Introduction
Oral epithelial dysplasia (OED) is the diagnostic
histopathological term used to describe an oral preneoplastic
lesion (OPL), and is predictive of an increased rate of developing
oral squamous cell carcinoma (OSCC) (1). However, assessing the risk of the
malignant transformation of OPLs is challenging. Early diagnosis of
high-risk, potentially malignant lesions is a high priority for
reducing morbidity and mortality (1–3). Studies
with a median follow-up of >7 years have reported malignant
transformation rates between 17 and 20% (4). Although lesions with dysplastic features
are considered to be at an increased risk for malignant
transformation, the majority of the oral cancers develop from
lesions that lack dysplastic changes (4,5).
Therefore, objective biomarkers are required to evaluate the risk
of malignant transformation in OPL, and for the prophylactic
intervention and proper management of high risk patient groups.
ELAV like RNA binding protein 1 (HuR) is an
ubiquitously expressed mRNA-binding protein. Intracellularly, HuR
is localized predominantly in the nucleus, but shuttles between the
nucleus and the cytoplasm (6). The
export of HuR is mediated by the association with transportin 1 and
2, via the HuR nucleocytoplasmic shuttling sequence in the hinge
region, and the association with the acidic nuclear phosphoprotein
32 family member A, a proliferation-inducing ligand and the SET
nuclear proto-oncogene α/β protein, which includes the nuclear
export signal recognized by the export receptor chromosome
maintenance region 1 (6–8). AU-rich elements (ARE) are located in the
untranslated regions of numerous proto-oncogenes, growth factors
and cytokine mRNAs as the core sequence of AUUUA. HuR binds to AREs
to protect ARE-mRNAs against rapid degradation. As
nucleocytoplasmic translocation is necessary for the activity of
HuR and the cytoplasmic presence of HuR is indicated in several
carcinomas, cytoplasmic HuR expression is hypothesized to be a
prognostic marker in cancer patients (9,10).
Podoplanin is a mucin-type transmembrane
glycoprotein that is specifically expressed in lymphatic
endothelial cells, but not in blood endothelial cells (11). Podoplanin has been identified as a
potential marker for the progression of oral leukoplakia to
invasive carcinoma (12–14). Wicki and Christofori (15) suggested that podoplanin may act as a
mediator of tumor cell invasion and metastasis. Podoplanin is also
expressed in the hyperplastic and dysplastic regions that are
adjacent to primary tumors, which indicates that the abnormal
expression of podoplanin occurs early in oral tumorigenesis
(16). In addition, in oral
premalignant lesions certain molecular genetic traits are in common
with OSCC, even in the absence of histologically defined dysplasia
(17). Therefore, the purpose of the
present study was to investigate the potential association between
HuR and podoplanin expression in OPL, with or without malignant
transformation, and to determine the usefulness of the proteins as
biomarkers for cancer risk assessment.
Materials and methods
Patients and tissue specimens
All medical records of the 51 patients that were
diagnosed with OPL between March 2001 and May 2012 at Hokkaido
University Hospital (Sapporo, Japan) were retrieved and reviewed at
the Department of Oral Pathology and Biology, Hokkaido University
Graduate School of Dental Medicine (Sapporo, Japan). The clinical
data were obtained from the medical records and biopsy specimens
were obtained from formalin-fixed paraffin-embedded tissues. The
expression of HuR and podoplanin were determined in 51 patients
with OPL during the follow-up period using immunohistochemistry.
Associations between the protein expression patterns and
clinicopathological parameters, including oral cancer development,
during the follow-up were analyzed statistically. In the present
retrospective follow-up study, malignant transformation vs.
nontransformation was considered as the surrogate for the clinical
outcome of patients with OPL. Of the 51 patients with a median
follow-up of 55 months, 24 patients (47%) developed OSCC. The
present study was approved by the institutional review board.
Tissue processing and
immunohistochemistry
Serial tissue sections (5 µm thick) from
formalin-fixed, paraffin-embedded tissue blocks of OPL were mounted
on positively charged glass slides. Immunohistochemical staining
was performed using the streptavidin-peroxidase methods, as
previously described (18). In brief,
sections were deparaffinized in xylene, rehydrated in graded
alcohol and subjected to antigen retrieval by heat treatment in
Tris-ethylenediaminetetraacetic acid (TE) buffer. In order to
inhibit endogenous peroxidase activity, the slides were then
immersed in 3% hydrogen peroxide (Sigma-Aldrich, St. Louis, MO,
USA) for 5 min, followed by blocking solution [1% bovine serum
albumin (Sigma-Aldrich) in phosphated-buffered saline (PBS)] for 30
min. The immunohistochemical detection of HuR and podoplanin were
performed using anti-HuR (mouse immunoglobulin G anti-human; clone
3A2; dilution, 1:5,000; catalog no., sc-5261; catolog no., sc-5261;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
anti-podoplanin (mouse anti-human; clone, D2-40; dilution, 1:100;
catalog no., M3619; Vector Laboratories, Inc., Burlingame, CA, USA)
monoclonal antibodies, respectively, in blocking solution in a
humidified chamber at 4°C overnight. The sections were then
subjected to Simple Stain Max PO (M) (Nichirei Bioscience, Tokyo,
Japan) at 37°C for 30 min. Carefully, rinses were performed with
several changes of PBS between the stages of the procedure (5
minute washes repeated 3 times). Visualization was performed using
the ChemMate EnVision kit/HRP (Dako North America, Inc.,
Carpinteria, CA, USA). Cytoplasmic and cell membrane
immunoreactivity in the epithelium was considered to indicate the
reaction of HuR and podoplanin, respectively.
