer patients, HMGB1
overexpression has been shown to be positively correlated to tumor
infiltration, lymphatic metastasis, distant metastasis, survival
time and Dukes staging (9). After
blocking HMGB1 synthesis using siRNA, a significant reduction in
invasion ability was reported in human colorectal cancer cell line
SW620 (10). HMGB1 must be
phosphorylated for secretion and HMGB1 phosphorylation is
accomplished by protein kinase C (cPKC) and is secreted by a
calcium-dependent mechanism (10).
Results obtained from a study conducted on serum
HMGB1 levels in 219 colorectal cancer patients and 75 healthy
cases, revealed that the serum HMGB1 level in colorectal cancer
patients was increased by 1.5-fold, which was similar to the
diagnostic efficiency of carcinoembryonic antigen (CEA) in
colorectal cancer. Diagnostic accuracy of HMGB1 index in stage I
colorectal cancer was significantly better than that of CEA,
however, the serum HMGB1 level was not related to the prognosis of
colorectal cancer patients.
The functional mechanism of gemcitabine involves its
transformation into difluorodeoxygenation cytidine triphosphate
within the body through metabolism, consequently combining with
cell DNA to break it and prevent cells from progressing from G1 to
S phase of the cell cycle, thus, killing cells and inducing
apoptosis (11). Compared with
cytosine arabinoside, it is more difficult to be removed and it has
better membrane penetrability and longer residence time in cells.
It is more tolerable for patients as it has few unpleasant side
effects (4). Compared with
cis-platinum, oxaliplatin has broader antitumor activity and better
treatment effects with no cross resistance (12). In the effective group, HMGB1
expression in cancer tissues was obviously lower than that in the
ineffective group. HMGB1 expression in para-carcinoma tissues in
the two groups demonstrated very small and insignificant
differences. Survival time of the patients with HMGB1-positive
expression was shorter than that found in patients with negative
expression. In the effective group, HMGB1 mRNA and protein
expression levels were lower than those in the ineffective group
and differences were statistically significant. In conclusion, low
expression of HMGB1 in terminal colorectal cancer may be related to
treatment effects of oxaliplatin combined with gemcitabine.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacchus CM, Dunfield L, Gorber S Connor,
Holmes NM, Birtwhistle R, Dickinson JA, Lewin G, Singh H,
Klarenbach S, Mai V, et al: Recommendations on screening for
colorectal cancer in primary care. CMAJ. 22:13–15. 2016.
|
|
3
|
van Hazel GA, Heinemann V, Sharma NK,
Findlay MP, Ricke J, Peeters M, Perez D, Robinson BA, Strickland
AH, Ferguson T, et al: SIRFLOX: Randomized phase III trial
comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus
mFOLFOX6 (plus or minus bevacizumab) plus selective internal
radiation therapy in patients with metastatic colorectal cancer. J
Clin Oncol. 34:1723–1731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bai M, Deng T, Han R, Zhou L and Ba Y:
Gemcitabine plus S-1 versus cetuximab as a third-line therapy in
metastatic colorectal cancer: An observational trial. Int J Clin
Exp Med. 8:21159–21165. 2015.PubMed/NCBI
|
|
5
|
Sims GP, Rowe DC, Rietdijk ST, Herbst R
and Coyle AJ: HMGB1 and RAGE in inflammation and cancer. Annu Rev
Immunol. 28:367–388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stros M: HMGB proteins: Interactions with
DNA and chromatin. Biochim Biophys Acta. 1799:101–113. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang D, Kang R, Zeh HJ III and Lotze MT:
High-mobility group box 1 and cancer. Biochim Biophys Acta.
1799:131–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang JW, Koh EJ and Lee SM: Melatonin
protects liver against ischemia and reperfusion injury through
inhibition of toll-like receptor signaling pathway. J Pineal Res.
50:403–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao X, Zhao G, Yang H, Hong X, Bie L and
Liu G: Overexpression of high-mobility group box 1 correlates with
tumor progression and poor prognosis in human colorectal carcinoma.
J Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee H, Park M, Shin N, Kim G, Kim YG, Shin
JS and Kim H: High mobility group box-1 is phosphorylated by
protein kinase C zeta and secreted in colon cancer cells. Biochem
Biophys Res Commun. 424:321–326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hosokawa M, Saito M, Nakano A, Iwashita S,
Ishizaka A, Ueda K and Iwakawa S: Acquired resistance to decitabine
and cross-resistance to gemcitabine during the long-term treatment
of human HCT116 colorectal cancer cells with decitabine. Oncol
Lett. 10:761–767. 2015.PubMed/NCBI
|
|
12
|
Hosokawa Y, Watanabe M, Makino H, Mushiake
H, Katsumata K, Maruno K, Fujino S and Sugiyama Y: Serum type IV
collagen concentration correlates with indocyanine green retention
rate and is an indicator of hepatotoxicity in patients receiving
FOLFOX for colorectal cancer. Hepatogastroenterology. 62:653–656.
2015.PubMed/NCBI
|