Introduction
Astrocytomas are one of the most common brain
tumours, and are classified as grades I to IV based on histology
and clinical criteria (1). Although
low-grade astrocytoma patients have better survival compared with
high-grade astrocytoma patients (World Health Organization grade
III or IV), the majority of tumours eventually progress to
high-grade astrocytoma and cause patient mortality (2). Various clinical characteristics fail to
explain a variation in disease progression in histologically
diagnosed same-grade astrocytomas (3). It has been proposed that historically
used clinical variables are not sufficient prognostic or predictive
indicators, as compared with the information provided by molecular
markers of tumours (2). Furthermore,
the discovery of molecular markers may identify suitable treatments
for sensitive patients and novel targets for improved treatment in
the future (2).
Gliomagenesis is a multistep process resulting from
the accumulation of genetic and epigenetic changes. DNA methylation
is one of the major epigenetic modifications, and leads to gene
silencing (4). The frequency of
hypermethylation of CpG dinucleotides varies significantly between
different malignancy grades of gliomas (5). Previous data in breast cancer suggest
the possibility that down-regulation of testin (TES) is associated
with alterations in cell adhesion and motility, and therefore may
lead to the development of tumours with an aggressive phenotype
(6).
The TES gene, also known as testin LIM domain
protein, is located on chromosome 7q31.2 (7). The protein encoded by the TES gene is a
negative regulator of cell growth and may act as a tumour
suppressor. This protein may also play a role in cell adhesion,
cell spreading and reorganization of the actin cytoskeleton
(8). The loss of TES
expression occurs frequently in various cancers (7,9). Missense
mutations are scarce, and homozygous deletions have not been
observed, which is consistent with the fact that CpG promoter
hypermethylation is a mechanism of TES inactivation
(9). TES methylation has been
reported in primary tumours, including glioblastomas (10). Promoter methylation is closely
associated with the loss of TES expression in glioblastoma
cell lines (10). The role of
TES promoter methylation and TES protein expression in
low-grade astrocytomas has not been studied thus far. Additionally,
there are limited data on TES molecular alterations in high-grade
astrocytomas and on the influence of these alterations in patient
clinical outcome. The present study investigated whether TES
gene promoter methylation and TES expression are associated with
glioma malignancy and participate in gliomagenesis.
Materials and methods
Patients and tissue samples
In total, 138 post-operative glioma tumour samples
of different malignancy grade were collected in the Neurosurgery
Clinic Hospital of the Lithuanian University of Health Sciences
(Kaunas, Lithuania) between March 2003 and January 2013. Informed
consent was obtained from patients. The study was performed in
accordance with the principles of the Declaration of Helsinki, and
was approved by the Ethics Committee for Biomedical Research of the
Lithuanian University of Health Sciences.
In total, 138 patients diagnosed with different
malignancy grade of astrocytoma tumour were involved in the
analysis: Grade I, 14 samples; grade II, 46 samples; grade III, 29
samples; and grade IV (glioblastoma), 49 samples. Diagnoses were
established by experienced pathologists according to the WHO
classification (11). Following
resection, glioma samples were immediately stored in liquid
nitrogen (snap frozen) until DNA extraction and protein lysate
preparation. The following clinical data were collected for each
patient: Age at the time of the operation, gender, time of the last
follow-up and patient status. For the prognostic analysis, patient
survival was calculated from the date of the operation until
mortality, or until the date of termination of the study. None of
the patients had received chemotherapy or radiation prior to
surgery.
DNA isolation and bisulfate
modification
Tumour DNA was extracted from 50–150 mg of
snap-frozen tissue. Cryogenic mechanical tissue disruption was used
for homogenization of tissue. DNA was purified using the
salting-out method, as described by Miller et al (12) with one modification: 50 µl chloroform
was used for 850 µl of the total lysate following the 6M NaCl step.
The methylation status of the TES gene promoter was
determined upon bisulfite treatment of the DNA. A total of 400 ng
DNA was used for bisulfite modification. DNA modification was
performed using the EZ DNA Methylation kit (Zymo Research
Corporation, Irvine, CA, USA), and all procedures were conducted
according to the manufacturer's protocol. Bisulfite-treated DNA was
eluted in 40 µl distilled water, and stored at −80°C until
subjected to methylation-specific polymerase chain reaction
(MS-PCR).