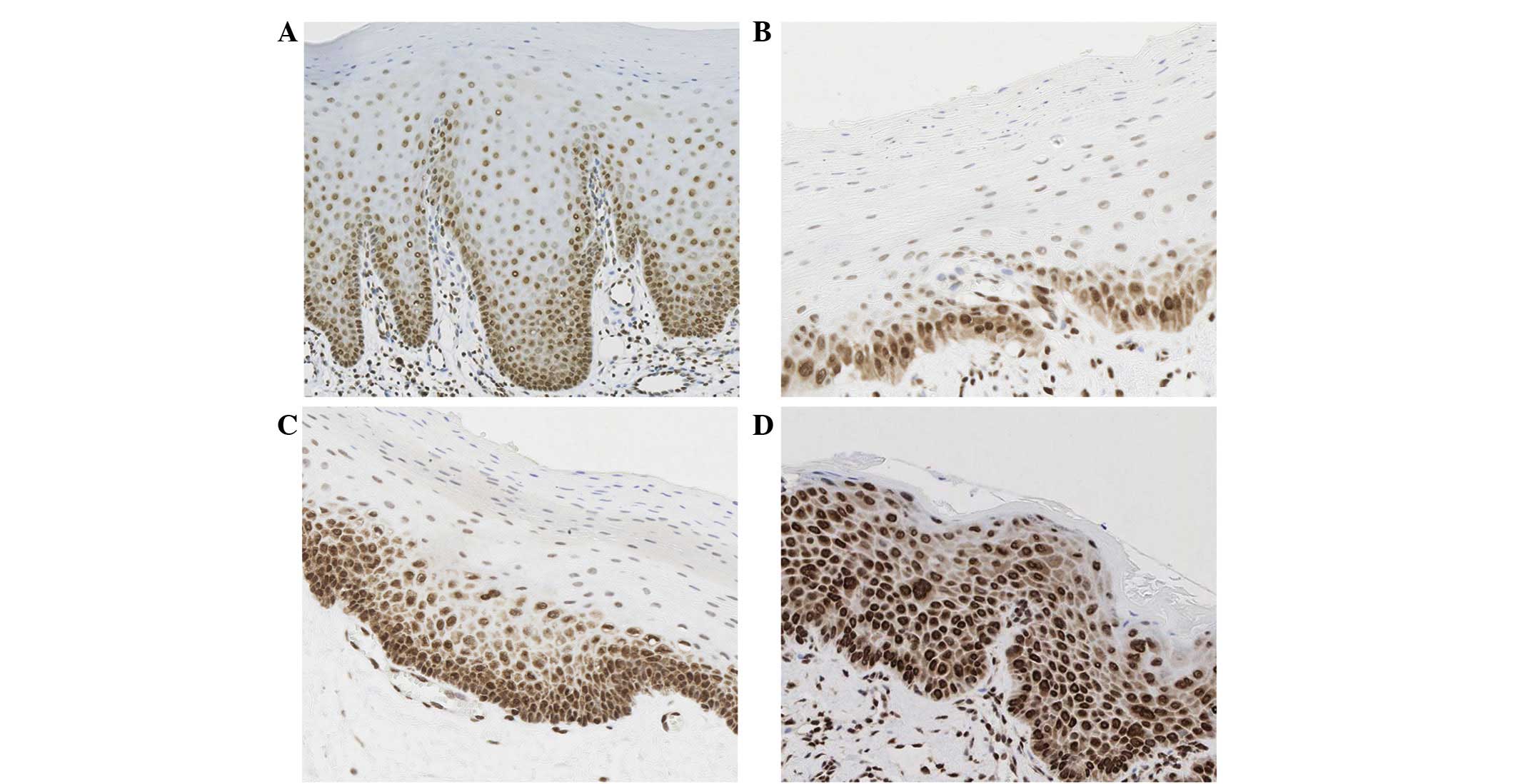
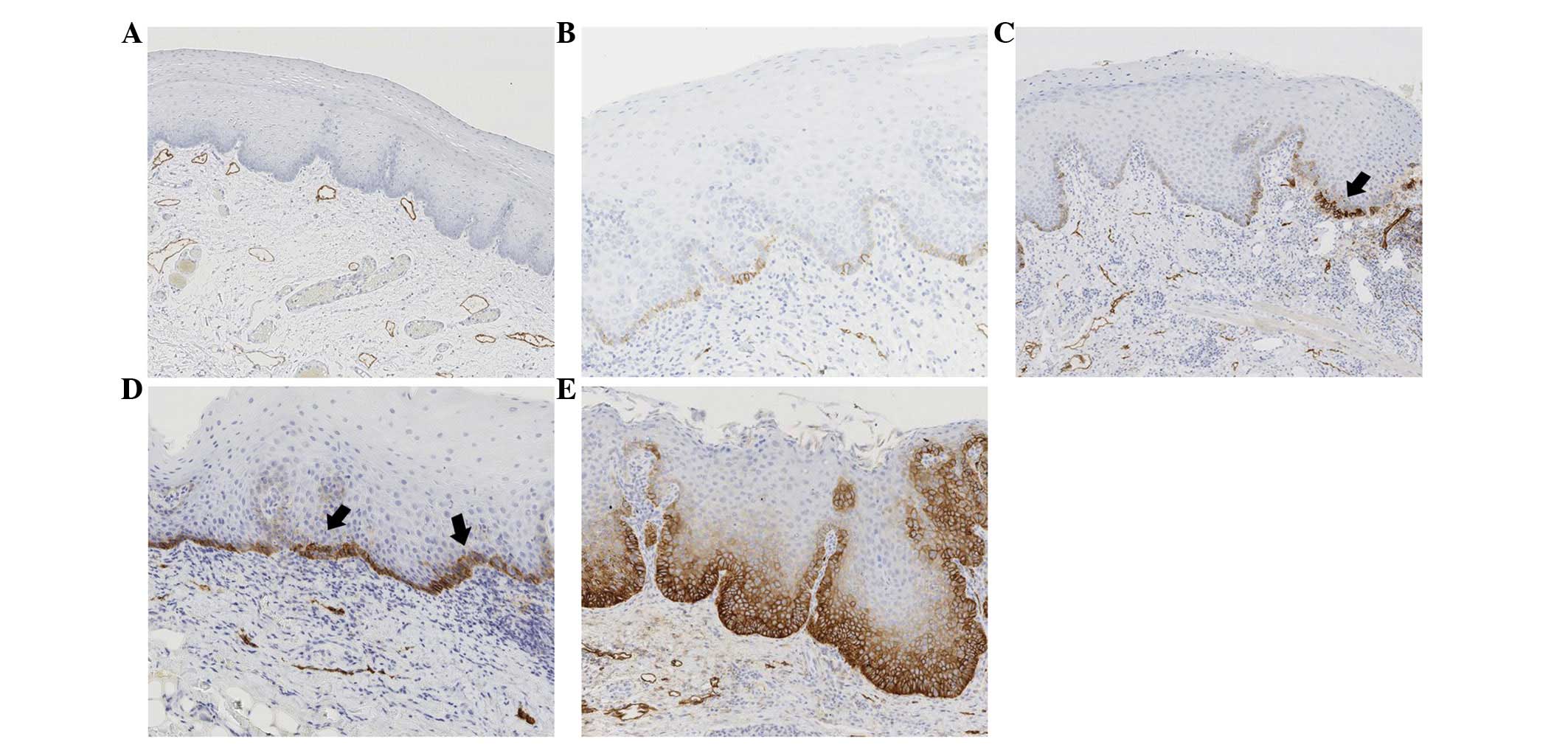
In order to analyze the prognostic values for cancer
development, the immunoreactivity of HuR and podoplanin was
classified into two categories (Figs.
1 and 2). According to the
criteria that were described in a previous study (18), the distribution of the HuR protein in
the cell was categorized into the three levels of the epithelium:
Level 1, lower one-third; level 2, lower two-thirds; and level 3,
extending to the upper one-third of the epithelium. A sample
evaluation of the immunoreactivity of podoplanin was performed
according to the criteria based on staining score, previously
described by Kawaguchi et al (14). In accordance with these criteria,
scores between 0–4 were determined. No expression resulted in the
score 0, podoplanin expression limited to the basal layer was
scored 1, expression in the basal layer and suprabasal layers in
one region was scored 2 and podoplanin expression at 2–3 suprabasal
regions was scored 3. Podoplanin expression observed in >3 areas
in the suprabasal layer was scored 4. The scores were based on the
examination of the entire section in each biopsy. Lesions that were
classified with level 2 and 3 distributions (positive expression up
to lower two-thirds and upper one-third, respectively) were
considered to express HuR, and lesions with scores of ≥2 (positive
expression in the suprabasal layer in 1 or more areas) were
considered to express podoplanin. Accordingly, 28 (55%) and 36
(71%) of lesions were considered to express HuR and podoplanin,
respectively, and the remaining 23 (45%) and 15 (29%) were not.
Statistical analysis
Associations between HuR and podoplanin expression
and the clinicopathological variables were assessed using the
Wilcoxon rank-sum test for continuously distributed variables and
the χ2 test for categorical variables. The Kaplan-Meier
survival analysis was used to investigate the associations with
oral cancer-free survival (OCFS) time, which is the time interval
between the histopathological diagnosis and the development of
OSCC. Patients that did not develop invasive OSCC were censored at
the final date of follow-up. The log-rank test was used to compare
survival times among patients with different characteristics. The
Cox proportional hazards regression model was applied to evaluate
the hazard ratio (HR) for the malignant transformation of OPLs. HRs
