Introduction
Ganoderma lucidum, also called
Ganoderma or Lingzhi, is one of most frequently used fungi
in Chinese medicine. Modern pharmacological and clinical studies
have confirmed that Ganoderma contains abundant biologically
active substances in its fruiting body, mycelia and spores, and
possesses variable functions, including immunomodulation,
anti-aging, reducing blood lipids, anti-viral and anti-tumor
activities (1–6). The present study examined the antitumor
activity of a mixture of aqueous and ethanol extracts of the
Ganoderma fruiting body and Ganoderma spores oil,
which was extracted from broken spores by supercritical
CO2 extraction technology and explored the possible
underlying mechanisms. DNA topoisomerases are a class of enzymes
involved in the regulation of DNA supercoiling. Topoisomerase
overexpression has been linked to a number of human malignancies
and is the target for numerous chemotherapeutic agents (7). In the event that topoisomerases are
blocked, the cell encounters problems during transcription of the
DNA and during cell division. The widely-used antitumor drug,
campothecin, blocks the relaxing action of class I topoisomerases
and induces significant G1 cell cycle arrest (8). A previous study indicated that the
active components of Ganoderma exhibited inhibition of
topoisomerases (9). The present study
examined whether Ganoderma extracts and spore oil affected
the cell cycle and topoisomerases I and II.
Materials and methods
Preparations of Ganoderma extracts and
spores oil
Ganoderma extracts (GanoHerb™) and
Ganoderma spores oil were provided by Fujian Xianzhilou
Biological Science and Technology Co., Ltd. (Fuzhou, China).
Ganoderma extract, a brown powder, was dissolved in double
distilled water to prepare solutions of various concentrations,
which were brown suspensions. Ganoderma spores oil was a
soft capsule with 0.5 g/0.5 ml golden oil in each capsule. The
stock solution of Ganoderma spores oil were prepared using
double distilled water that contained 6 µl/ml (v/v) Tween 80.
Cell lines and culture
The human acute myeloid leukemia HL-60, human
chronic myeloid leukemia K562, human gastric carcinoma SGC7901,
murine sarcoma S180 and murine hepatoma H22 cell lines were
purchased from the Shanghai Cell Institute of the Chinese Academy
of Sciences (Shanghai, China). All tumor cells were maintained in
RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal calf serum (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) and antibiotics (100 kU/l penicillin and
100 mg/l streptomycin) at 37°C in a humidified atmosphere of 5%
CO2.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The cell cytotoxicity of Ganoderma spores oil in
vitro was detected by MTT assay. The MTT assay was based on the
ability of live cells to utilize thiazolyl blue and convert it into
dark blue formazan. Exponentially growing cells
(2×104/well) were seeded into 96-well plates and treated
with a series of concentrations of Ganoderma spores oil
(0.31, 0.63, 1.25, 2.5, 5 and 10 mg/ml) for 24 h. Control cells
were exposed to double distilled water containing the same
concentration of Tween 80. Subsequently, 20 µl of 5 mg/ml MTT
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well and
incubated for 4 h. The dark blue formazan crystals were solubilized
in lysis buffer containing 10% sodium dodecyl sulfate (SDS), 5%
isobutyl alcohol and 12 mmol/l HCl, and the optical density (OD)
was detected at 492 nm with a spectrophotometer. The rate of growth
inhibition was calculated according to the formula: Inhibition rate
(%) = (1 - mean OD value of treated group/mean OD value of control
group) × 100. The half maximal inhibitory concentration
(IC50) value was calculated by linear regression
method.
Trypan blue exclusion method
The cell cytotoxicity of Ganoderma extract
in vitro was assessed using the trypan blue exclusion
method. Briefly, exponentially growing cells were treated with a
series of concentrations of Ganoderma extract (0.22, 0.44,
0.67, 0.89 and 1.10 mg/ml) for 24 h. Control cells were exposed to
double distilled water. Then cells were dyed with 0.4% trypan blue.
Viable (trypan blue dye-excluding) cells were counted using a light
microscope. Inhibition rate was calculated according to the
formula: Inhibition rate (%) = (1 - viable cells in treated group /
viable cells in control group) × 100.
Antitumor effects in vivo
A total of 60 ICR mice (6–8 weeks old; male and
female; body weight, 18–20 g) were obtained from the Animal Centre
of Fujian Medical University (Fuzhou, China) and maintained in
specific pathogen-free conditions. Animals were exposed to a 12 h
light/dark cycle and fed a standard diet. All animal studies were
approved by the ethics committee of Fujian Medical University. S180
or H22 tumor cells in suspension (1×106 cells in 200 µl
solution) were carefully inoculated intradermally into the left
axilla of mice. The tumor-bearing mice were then randomly assigned
to treatment experimental groups (n=10 per group). At 24 h
following the tumor inoculation, mice were intragastrically
administrated with i) extract of Ganoderma, 4 g/kg; ii)
extract of Ganoderma, 2 g/kg; iii) extract of
Ganoderma, 1 g/kg; iv) Ganoderma spores oil, 1.2
g/kg; v) fluorouracil (5-FU), 25 mg/kg (positive control); or vi)
0.9% NaCl solution 0.2 ml/10 g (negative control) once per day for
10 consecutive days. At the end of the experiments (day 12), mice
were weighed and then sacrificed by cervical dislocation. The
tumors, thymus glands and spleens of the mice were excised
carefully and weighed. The following formulae were used: i) Tumor
suppression rate (%) = (1 - mean tumor weight in treated group /
mean tumor weight in control group) × 100; ii) Thymus index = 100 ×
thymus weight / body weight; and iii) Spleen index = 100 × spleen
weight / body weight.