MS-PCR
The methylation status of the TES promoter
region was determined by MS-PCR. Primers distinguishing
unmethylated from methylated alleles were previously published by
Ma et al (13), and their
sequences were as follows: Methylated forward:
5′-TATTGAGTTTGTTTAGTAGGGCGTC-3′ and reverse:
5′-AATAACAACCGAACAACTCCG-3′; and unmethylated forward:
5′-TGAGTTTGTTTAGTAGGGTGTTG-3′ and reverse:
5′-ATAACAACCAAACAACTCCAA-3′. Each PCR reaction incorporated ~20 ng
of sodium bisulphite-modified DNA. MS-PCR was performed in a total
volume of 15 µl, using 7.5 µl Maxima Hot Start Green PCR Master Mix
(2X) with Hot Start Taq DNA Polymerase (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and 10 pmol of each primer (Metabion
International AG, Steinkirchen, Germany). The cycling conditions
were as follows: Initial denaturation at 95°C for 5 min, followed
by 36 cycles of 94°C for 15 sec, 62°C for 30 sec and 72°C for 15
sec, and a final step at 72°C for 5 min. For each set of MS-PCR,
human blood lymphocyte DNA treated with bisulfite (Zymo Research
Corporation) served as an unmethylated DNA control, and as a
positive methylation control, Bisulfite-converted Universal
Methylated Human DNA Standard (Zymo Research Corporation) was used.
Human Matched DNA (Zymo Research Corporation) served as a normal
brain tissue control. A water blank control was also included. The
PCR products were analysed on 2% agarose gels with ethidium
bromide, and visualized under ultraviolet illumination. PCR
analyses were repeated twice for each sample.
Whole-tissue extract preparation and
western blot analysis
Whole-tissue extracts of cryogenically homogenized
tumour samples were routinely prepared by resuspending the
disaggregated samples (100–200 mg) in radioimmunoprecipitation
assay lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1%
IGEPAL® CA-630 (Cat. No. I3021; Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany), 0.5% sodium deoxycholate and 0.1%
sodium dodecyl sulfate (SDS)] supplemented with a protease
inhibitor cocktail (Cat. No. S8820; Sigma-Aldrich; Merck
Millipore), and next homogenized using an ultrasonic sonicator
(500-Watt Ultrasonic Processor; Cole-Parmer Instrument Co. Ltd.,
London, UK). Subsequently, the extracts were cleared by
centrifugation for 30 min at 13,000 × g at 4°C. In total, 80 µg of
the total extract of protein were fractionated by 7.5%
SDS-polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes. Each membrane incorporated a full set of
analysed astrocytoma malignancies of grades I–IV. The immobilized
proteins were incubated for 4 h at room temperature under rotation
with a primary rabbit antibody against TES (dilution 1:500; Cat.
No. 10258-1-AP; ProteinTech Group, Inc., Chicago, IL, USA) in
blocking solution [5% non-fat milk in phosphate-buffered saline
(PBS)]. Upon extensive washing in PBS supplemented with 0.5%
Tween-20 buffer, membranes were incubated with a horseradish
peroxidase (HRP)-conjugated anti-rabbit secondary antibody
(dilution 1:2,000; Cat. No. 656120; Thermo Fisher Scientific, Inc.)
for 1 h at room temperature under rotation. For detection of
β-actin on the same membranes, the the TES antibody complexes were
first cleared from the membranes by washing in a mild striping
buffer (25 mM glycine and 2% SDS, pH 2.0), and then the membranes
were re-probed with a primary monoclonal mouse antibody against
β-actin (dilution 1:2,000; Cat. No. ABIN559692; Antibodies-online
Inc., Atlanta, GA, USA) for 1 h at room temperature under rotation,
followed by incubation with an HRP-conjugated anti-mouse secondary
antibody (dilution 1:2,000; Cat. No. 626520; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Immunocomplexes were
visualized using liquid 3,3′,5,5′-tetramethylbenzidine substrate
(Cat. No. T0565-100ML; Sigma-Aldrich; Merck Millipore) and
documented using an ordinary scanner.
The values of TES and β-actin signals were
calculated using the image analysis program ImageJ version 1.47
(National Institutes of Health, Bethesda, USA). It should be noted
that when calculating TES signals, all bands (~48 kDa full-length
TES, as described in the literature (8), and possible degradation products of the
protein) were included in the analysis.