with a 95% confidence interval (CI) and the P-values were reported.
All tests were two sided, and P-values of <0.05 were considered
to indicate a statistically significant difference. The
JMP® Pro version 10.0.2 (SAS Institute Inc., Cary, NC,
USA) was used for statistical analysis.
Results
Patient characteristics and HuR and
podoplanin expression
The present study comprised of 51 patients, of which
39 were female and 12 were male. HuR and podoplanin was not
expressed in 45% and 29% of the patients, whereas expression was
observed in 55% and 71% of the patients, respectively (Table I). The expression patterns of HuR and
podoplanin in serial tissue samples of OPLs are shown in Figs. 1 and 2.
With regards to HuR, 23 (45.1%), 19 (37.3%) and 9 (17.6%) cases of
OPL showed level 1, level 2 and level 3 expression within the
epithelium, respectively; for podoplanin expression, 5 (9.8%)
samples were scored as 0, 10 (19.6%) were scored as 1, 9 (17.6%)
were scored as 2, 6 (11.8%) were scored as 3 and 21 (41.2%) were
scored as 4.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| Characteristic | No. of patients
(%) |
|---|
| All patients | 51
(100.0) |
| Age, years |
|
| Mean ±
SD | 70.9±10.2 |
|
Median | 72 |
| Gender |
|
|
Female | 39 (76.5) |
| Male | 12 (23.5) |
| Follow-up,
months |
|
| Mean ±
SD | 42.2±35.6 |
|
Median | 43 |
| Site |
|
|
Tongue | 21 (41.2) |
|
Gingiva | 8
(15.7) |
| BM | 14 (27.5) |
| FOM | 4 (7.8) |
|
Others | 4 (7.8) |
| Dysplasia |
|
| LGD | 17 (33.3) |
| HGD | 34 (66.7) |
| HuR expression |
|
| Level
1 | 23 (45.1) |
| Level
2 | 19 (37.3) |
| Level
3 | 9
(17.6) |
| Podoplanin
expression |
|
| Score
0 | 5 (9.8) |
| Score
1 | 10 (19.6) |
| Score
2 | 9
(17.6) |
| Score
3 | 6
(11.8) |
| Score
4 | 21 (41.2) |
| Malignant
transformation |
|
|
Yes | 24 (47.1) |
| No | 27 (52.9) |
The association between HuR and podoplanin
expression and the clinicopathological parameters are summarized in
Table II. HuR and podoplanin
expression was significantly associated with grades of dysplasia
(P<0.05). While 71 and 82% of OPLs with HGD were associated with
HuR and podoplanin expression, 29 and 18% of the patients with HGD
were not associated with HuR and podoplanin expression,
respectively. However, 23 and 47% of patients with OPLs with LGD
exhibited the expression of HuR and podoplanin, respectively. No
significant association between protein expression and age, gender
or site of the lesion was observed.