Catalytic activity of
topoisomerases
The catalytic activity of topoisomerases was
measured as previously described (10). K562 cells (107) were washed
with phosphate-buffered saline (PBS) and treated with lysis buffer
containing 20 mM Tris, 1 mM ethylene glycol-bis(β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid (EGTA), 25 mM KCl, 5 mM
MgCl2, 250 mM sucrose and 0.5% NP40 (pH 7.2) for 10 min
at 4°C, then centrifuged at 4,000 × g for 2 min. The pellet was
resuspended in 50 µl of extraction buffer containing 20 mM Tris, 1
mM EGTA, 2 mM ethylenediaminetetraacetic acid (EDTA), 2 mM
dithiothreitol (DTT) and 400 mM NaCl (pH 7.2) and incubated for 30
min at 4°C. After centrifugation at 22,000 × g for 15 min, the
supernatant containing extracted topoisomerases was obtained and
its protein concentration was determined using the Bradford Protein
Assay kit (Beyotime Institute of Biotechnology, Shanghai, China)
according to the manufacturer's instructions, prior to storage at
−20°C.
The catalytic activity of topoisomerase II in
Ganoderma extracts was evaluated as follows: The total
reaction volume was 10 µl, containing 2.5 µl 4X reaction buffer [50
mM Tris (pH 8.0), 120 mM KCl, 0.5 mM DTT, 0.5 mM adenosine
triphosphate (ATP), 10 mM MgCl2 and 30 µg/ml bovine
serum albumin (BSA; Beyotime Institute of Biotechnology)], 0.1 mM
ATP (Promega Corporation, Madison, WI, USA), 0.2 µg pBR322 DNA
(Promega Corporation), various amounts of topoisomerase
II-containing crude extract and double distilled water to make the
total volume up to 10 µl. Following incubation at 37°C for 30 min,
the reaction was stopped by the addition of 5 µl of a solution (50
mM EDTA, 50% glycerol and 0.25 mg/ml bromphenol blue) maintained at
4°C. Samples were separated through a 1% agarose gel. After
staining with ethidium bromide, gels were photographed under
ultraviolet (UV) illumination and analyzed using Syngene Genesnap
Tools software (version 3.00.22; Syngene; Synoptics Ltd.,
Cambridge, UK).
The catalytic activity of topoisomerase I in
Ganoderma extracts was evaluated as follows: The total
reaction volume was 10 µl, containing 1 µl 10X reaction buffer [10
mM Tris-HCl (pH 7.9), 0.15 M NaCl, 1 mM EDTA, 0.1 mM spermidine,
0.1% BSA and 5% glycerol], 0.2 µg pBR322 DNA, various amounts of
topoisomerase I-containing crude extract and double distilled water
to make the total volume up to 10 µl. The rest of the procedures
were the same as the aforementioned topoisomerase II method.
The inhibitory effects of Ganoderma extracts
and Ganoderma spores oil on topoisomerase I and II were then
detected. After selecting the amounts of crude extract that unwound
0.2 µg pBR322 DNA to establish topoisomerase I and topoisomerase II
reaction systems, 1, 2 and 4 mg/ml extracts and 4.5, 9 and 18 mg/ml
spores oil was added to each reaction system and double distilled
water was used to make the total volume up to 10 µl. Following
incubation at 37°C for 30 min, the reaction was stopped and
separated through 1% agarose gel. The staining gel was photographed
under ultraviolet (UV) illumination and analyzed using Syngene
Genesnap Tools software (version 3.00.22; Syngene).
Cell cycle analysis
The cell cycle was analyzed by flow cytometry. K562
cells were seeded in 6-well plates at a density of
1×105/ml, and treated with 1.25 and 2.5 mg/ml
Ganoderma spores oil for 12 h. Then cells were washed twice
with PBS and fixed in 70% ethanol at 4°C overnight. Prior to
analysis, cells were washed and resuspended in PBS, then incubated
with 10 mg/ml RNase A for 3–5 min and 50 µg/ml propidium iodide
(Sigma-Aldrich) at 4°C for 30 min in a dark chamber. The cell cycle
distribution (percentage of cells in various phases) was analyzed
by flow cytometry (Epics XL; Beckman Coulter, Inc., Brea, CA, USA)
using Multicycle software (version 3.11; Phoenix Flow Systems; San
Diego, CA, USA).