Statistical analysis
SPSS version 19 software (IBM SPSS, Armonk, NY, USA)
was used for statistical analysis. Associations between gene
methylation data and clinical features of glioma patients were
analysed by the χ2 test. To estimate survival functions, the
Kaplan-Meier method was used. For comparing survival time
distributions between groups, the log-rank test was applied. For
determination of the independence of prognostic factors, the Cox
regression analysis was used. For western blot assays, differences
across groups were analysed using the Kruskal-Wallis test
(comparison of >2 groups). To evaluate expression differences
across different gene methylation groups, the Mann-Whitney test was
used (comparison of two groups). For the analysis of patients
prognosis, survival was calculated from the date of operation until
mortality or until the date of the last follow-up. P<0.05 was
considered to indicate a statistically significant difference.
Results
TES promoter is hypermethylated in
glioblastoma tumours
The methylation status of the TES promoter in
glioblastoma tumour samples was detected by MS-PCR assay. The
methylation status of the TES promoter was evaluated in 138
astrocytoma tumours: Grade I, 14 samples; grade II, 46 samples;
grade III, 29 samples; and grade IV (glioblastoma), 49 samples.
Promoter hypermethylation was detected in 57.25% (79/138) of all
analysed astrocytoma tumours, but not in normal brain tissue. The
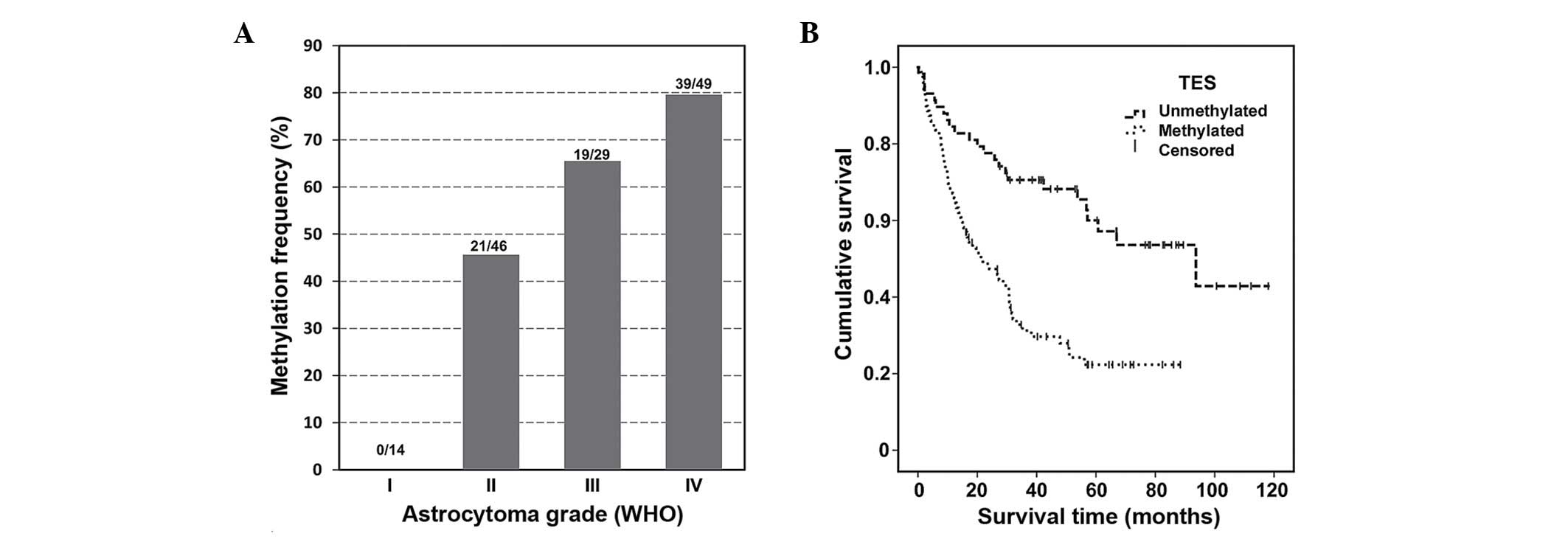
results revealed that the TES gene methylation frequency increases
with the degree of malignancy of the tumour (Fig. 1A).