 | Table II.Association between HuR and
podoplanin expression and clinicopathological parameters. |
Table II.
Association between HuR and
podoplanin expression and clinicopathological parameters.
|
|
| HuR expression |
| Podoplanin
expression |
|
|---|
|
|
|
|
|
|
|
|---|
|
Characteristics | No. of
patients | Not expressed
(level 1), n (%) | Expressed (levels 2
& 3), n (%) | P-value | Not expressed
(scores 0–1), n (%) | Expressed (scores
2–4), n (%) | P-value |
|---|
| All patients | 51 | 23 (45) | 28 (55) |
| 15 (29) | 36 (71) |
|
|---|
| Age, years |
|
|
| 0.676 |
|
| 0.702 |
|
Mean |
| 72±12 | 70±09 |
| 72±13 | 71±09 |
|
|
Median |
| 75 | 71 |
| 75 | 71 |
|
|
Max/Min |
| 90/50 | 82/55 |
| 90/50 | 82/54 |
|
| Gender |
|
|
| 0.349 |
|
| 0.067 |
|
Female | 39 | 19 (49) | 20 (51) |
| 14 (36) | 25 (64) |
|
|
Male | 12 | 4
(33) | 8
(67) |
| 1 (8) | 11 (92) |
|
| Site |
|
|
| 0.497 |
|
| 0.393 |
|
Tongue | 21 | 8
(38) | 13 (62) |
| 5
(24) | 16 (76) |
|
|
Gingiva | 8 | 3
(38) | 5
(63) |
| 2
(25) | 6
(75) |
|
| BM | 14 | 9
(64) | 5
(36) |
| 6
(43) | 8
(57) |
|
|
FOM | 4 | 1
(25) | 3
(75) |
| 0 (0) | 4
(100) |
|
|
Others | 4 | 2
(50) | 2
(50) |
| 2
(50) | 2
(50) |
|
| Dysplasia |
|
|
| 0.001 |
|
| 0.009 |
|
LGD | 17 | 13 (76) | 4
(23) |
| 9
(53) | 8
(47) |
|
|
HGD | 34 | 10 (29) | 24 (71) |
| 6
(18) | 28 (82) |
|
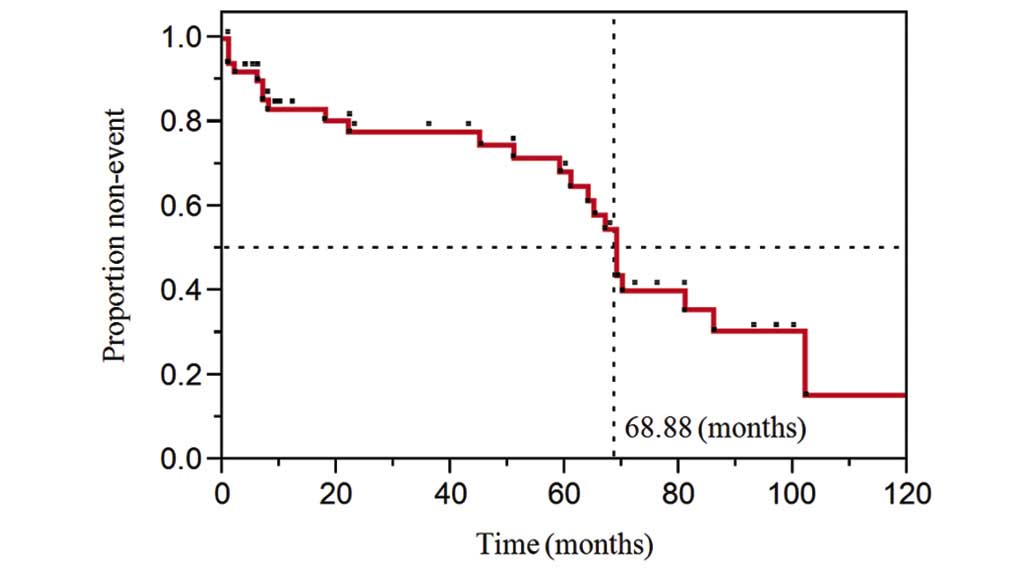
Median follow-up times were 43 months for the
patients that did not develop an OSCC. During the follow-up period,
24/51 patients (47%; 18 female and 6 male), developed OSCC.
Malignant transformation occurred at a median of 55 months
subsequent to diagnosis with a premalignant lesion. The estimated
time for malignant transformation was 69 months from the primary
diagnosis for 50% of the patients (Fig.
3). The calculated annual transformation rate was 8.7%.
HuR and podoplanin expression and the
risk of oral cancer
One of the primary aims of the present study was to
determine whether protein expression (HuR and podoplanin) in a
premalignant lesion is a feasible parameter to predict the
patient's clinical outcome in terms of malignant transformation.