Western blot analysis
K562 cells (1×107) were washed with PBS
and then treated with lysis buffer containing 50 mM Tris-HCl (pH
8.0), 150 mM NaCl, 1 mM phenylmethane sulfonyl fluoride, 1 mM
aprotitin and 1% NP40 for 30 min at 4°C. Following centrifugation
at 11,000 × g for 20 min, the protein concentration of the
supernatant was determined; equal amounts (30 µg) of protein were
separated by 10% SDS-polyacrylamide gel electrophoresis and then
electro- transferred onto nitrocellulose membranes in transfer
buffer. The membrane was blocked with 5% BSA for 2 h and then
incubated with monoclonal mouse anti-human cyclin D1 (cat. no.
sc-20044; 1:500), monoclonal mouse anti-human cyclin E (cat. no.
sc-247; 1:500) and polyclonal rabbit anti-human actin (cat. no.
sc-7210; 1:2,000) primary antibodies (Santa Cruz Biotechnology
Inc., Dallas, TX, USA) in blocking solution at 4°C for 2 h,
followed by extensive washing with PBS twice. The membranes were
then incubated with alkaline-phosphatase conjugated goat anti-mouse
(cat. no. sc-2047; 1:2,500) and goat anti-rabbit (cat. no. sc-2034;
1:2,000) IgG secondary antibodies (Santa Cruz Biotechnology Inc.)
in blocking solution at room temperature, followed by washing with
Tris-buffered saline 3 times. The target proteins became visible
following the addition of Alkaline Phosphatase Substrate Solution
BCIP/NBT (Promega Corporation).
Statistical analysis
Statistical analysis of the data was performed with
the Student's t-test. Data were expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibitory effects on tumor cells in
vitro
The anti-proliferative activities of
Ganoderma extracts and spores oil on the K562, HL60 and
SGC-7901 cell lines were determined. Using the trypan blue
exclusion method, dose-dependent inhibitory effects were observed
when the Ganoderma extract concentrations were in the range
of 0.22–1.10 mg/ml. The IC50 value of Ganoderma
extracts for the K562, HL60 and SGC-7901 cells were 0.44, 0.39 and
0.90 mg/ml, respectively (Table
I).
 | Table I.Growth inhibition rate of
Ganoderma extracts on tumor cell lines. |
Table I.
Growth inhibition rate of
Ganoderma extracts on tumor cell lines.
|
| Inhibitory rate,
% |
|
|---|
|
|
|
|
|---|
| Cell lines | 1.10 mg/ml | 0.89 mg/ml | 0.67 mg/ml | 0.44 mg/ml | 0.22 mg/ml | IC50,
mg/ml |
|---|
| K562 |
97.34 | 90.55 | 85.71 | 40.48 |
9.52 | 0.44 |
| HL60 | 100.00 | 95.35 | 95.20 | 56.16 | 12.33 | 0.39 |
| SGC-7901 |
73.56 | 38.92 | 18.37 |
3.43 |
−1.64 | 0.90 |
The MTT assays revealed that treatment with
Ganoderma spores oil concentrations of 0.31–10.0 mg/ml
caused dose-dependent cytotoxicity in K562, HL60 and SGC-7901
cells, with IC50 values of 1.13, 2.27 and 6.29 mg/ml,
respectively (Table II).
 | Table II.Growth inhibition rate of
Ganoderma spores oil on tumor cell lines. |
Table II.
Growth inhibition rate of
Ganoderma spores oil on tumor cell lines.
|
| Inhibitory rate,
% |
|
|---|
|
|
|
|
|---|
| Cell lines | 10 mg/ml | 5 mg/ml | 2.5 mg/ml | 1.25 mg/ml | 0.63 mg/ml | 0.31 mg/ml | IC50,
mg/ml |
|---|
| K562 | 83.14 | 74.47 | 70.21 | 52.63 | 32.31 | 11.49 | 1.13 |
| HL60 | 89.62 | 87.76 | 55.50 | 15.50 |
8.88 |
3.54 | 2.27 |
| SGC-7901 | 68.31 | 41.27 | 12.84 |
7.64 |
−1.30 |
2.67 | 6.29 |
Suppression of tumor growth in
vivo
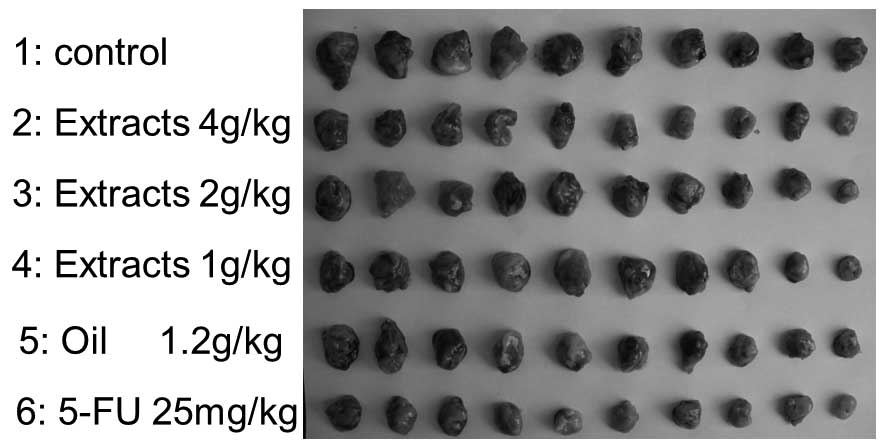
The effects of intragastric administration of
Ganoderma extracts and spores oil on S180 cells in mice were
examined (Fig. 1; Table III). Table III shows that the 4 g/kg extracts
and 1.2 g/kg oil groups resulted in significant inhibitions of
tumor growth by comparing the average tumor weight with the control
group at the end of the experiment (P=0.013 and P=0.032,
respectively); however, the inhibitory rates of the 1 and 2 g/kg
extract groups were 15.6 and 19.0%, respectively (P=0.253 and
P=0.170, respectively). The average body weights were not
significantly different among treatment groups, with the exception
of the 5-FU group (positive control).
 | Table III.Inhibitory effects of
Ganoderma extracts and spores oil on S180 in mice. |
Table III.