Therefore, in order to estimate the time to malignant
transformation of OPL, the OCFS time was assessed by the
Kaplan-Meier method, using clinicopathological factors and HuR and
podoplanin expression. In this analysis, HuR and podoplanin
expression and the grade of dysplasia were established to be
significant indicators using the log-rank test. All findings are
summarized in Table III and
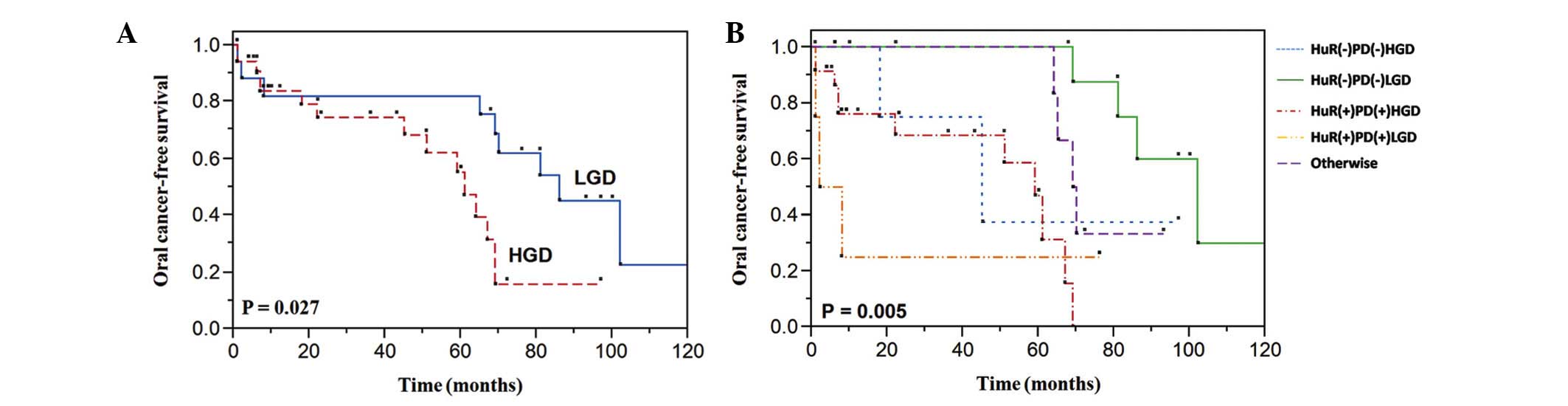
presented in Figs. 4 and 5. Patients demonstrating HuR (Fig. 4A) and podoplanin (Fig. 4B) expression experienced a
significantly increased oral cancer incidence compared with
patients that did not express the proteins (P=0.001 and P=0.006,
respectively). The results showed that 58 and 75% of the OPLs
expressing HuR and podoplanin became malignant, respectively, and
the expression demonstrates a stepwise pattern of malignant
transformation (Fig. 4C and D;
Table III). In addition, a
statistically significant association existed between the
histopathological grade and the risk of progression from dysplasia
to oral cancer (P=0.027) (Fig. 5A).
In order to determine whether protein expression augments oral
cancer risk, the combination of HuR and podoplanin expression and
the degree of dysplasia in OPLs was also analyzed. The lesions that
expressed the two proteins turned malignant in a significantly
shorter period, regardless of histopathology (P=0.005) (Fig. 5B; Table
III).
 | Table III.Univariate survival analysis by
significant prognostic factors using Kaplan-Meier modeling. |
Table III.
Univariate survival analysis by
significant prognostic factors using Kaplan-Meier modeling.
| Prognostic
factors | No. of
patients | Patients without
malignant transformation, n (%) | Patients with
malignant transformation, n (%) | Mean survival time,
months, standard error (95% CI) | P-value |
|---|
| HuR |
|
|
|
| 0.001 |
|
Negative (level 1) | 23 | 13 (57) | 10 (43) | 85.8,
7.1 (72.1–99.7) |
|
|
Positive (levels 2–3) | 28 | 14 (50) | 14 (50) | 43.8,
6.1 (32.1–55.5) |
|
| Podoplanin |
|
|
|
| 0.006 |
|
Negative (score 0–1) | 15 | 9
(60) | 6
(40) | 89.3,
8.9 (71.7–106.8) |
|
|
Positive (score 2–4) | 36 | 18 (50) | 18 (50) | 53.1,
5.9 (41.5–64.8) |
|
| HuR level |
|
|
|
| 0.000 |
| Level
1 | 23 | 13 (57) | 10 (43) | 85.8,
7.1 (71.9–99.7) |
|
| Level
2 | 19 | 11 (58) | 8
(42) | 49.9,
7.2 (35.8–63.9) |
|
| Level
3 | 9 | 3
(33) | 6
(67) | 31.6,
6.4 (54.9–80.1) |
|
| PDS |
|
|
|
| 0.020 |
|
PDS-0 | 5 | 3
(60) | 2
(40) | 92.4,
17.4 (58.3–126.5) |
|
|
PDS-1 | 10 | 6
(60) | 4
(40) | 79.9,
7.0 (66.2–93.7) |
|
|
PDS-2 | 9 | 6
(67) | 3
(33) | 72.0,
6.2 (59.8–84.2) |
|
|
PDS-3 | 6 | 2
(33) | 4
(67) | 44.8,
13.7 (17.9–71.7) |
|
|
PDS-4 | 21 | 10 (48) | 11 (52) | 43.3,
6.9 (29.8–56.8) |
|
| Dysplasia |
|
|
|
| 0.027 |
|
LGD | 17 | 8
(47) | 9
(53) | 78.4,
10.2 (58.5–98.3) |
|
|
HGD | 34 | 19 (56) | 15 (44) | 53.9,
6.2 (41.7–66.1) |
|
| HuR and PD
expression and histopathology |
|
|
|
| 0.005 |
|
HuR+ PD+
HGD | 23 | 12 (52) | 11 (48) | 45.5,
6.3 (33.1–58.1) |
|
|
HuR+ PD+
LGD | 4 | 1
(25) | 3
(75) | 21.7, 15.7
(0.0–52.5) |
|
|
HuR− PD−
HGD | 5 | 3
(60) | 2
(40) | 57.7,
17.9 (22.5–92.9) |
|
|
HuR− PD−
LGD | 9 | 5
(56) | 4
(44) | 98.2,
7.0 (84.5–112.0) |
|
|
Otherwisea | 10 | 6
(60) | 4
(40) | 76.1,
5.1 (65.7–85.6) |
|
To evaluate the oral cancer risk in patients with
OPLs, clinicopathological parameters and HuR and podoplanin
expression were analyzed using the Cox proportional hazards model
(Table IV). For the univariate
analysis, the expression of HuR (HR, 4.99; 95% CI, 1.93–14.01;
P=0.001), podoplanin (HR, 4.01; 95% CI, 1.49–12.92; P=0.005) and
the grade of dysplasia (HR, 2.75; 95% CI, 1.11–7.32; P=0.029) were
significantly associated with an increased risk of malignant
transformation. A multivariate analysis was performed in order to
assess the factors that had a significant impact on the OCFS time
in the univariate analysis, including histology, podoplanin and HuR
expression. For the multivariate analysis, the adjusted HR for
malignant transformation was 2.93 for HuR expression (95% CI,
0.98–10.34; P=0.055). Notably, when the histology and coexpression
of HuR and podoplanin were considered as cofactors, the risk of the
malignant transformation of OPLs was considerably increased
compared with OPLs without coexpression and histology (HR, 5.79;
95% CI, 1.64–23.59; P=0.005).
 | Table IV.Cox proportional hazard regression
models in estimating cancer development. |
Table IV.
Cox proportional hazard regression
models in estimating cancer development.
|
Characteristics | Hazard ratio | 95% CI | P-value |
|---|
| Univariate
analysis |
|
|
|
| Age,
≤71 vs. >71 years | 1.18 | 0.51–2.65 | 0.696 |
| Sex,
male vs. female | 1.38 | 0.49–3.36 | 0.051 |
|
Histology, HGD vs. LGD | 2.75 | 1.11–7.32 | 0.029 |
|
Podoplanin, expressed vs. not
expressed | 4.01 |
1.49–12.92 | 0.005 |
| HuR,
expressed vs. not expressed | 4.99 |
1.93–14.01 | 0.001 |
| Multivariate
analysis, histology, podoplanin and HuR |
|
|
|
|
Histology, HGD vs. LGD | 1.45 | 0.52–4.36 | 0.486 |
|
Podoplanin, expressed vs. not
expressed | 2.06 | 0.55–8.01 | 0.283 |
| HuR,
expressed vs. not expressed | 2.93 |
0.98–10.34 | 0.055 |
| Multivariate
analysis, histology, coexpression of podoplanin and HuR |
|
|
|
|
Histology, HGD vs. LGD | 1.47 | 0.52–4.44 | 0.477 |
| Podoplanin and
HuR |
|
|
|
|
Both | 5.79 |
1.64–23.59 | 0.005 |
|
Either | 1.78 | 0.41–7.31 | 0.424 |
Discussion
The present study aimed to determine the usefulness
of HuR and podoplanin in predicting the risk of malignant
transformation in patients with OPL. Dysplasia and protein
expression (HuR and podoplanin) were indicated to be significant
predictors for malignant transformation in OPL (Table III).
Increasing numbers of studies regarding the human
ELAV-like protein HuR are being undertaken, as HuR regulates the
mRNA stability of numerous growth-promoting genes (8). As increased cytoplasmic HuR expression
has been detected in human malignant tumors, a deregulated HuR
pathway has been suggested to have implications in cancer biology,
by promoting the abnormal expression of several proteins (10). Cytoplasmic HuR expression has been
indicated in colon, ovary, breast, salivary gland, uterus, larynx
and prostate malignancies, and has been postulated to contribute to
the cancerous malignant phenotype (19–23). In
agreement with previous studies, the present study also indicated
that HuR may be used as a diagnostic marker for oral cancer
(24). A recently published study
(18) reported the significant role
of HuR in assessing the risk of malignant transformation in
patients with oral verrucous lesions. The present study reported
that HuR expression in OPL was associated with an increased risk of
malignant transformation (P<0.05).
Podoplanin as a cancer stem cell marker has been
reported to be expressed in approximately 90% of OSCCs and
restricted to the invasive front of squamous cell carcinoma
(15). Podoplanin was also reported
to be overexpressed in the basal cell layers of certain
hyperplastic and dysplastic regions located adjacent to OSCC
(25–27). Kawaguchi et al (14) reported podoplanin as a marker of
malignant transformation in oral leukoplakia and other oral
precancerous lesions. The present results are in agreement with
Kawaguchi's observation, suggesting that podoplanin expression in
OPL is associated with an increased risk of malignant
transformation (P=0.006). Other studies also support that
podoplanin has a relevant role in early oral tumorigenesis, even
considering that podoplanin expression alone may not be sufficient
to promote carcinogenesis (12–15).