Inhibitory effects of
Ganoderma extracts and spores oil on S180 in mice.
|
| Body weight, g |
|
|
|---|
|
|
|
|
|
|---|
| Groupsa | Start | End | Difference | Tumor weight,
g | Inhibitory rate,
% |
|---|
| Control | 21.1±1.3 | 26.3±2.7 | 5.2±1.7 | 1.4±0.5 | – |
| Extracts, 4
g/kg | 21.6±1.2 | 26.3±2.4 | 4.7±1.8 | 0.9±0.5 |
39.1b |
| Extracts, 2
g/kg | 21.6±0.7 | 26.6±2.0 | 5.0±1.8 | 1.2±0.5 | 19.0 |
| Extracts, 1
g/kg | 21.6±0.7 | 26.3±1.6 | 4.7±1.6 | 1.2±0.4 | 15.6 |
| Oil, 1.2 g/kg | 21.1±1.0 | 25.9±2.3 | 4.8±2.2 | 1.0±0.4 |
30.9b |
| 5-FU, 25 mg/kg | 21.7±0.8 | 22.0±2.6 |
0.3±2.4c | 0.7±0.2 |
54.1c |
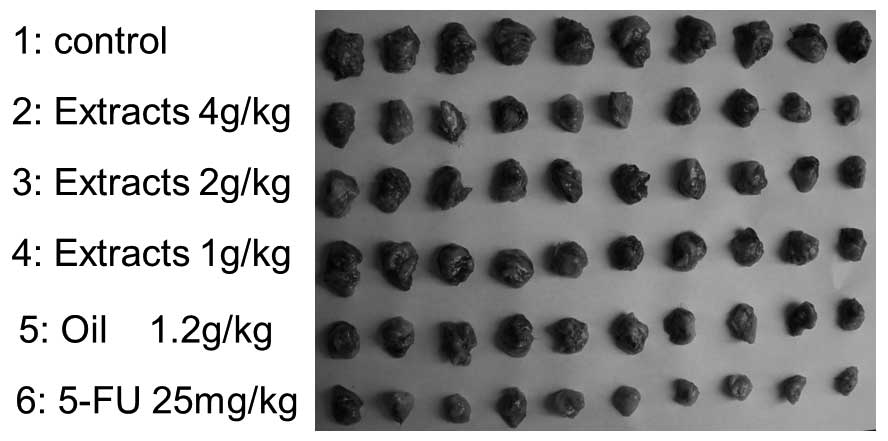
The suppressive effects of Ganoderma extracts
and spores oil on H22 cells in mice are shown in Fig. 2 and Table
IV. Data in Table IV showed that
Ganoderma extracts and spores oil exhibited relatively
strong inhibitory effects on H22 cells in mice. The inhibitory
rates of the 1, 2 and 4 g/kg extract groups on H22 were 28.8, 38.5
and 44.6%, respectively (P=0.008, P=0.008 and P=0.002,
respectively, compared with control group), and of the 1.2 g/kg oil
group was 44.9% (P=0.004). The average body weights were
significantly decreased in the 4 g/kg extracts (P=0.038) and 5-FU
(P=0.002) groups compared with the control group.
 | Table IV.Inhibitory effects of
Ganoderma extracts and spores oil on H22 in mice. |
Table IV.
Inhibitory effects of
Ganoderma extracts and spores oil on H22 in mice.
|
| Body weight, g |
|
|
|---|
|
|
|
|
|
|---|
| Groupsa | Start | End | Difference | Tumor weight,
g | Inhibitory rate,
% |
|---|
| Control | 19.9±1.0 | 29.8±1.6 | 9.9±1.7 | 2.0±0.4 | – |
| Extracts, 4
g/kg | 19.6±1.2 | 26.9±2.2 |
7.3±1.7b | 1.1±0.2 | 44.6c |
| Extracts, 2
g/kg | 19.6±1.2 | 29.2±3.4 | 9.7±3.3 | 1.2±0.3 | 38.5c |
| Extracts, 1
g/kg | 19.3±0.7 | 27.4±2.5 | 8.1±2.5 | 1.4±0.5 | 28.8c |
| Oil, 1.2 g/kg | 19.5±1.2 | 27.2±3.6 | 7.8±2.8 | 1.1±0.2 | 44.9c |
| 5-FU, 25 mg/kg | 19.1±1.1 | 25.0±2.2 |
5.9±2.1c | 0.7±0.2 | 64.8c |
Effects on immunity indexes of mice
bearing tumors
Table V shows that
Ganoderma extracts increased the immunity indexes of mice
bearing S180: The spleen index of the 1 g/kg extracts group was
significantly increased compared with the control group (P=0.041);
the thymus indexes in the 1, 2 and 4 g/kg extracts groups were all
improved significantly (P=0.029, P=0.021 and P=0.038,
respectively); while in the 5-FU group, the thymus index was
decreased significantly (P=0.002). Ganoderma spores oil
exerted no significant effects on spleen index and thymus
index.
 | Table V.Effect of Ganoderma extracts
and Ganoderma spores oil on immunity indexes of mice bearing
S180. |
Table V.