The present study included podoplanin and performed
immunohistochemical staining of HuR and podoplanin to evaluate oral
cancer risk in patients with OPL. In the univariate analysis, HuR
and podoplanin expression was associated with a 4.99-fold and
4.01-fold increased risk of malignant transformation, respectively
(P<0.05). HuR and podoplanin expression in OPL showed a
significant impact on OCFS, with a decreasing 5-year OCFS rate of
100%, for patients with no expression, and of 35 and 42% for
patients with increased HuR (level 3) and podoplanin (score 4)
expression, respectively (P<0.05) (Fig. 4C and D; Table III). Additionally, in the
multivariate analysis, a significant difference in the coexpression
of HuR and podoplanin and histological features was indicated to be
associated with the malignant transformation of OPL (P<0.05).
Overall, these data support the potential importance of HuR and
podoplanin in oral carcinogenesis and also suggest that each may be
used as biomarkers for evaluating malignant transformation risk in
oral premalignancy. However, contrary to the previous findings, 10
(42%) and 6 (25%) patients without HuR and podoplanin expression,
respectively, developed cancers in the present study (Table III), although development was
delayed compared with patients that expressed HuR and podoplanin.
The plausible reasons behind the delayed but cancerous
transformation may be attributed to the lesions being biopsied
prior to the abnormality occurring, or to the cancers originating
from lesions that were not clinically visible at the time of biopsy
and therefore remained unexamined. Another possibility is that the
biopsies were taken from other clonal sites compared with the sites
from which the cancers eventually developed (14).
Although there is an almost general agreement that
the rate of malignant transformation increases with the severity of
the dysplasia, certain studies did not observe a significant
association between epithelial dysplasia and malignant
transformation (28). In addition,
substantial interobserver and intraobserver variation exists, in
terms of evaluating the presence and severity of epithelial
dysplasia (29,30). In the samples in the present study, 9
(53%) of 17 cases of LGD developed OSCC during the follow-up
period. Conversely, 19 (56%) of 34 cases showing severe dysplasia
did not develop OSCC. The combination of HuR and podoplanin
expression and the degree of dysplasia in association with
malignant transformation was also analyzed. As shown in Table III, >50% of the cases that
underwent malignant transformation expressed the two proteins
regardless of histopathology (P=0.005). Notably, the tumors of only
2 (8%) patients with HGD, who did not express the proteins, became
malignant. Surprisingly, the tumors of patients with LGD that
demonstrated the expression of the two proteins turned malignant
within a shorter period of time compared with patients with HGD. In
the univariate analysis, histology was one of the significant
factors for malignant transformation; however, the HR was 1.6-fold
decreased compared with the protein expression. Notably, when
histology and the coexpression of HuR and podoplanin were
considered as cofactors, the risk of OPL malignant transformation
was considerably increased compared with OPL without coexpression
(HR=5.79; 95% CI, 1.64–23.59; P=0.005) and histology. This finding
suggests that the coexpression of the two biomarkers may be more
informative compared with the histological examination alone.
Therefore, immunohistochemical staining of HuR and podoplanin may
contribute to augmenting the predictability and reliability in
cancer risk assessment of OPL, in association with a
histopathological assessment of epithelial dysplasia.
Cancer of the oral cavity results in severe
morbidity, compromised quality of life and short overall survival.
Therefore, there is a strong need to understand oral carcinogenesis
and to establish accurate and reliable predictors of oral cancer
risk (1–3,31). To the
best of our knowledge, the present study is the first to
investigate the role of HuR in oral cancer risk assessments and to
evaluate the combined expression of HuR and podoplanin in patients
with OPL. The present data showed that patients with OPL that
demonstrate the expression of HuR and podoplanin were significantly
associated with malignant transformation risk; consequently, these
patients are recommended for careful follow-up. In summary, HuR and
podoplanin may be used as biomarkers for the risk assessment of
oral malignant transformation in patients with OPL. However,
additional studies are required in order to fully define the
functional role of these biomarkers in oral cancer initiation and
disease progression.
Acknowledgements
The author was a recipient of the Iwadare
Scholarship. The authors thank Dr Yoichi M.ITO (Department of
Biostatistics, Hokkaido University Graduate School of Medicine) for
statistical support, and thank Mr. Yohei Murayama and Ms. Tomomi
Takahashi (Support Section for Education and Research, Hokkaido
University Graduate School of Dental Medicine) for the technical
assistance.
References
|
1
|
Ganly I, Patel S and Shah J: Early stage
squamous cell cancer of the oral tongue - clinicopathologic
features affecting outcome. Cancer. 118:101–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zini A, Czerninski R and Sgan-Cohen HD:
Oral cancer over four decades. Epidemiology, trends, histology and
survival by anatomical sites. J Oral Pathol Med. 39:299–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Warnakulasuriya S: Living with oral
cancer: Epidemiology with particular reference to prevalence and
life-style changes that influence survival. Oral Oncol. 46:407–410.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Silverman S Jr, Gorsky M and Lozada F:
Oral leukoplakia and malignant transformation. A follow-up study of
257 patients. Cancer. 53:563–568. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Papadimitrakopoulou VA, Hong WK, Lee JS,
Martin JW, Lee JJ, Batsakis JG and Lippman SM: Low-dose
isotretinoin versus beta-carotene to prevent oral carcinogenesis:
Long-term follow-up. J Natl Cancer Inst. 89:257–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan XC and Steitz JA: HNS, a
nuclear-cytoplasmic shuttling sequence in HuR. Proc Natl Acad Sci
USA. 95:15293–15298. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rebane A, Aab A and Steitz JA: Transportin
1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR.