Effect of Ganoderma extracts
and Ganoderma spores oil on immunity indexes of mice bearing
S180.
| Groups | Spleen index | Thymus index |
|---|
| Control | 11.47±1.88 | 2.13±0.59 |
| Extracts, 4
g/kg | 13.31±2.89 |
2.79±0.67a |
| Extracts, 2
g/kg | 13.17±3.69 |
2.83±0.59a |
| Extracts, 1
g/kg |
13.75±1.95a |
2.92±0.75a |
| Oil, 1.2 g/kg | 12.29±2.55 | 2.51±0.85 |
| 5-FU, 25 mg/kg | 10.57±3.74 |
1.21±0.50b |
After treatment with Ganoderma extract and
spores oil, there were no significant changes in the immunity
indexes of mice bearing H22 cells (Table
VI).
 | Table VI.Effect of Ganoderma extracts
and Ganoderma spores oil on immunity indexes of mice bearing
H22. |
Table VI.
Effect of Ganoderma extracts
and Ganoderma spores oil on immunity indexes of mice bearing
H22.
| Groups | Spleen index | Thymus index |
|---|
| Control | 13.67±2.89 | 3.26±0.73 |
| Extracts, 4
g/kg | 16.48±4.25 | 2.89±0.78 |
| Extracts, 2
g/kg | 15.39±3.78 | 3.10±0.86 |
| Extracts, 1
g/kg | 14.25±3.84 | 2.86±0.61 |
| Oil, 1.2 g/kg | 14.65±4.03 | 2.60±0.78 |
| 5-FU, 25 mg/kg |
9.32±2.26a |
1.32±0.77b |
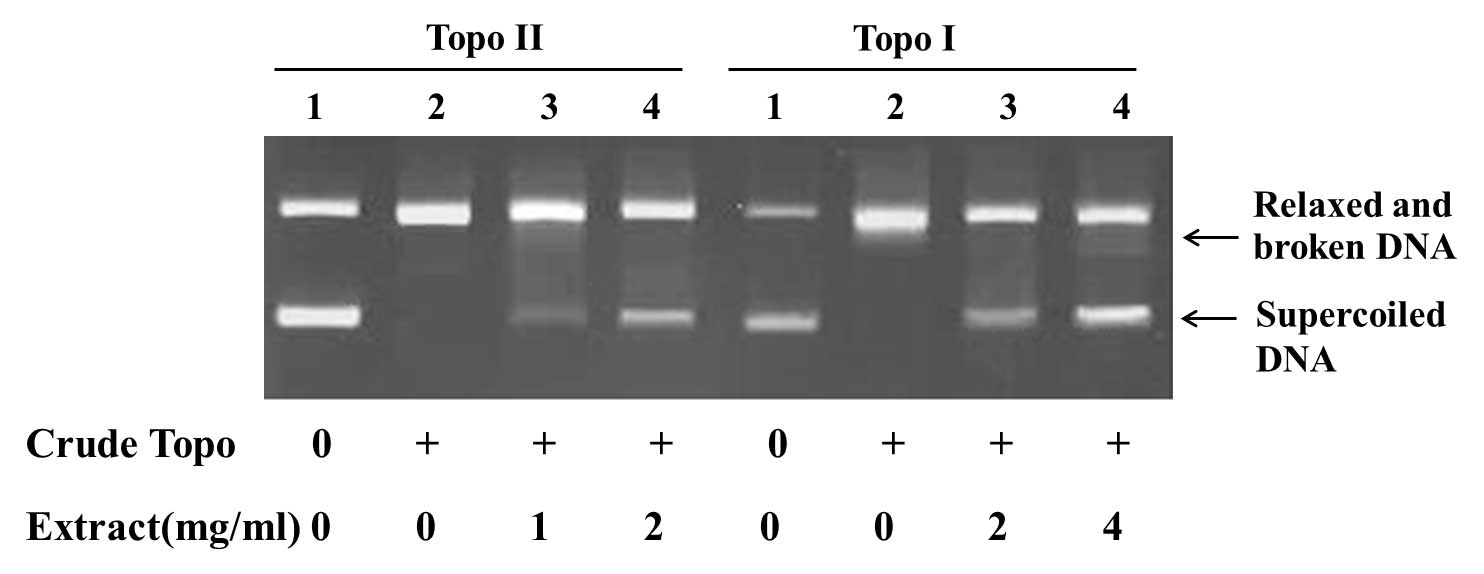
Inhibitory effects on topoisomerases
activities
The activities of topoisomerase I and II are
determined by the conversion of a fixed amount of supercoiled DNA
into relaxed and broken DNA. Topoisomerase inhibitors could
decrease the amount of relaxed and broken DNA and increased
supercoiled DNA by inhibiting the catalytic activities of
topoisomerases. The minimal concentration of crude topoisomerases
from K562 cells required to fully relax 0.2 µg pBR322 DNA was used
to test Ganoderma extracts and spores oil.
The results showed that the Ganoderma
extracts and spores oil inhibited the activities of topoisomerase I
and II. Treated with Ganoderma extracts, 2 mg/ml,
topoisomerase I and II were inhibited, as reflected by the
increased amount of supercoiled DNA and decreased amount of relaxed
and broken DNA (Fig. 3). When
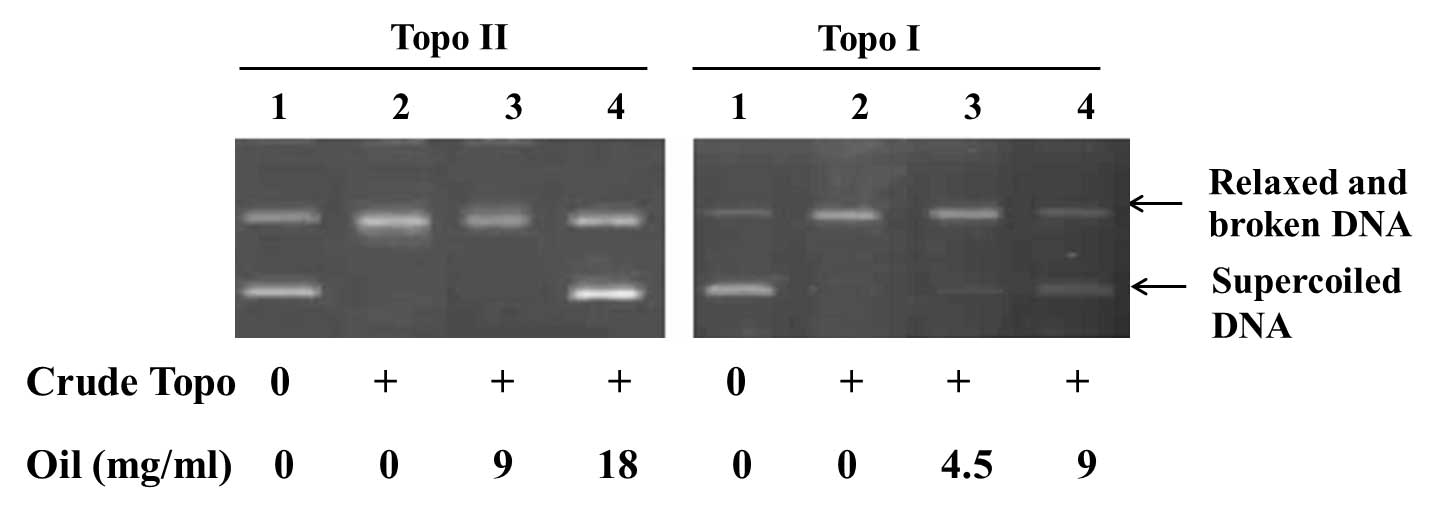
Ganoderma spores oil, 4.5 mg/ml, was added into the reaction
system of topoisomerase I, supercoiled DNA was observed. As the
concentration of Ganoderma spores oil was increased, the
inhibition of topoisomerase in dose-dependent manner was observed.
Using Ganoderma spores oil, 18 mg/ml, the activity of
topoisomerase II was then suppressed (Fig. 4).
Effect of Ganoderma spores oil on cell
cycle
The cell cycle was analyzed by flow cytometry. As
shown in Table VII,
Ganoderma spores oil induced a G1 arrest in K562 cells. With
the treatment of Ganoderma spores oil, 1.25 mg/ml, for 12 h,
the percentage of S cells in K562 was decreased from 55.7±0.6 to
42.3±0.2 (P=0.038). When the concentration of Ganoderma
spores oil was raised to 2.5 mg/ml, the percentage of S cells was
further decreased to 38.7±3.2 (P=0.017), and the percentage of G1
cells rose from 32.7±0.5 to 43.7±2.2 (P=0.022).
 | Table VII.Effects of Ganoderma spores
oil on K562 cell cycle. |
Table VII.
Effects of Ganoderma spores
oil on K562 cell cycle.