RNA. 10:590–599. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brennan CM, Gallouzi IE and Steitz JA:
Protein ligands to HuR modulate its interaction with target mRNAs
in vivo. J Cell Biol. 151:1–14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erkinheimo TL, Lassus H, Sivula A,
Sengupta S, Furneaux H, Hla T, Haglund C, Butzow R and Ristimäki A:
Cytoplasmic HuR expression correlates with poor outcome and with
cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer
Res. 63:7591–7594. 2003.PubMed/NCBI
|
|
10
|
Denkert C, Weichert W, Pest S, Koch I,
Licht D, Köbel M, Reles A, Sehouli J, Dietel M and Hauptmann S:
Overexpression of the embryonic-lethal abnormal vision-like protein
HuR in ovarian carcinoma is a prognostic factor and is associated
with increased cyclooxygenase 2 expression. Cancer Res. 64:189–195.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kahn HJ and Marks A: A new monoclonal
antibody, D2-40, for detection of lymphatic invasion in primary
tumors. Lab Invest. 82:1255–1257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inoue H, Miyazaki Y, Kikuchi K, Yoshida N,
Ide F, Ohmori Y, Tomomura A, Sakashita H and Kusama K: Podoplanin
expression during dysplasia-carcinoma sequence in the oral cavity.
Tumor Biol. 33:181–194. 2012. View Article : Google Scholar
|
|
13
|
Saintigny P, El-Naggar AK,
Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim ES, Hong
WK, Lippman SM and Mao L: DeltaNp63 over expression, alone and in
combination with other biomarkers, predicts the development of oral
cancer in patients with leukoplakia. Clin Cancer Res. 15:6284–6291.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawaguchi H, El-Naggar A,
Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong
WK, Lippman SM and Mao L: Podoplanin: A novel marker for oral
cancer risk in patients with oral premalignancy. J Clin Oncol.
26:354–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wicki A and Christofori G: The potential
role of podoplanin in tumour invasion. Br J Cancer. 96:1–5. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martín-Villar E, Scholl FG, Gamallo C,
Yuritta MM, Muñoz-Guerra M, Cruces J and Quintanilla M:
Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a
small membrane mucin induced in oral squamous cell carcinomas. Int
J Cancer. 113:899–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mithani SK, Mydlarz WK, Grumbione FL,
Smith IM and Califano JA: Molecular genetics of premalignant oral
lesions. Oral Diseases. 13:126–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Habiba U, Kitamura T, Yanagawa-Matsuda A,
Hida K, Higashino F, Ohiro Y, Totsuka Y and Shindoh M: Cytoplasmic
expression of HuR may be a valuable diagnostic tool for determining
the potential for malignant transformation of oral verrucous
borderline lesions. Oncol Rep. 31:1547–1554. 2014.PubMed/NCBI
|
|
19
|
Cho NP, Han HS, Soh Y, Lee KY and Son HJ:
Cytoplasmic HuR over-expression is associated with increased
cyclooxygenase-2 expression in laryngeal squamous cell carcinomas.
Pathology. 39:545–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho NP, Han HS, Soh Y and Son HJ:
Overexpression of cyclooxygenase-2 correlates with cytoplasmic HuR
expression in salivary mucoepidermoid carcinoma but not in
pleomorphic adenoma. J Oral Pathol Med. 36:297–303. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heinonen M, Bono P, Narko K, Chang SH,
Lundin J, Joensuu H, Furneaux H, Hla T, Haglund C and Ristimäki A:
Cytoplasmic HuR expression is a prognostic factor in invasive
ductal breast carcinoma. Cancer Res. 65:2157–2161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niesporek S, Kristiansen G, Thoma A,
Weichert W, Noske A, Buckendahl AC, Jung K, Stephan C, Dietel M and
Denkert C: Expression of the ELAV-like protein HuR in human
prostate carcinoma is an indicator of disease relapse and linked to
COX-2 expression. Int J Oncol. 32:341–347. 2008.PubMed/NCBI
|
|
23
|
Gallouzi IE and Steitz JA: Delineation of
mRNA export pathways by the use of cell-permeable peptides.
Science. 294:1895–1901. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hasegawa H, Kakuguchi W, Kuroshima T,
Kitamura T, Tanaka S, Kitagawa Y, Totsuka Y, Shindoh M and
Higashino F: HuR is exported to the cytoplasm in oral cancer cells
in a different manner from that of normal cells. Br J Cancer.
100:1943–1948. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpressin of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kreppel M, Scheer M, Drebber U, Ritter L
and Zöller JE: Impact of podoplanin expression in oral squamous
cell carcinoma: Clinical and histopathologic correlations. Virchows
Arch. 456:473–482. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Villaret A Bolzoni, Schreiber A, Facchetti
F, Fisogni S, Lonardi S, Lombardi D, Cocco D, de Zinis LO Redaelli
and Nicolai P: Immunostaining patterns of CD31 and podoplanin in
previously untreated advanced oral/oropharyngeal cancer: Prognostic
implications. Head neck. 32:786–792. 2010.PubMed/NCBI
|
|
28
|
Holmstrup P, Vedtofte P, Reibel J and
Stoltze K: Long term treatment outcome of oral premalignant
lesions. Oral Oncol. 42:461–474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karrabul A, Reibel J, Therkildsen MH,
Praetorius F, Nielsen HW and Dabelsteen E: Observer variability in
the histologic assessment of oral premalignant lesions. J Pathol
Med. 24:198–200. 1995. View Article : Google Scholar
|
|
30
|
Abbey LM, Kaugars GE, Gunsolley JC, Burns
JC, Page DG, Svirsky JA, Eisenberg E, Krutchkoff DJ and Cushing M:
Intraexaminer and interexaminer reliability in diagnosis of oral
epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 80:188–191. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Vicente JC, Rodrigo JP,
Rodriguez-Santamarta T, Lequerica-Fernández P, Allonca E and
Garcia-Pedrero JM: Podoplanin expression in oral leukoplakia:
Tumorigenic role. Oral Oncol. 49:598–603. 2013. View Article : Google Scholar : PubMed/NCBI
|