| Groupsa | G1 phase, % | S phase, % |
|---|
| Control | 32.7±0.5 | 55.7±0.6 |
| Oil, 1.25
mg/ml | 39.9±1.3 |
42.3±0.2b |
| Oil, 2.5 mg/ml |
43.7±2.2b |
38.7±3.2b |
Results of Ganoderma spores oil on
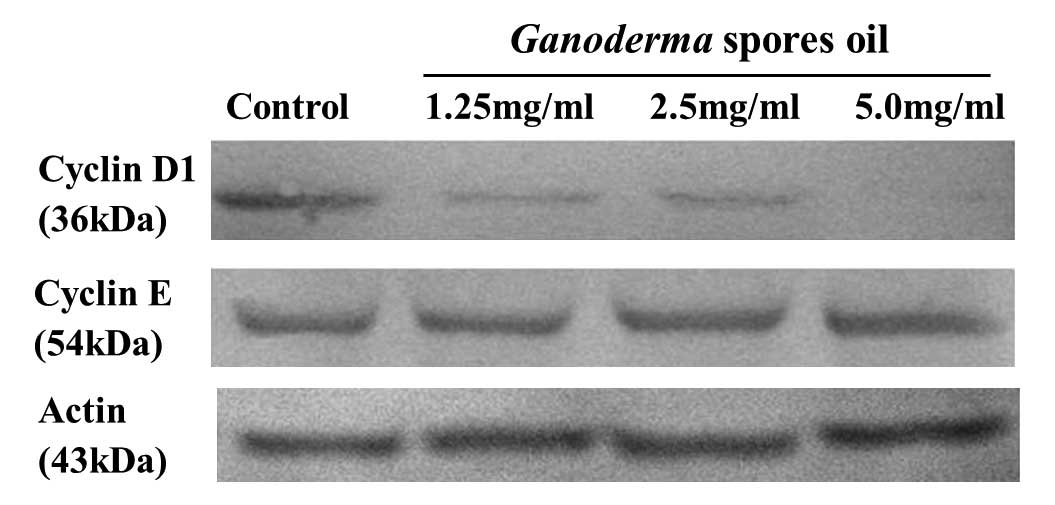
cyclin D1 and cyclin E
Cyclin D1 and E are important regulators of G1-S
phase cell cycle transition. The expression levels of cyclin D1 and
E were determined by Western blotting. The results showed that
after the treatment of Ganoderma spores oil 1.25–5.0 mg/ml
for 12 h, cyclin D1 expression in K562 decreased, but cyclin E
expression exhibited no obvious change (Fig. 5).
Discussion
Recently, the effect of Ganoderma on tumors
has been increasingly studied. The present study revealed that
Ganoderma extracts and spores oil inhibited the growth of
human leukemia cells (K562 and HL60) and human gastric carcinoma
cells (SGC-7901) in a dose-dependent manner. In addition,
Ganoderma extracts and spores significantly suppressed the
growth of the S180 and H22 transplant tumors in mice. Therefore,
Ganoderma extracts and spores oil demonstrated definite
antitumor effects in the in vitro and in vivo
studies.
Since ancient times, Ganoderma has been
widely used as a popular herbal medicine for the promotion of
health (11). Numerous previous
studies examined the immunomodulatory activities of
Ganoderma (12,13). By detecting the immunity indexes of
mice bearing S180 or H22 cells, Ganoderma extracts were
concluded to have a certain effect on improving immune function,
while Ganoderma spores oil had no significant effect on the
spleen or thymus indexes of mice. One of the main components of
Ganoderma extract is a polysaccharide that has been reported
as immune function enhancer (12–15). As
there were few polysaccharides (water-soluble substances) in the
Ganoderma spores oil, the spores oil exhibited no evident
effect on immunity. The present study also indicated that the
antitumor effects of Ganoderma may be safer compared with
5-FU, which resulted in the decreased body weight and immunity
indexes of mice (Tables I and
II).
To investigate the possible mechanism of
Ganoderma extracts and spores oil, the effects of extracts
and spores oil on topoisomerases and the effect of spores oil on
the cell cycle were examined.
DNA topoisomerases are a class of enzymes involved
in the regulation of DNA supercoiling. Type I topoisomerases change
the degree of supercoiling of DNA by causing single-strand breaks
and religation, whereas type II topoisomerases cause double-strand
breaks. These two activities are particularly crucial during DNA
transcription and replication, when the DNA helix must be unwound
to allow proper function of large enzymatic machinery. Cancer
chemotherapy takes advantage of this finding, using drugs that
block topoisomerases to kill rapidly-dividing cancer cells. For
instance, the widely-used anthracycline drugs, such as doxorubicin
and daunorubicin, attack class II topoisomerases and the plant
toxin, campothecin, blocks the relaxing action of class I
topoisomerases (16).
Li et al (9)
reported that Chinese Ganoderma lucidum essence, containing
Ganoderma licidum extracted powder and sporoderm-broken
spores powder, could inhibit the activities of topoisomerase I and
II. Consistent with these reports, the results of the present study
showed that the catalytic activities of topoisomerase I and II were
inhibited by Ganoderma extracts and spores oil. This finding
indicates that topoisomerase I and II may be target for the actions
of Ganoderma extracts and spores oil.
Extracting Ganoderma spores oil from broken
spores by supercritical CO2 extraction technology has
been developed in recent years, and several studies have since
examined the antitumor effect of Ganoderma spores oil
(17,18). In the present study, a G1 arrest was
detected in K562 cells treated with Ganoderma spores oil.
Ganoderma extracts are in a brown suspension and cannot be
detected by flow cytometry. Ganoderma spores oil mainly
contains triterpenes and fatty acids, and Tang et al
(19) reported that ganoderic acid T,
a triterpenoid chemical, markedly inhibited the proliferation of a
highly metastatic lung cancer cell line by apoptosis induction and
cell cycle arrest at the G1 phase. Therefore, the cell cycle
blocking effect of Ganoderma spores oil was assumed to
mainly be associated with triterpenes.
To the best of our knowledge, the present study
described, for first time, that the level of cyclin D1 decreased in
Ganoderma spores oil-treated K562 cells. Cyclin D1 is
critical for the G1-S transition of the cell cycle and induces G1
arrest when its level is too low. Therefore, a decrease in cyclin
D1 levels may be suggested as one of the reasons for the G1 arrest
of the K562 cell cycle, which resulted in the inhibition of cell
growth in the presence of Ganoderma spores oil. The active
substances in Ganoderma are worth studying further.
Acknowledgements
The authors gratefully acknowledge the Projects of
International Science and Technology Cooperation from the Ministry
of Science and Technology of China (grant no. 2013DFA30900) and the
Projects of Industry-Academy Cooperation for Science and Technology
of Fujian Province, China (grant no. 2016Y4005).
References
|
1
|
Kuo MC, Weng CY, Ha CL and Wu MJ:
Ganoderma lucidum mycelia enhance innate immunity by activating
NF-kappaB. J Ethnopharmacol. 103:217–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lai CS, Yu MS, Yuen WH, So KF, Zee SY and
Chang RC: Antagonizing beta-amyloid peptide neurotoxicity of the
anti-aging fungus Ganoderma lucidum. Brain Res. 1190:215–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong KJ, Dunn DM, Shen CL and Pence BC:
Effects of Ganoderma lucidum on apoptotic and anti-inflammatory
function in HT-29 human colonic carcinoma cells. Phytother Res.
18:768–770. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hajjaj H, Macé C, Roberts M, Niederberger
P and Fay LB: Effect of 26-oxygenosterols from Ganoderma lucidum
and their activity as cholesterol synthesis inhibitors. Appl
Environ Microbiol. 71:3653–3658. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang W, Tao J, Yang X, Yang Z, Zhang L,
Liu H, Wu K and Wu J: Antiviral effects of two Ganoderma lucidum
triterpenoids against enterovirus 71 infection. Biochem Biophys Res
Commun. 449:307–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loganathan J, Jiang J, Smith A, Jedinak A,
Thyagarajan-Sahu A, Sandusky GE, Nakshatri H and Sliva D: The
mushroom Ganoderma lucidum suppresses breast-to-lung cancer
metastasis through the inhibition of pro-invasive genes. Int J
Oncol. 44:2009–2015. 2014.PubMed/NCBI
|
|
7
|
Mariani A, Bartoli A, Atwal M, Lee KC,
Austin CA and Rodriguez R: Differential targeting of human
topoisomerase II isoforms with small molecules. J Med Chem.
58:4851–4856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu N, Wu XW, Agama K, Pommier Y, Du J, Li
D, Gu LQ, Huang ZS and An LK: A novel DNA topoisomerase I inhibitor
with different mechanism from camptothecin induces G2/M phase cell
cycle arrest to K562 cells. Biochemistry. 49:10131–10136. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CH, Chen PY, Chang UM, Kan LS, Fang WH,
Tsai KS and Lin SB: Ganoderic acid X, a lanostanoid triterpene,
inhibits topoisomerases and induces apoptosis of cancer cells. Life
Sci. 77:252–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruczynski A, Barret JM, Van Hille B,
Chansard N, Astruc J, Menon Y, Duchier C, Créancier L and Hill BT:
Decreased nucleotide excision repair activity and alterations of
topoisomerase II alpha are associated with the in vivo resistance
of a P388 leukemia subline to F11782, a novel catalytic inhibitor
of topoisomerases I and II. Clin Cancer Res. 10:3156–3168. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boh B, Berovic M, Zhang J and Zhi-Bin L:
Ganoderma lucidum and its pharmaceutically active compounds.
Biotechnol Annu Rev. 13:265–301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S, Nie S, Huang D, Li W and Xie M:
Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26
tumor-bearing mice. Food Chem. 136:1213–1219. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsin IL, Ou CC, Wu TC, Jan MS, Wu MF, Chiu
LY, Lue KH and Ko JL: GMI, an immunomodulatory protein from
Ganoderma microsporum, induces autophagy in non-small cell lung
cancer cells. Autophagy. 7:873–882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu XL and Lin ZB: Effects of Ganoderma
lucidum polysaccharides on proliferation and cytotoxicity of
cytokine-induced killer cells. Acta Pharmacol Sin. 26:1130–1137.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Gao H, Chan E, Tang W, Xu A, Yang
H, Huang M, Lan J, Li X, Duan W, et al: Antitumor activity and
underlying mechanisms of ganopoly, the refined polysaccharides
extracted from Ganoderma lucidum, in mice. Immunol Invest.
34:171–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofland K, Petersen BO, Falck J, Helin K,
Jensen PB and Sehested M: Differential cytotoxic pathways of
topoisomerase I and II anticancer agents after overexpression of
the E2F-1/DP-1 transcription factor complex. Clin Cancer Res.
6:1488–1497. 2000.PubMed/NCBI
|
|
17
|
Peng X, Liu J, Xia J, Wang C, Li X, Deng
Y, Bao N, Zhang Z and Qiu M: Lanostane triterpenoids from Ganoderma
hainanense J. D. Zhao. Phytochemistry. 114:137–145. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu YN and Zhong JJ: Impacts of calcium
signal transduction on the fermentation production of antitumor
ganoderic acids by medicinal mushroom Ganoderma lucidum. Biotechnol
Adv. 30:1301–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang W, Liu JW, Zhao WM, Wei DZ and Zhong
JJ: Ganoderic acid T from Ganoderma lucidum mycelia induces
mitochondria mediated apoptosis in lung cancer cells. Life
Sciences. 80:205–211. 2006. View Article : Google Scholar : PubMed/NCBI
|