Methylated TES gene promoter was not detected
in grade I astrocytoma [0.00% (0/14)], while almost half of the
grade II astrocytoma samples were methylated [45.65% (21/46)].
Grade III astrocytoma TES promoter methylation was detected
in 65.52% (19/29) of samples, and the highest methylation degree of
the TES gene was observed in glioblastoma samples [79.59%
(39/49)]. It should be mentioned that unmethylated promoter of the
TES gene was detected in the normal brain control.
The TES gene methylation status differed
significantly between astrocytomas of different malignancy grades
(P<0.001). The present study revealed a significant association
between patients' clinical data and survival, and TES gene promoter
methylation (P<0.05) (Table
I).
 | Table I.Association between TES
promoter methylation and clinicopathological features in human
astrocytoma tissue. |
Table I.
Association between TES
promoter methylation and clinicopathological features in human
astrocytoma tissue.
|
| TES gene
promoter status |
|
|---|
|
|
|
|
|---|
| Variables | Methylated, n
(%) | Unmethylated, n
(%) | P-value |
|---|
| Total patients | 79 (57.25) | 59 (42.75) |
|
| Age (years) |
|
| <0.001 |
| ≤55 | 39 (44.80) | 48 (55.20) |
|
|
>55 | 40 (78.40) | 11 (21.60) |
|
| Gender |
|
| 0.730 |
| Male | 34 (54.84) | 28 (45.16) |
|
|
Female | 45 (59.21) | 31 (40.79) |
|
| Survival
(months) |
|
| <0.001 |
| ≤24 | 44 (77.19) | 13 (22.81) |
|
|
>24 | 35 (43.21) | 46 (56.79) |
|
| Pathological
grade |
|
| <0.001 |
| I | 0 (0.00) | 14 (100.00) |
|
| II | 21 (45.65) | 25 (54.35) |
|
| III | 19 (65.52) | 10 (34.48) |
|
| IV | 39 (79.59) | 10 (20.41) |
|
The results revealed that methylated TES
promoter was more common (78.4%) in patients older than 55 years as
compared with younger patients (55.2%). In addition, methylation of
TES was significantly associated with patient survival. Patients
who survived <24 months after resection tended to have a
methylated TES allele (P=0.001).
However, there was no significant association
between TES methylation status and patient gender.
The use of the methylation of the TES gene promoter
as a prognostic value of overall survival was evaluated using
Kaplan-Meier analysis (Fig. 1B).
Promoter methylation of TES was noticed to be closely
associated with shorter survival (long-rank test, P<0.001).
TES protein expression in glioma
Hypermethylation typically occurs at CpG islands in
the promoter region of genes, and is associated with gene silencing
(4). To determine if TES protein
expression was associated with tumour histopathological grading and
TES gene promoter methylation, the aforementioned set of 86
human astrocytoma samples (13 of grade I, 31 of grade II, 17 of
grade III and 25 of grade IV) was used for TES western blot
analysis.
The protein expression levels of TES in glioblastoma
(grade IV) were significantly lower than those in lower grade
(II–III) glioma tissues (P<0.05) (Fig.
2A and B). To determine the significance of decreased TES
expression in glioblastoma, the association between TES expression
and clinicopathological features of astrocytoma patients was
assessed. For this purpose, the TES protein expression values
obtained by western blotting were ranked into three categories
irrespective of tumour grading: Values that were lower than or
equal to the 25th percentile were ranked as ‘low’ TES protein
expression; values ranging from the 25th to the 75th percentiles
were considered as ‘medium’ TES protein expression; and values that
were higher than or equal to the 75th percentile were ranked as
‘high’ TES protein expression. According to the western blot
results, low expression levels of TES were determined for 22
(25.6%) cases, medium expression levels were determined for 42
(48.8%) cases and high expression levels were determined for 22
(25.6%) cases of the total 86 glioma patients. As indicated in
Table II, according to the
Kruskal-Wallis test, TES expression differed significantly between
different astrocytoma grades (P=0.012). Pairwise comparison to
assess the differences for each group revealed that grade II and
grade III tumours tended to have significantly higher TES protein
expression than glioblastomas (P=0.028 and P=0.040, respectively)
(Fig. 2A). This suggests that
decreased TES expression could be positively associated with
glioma progression. The TES protein levels did not correlate
with patients' age or gender (both P>0.05) (Table II).
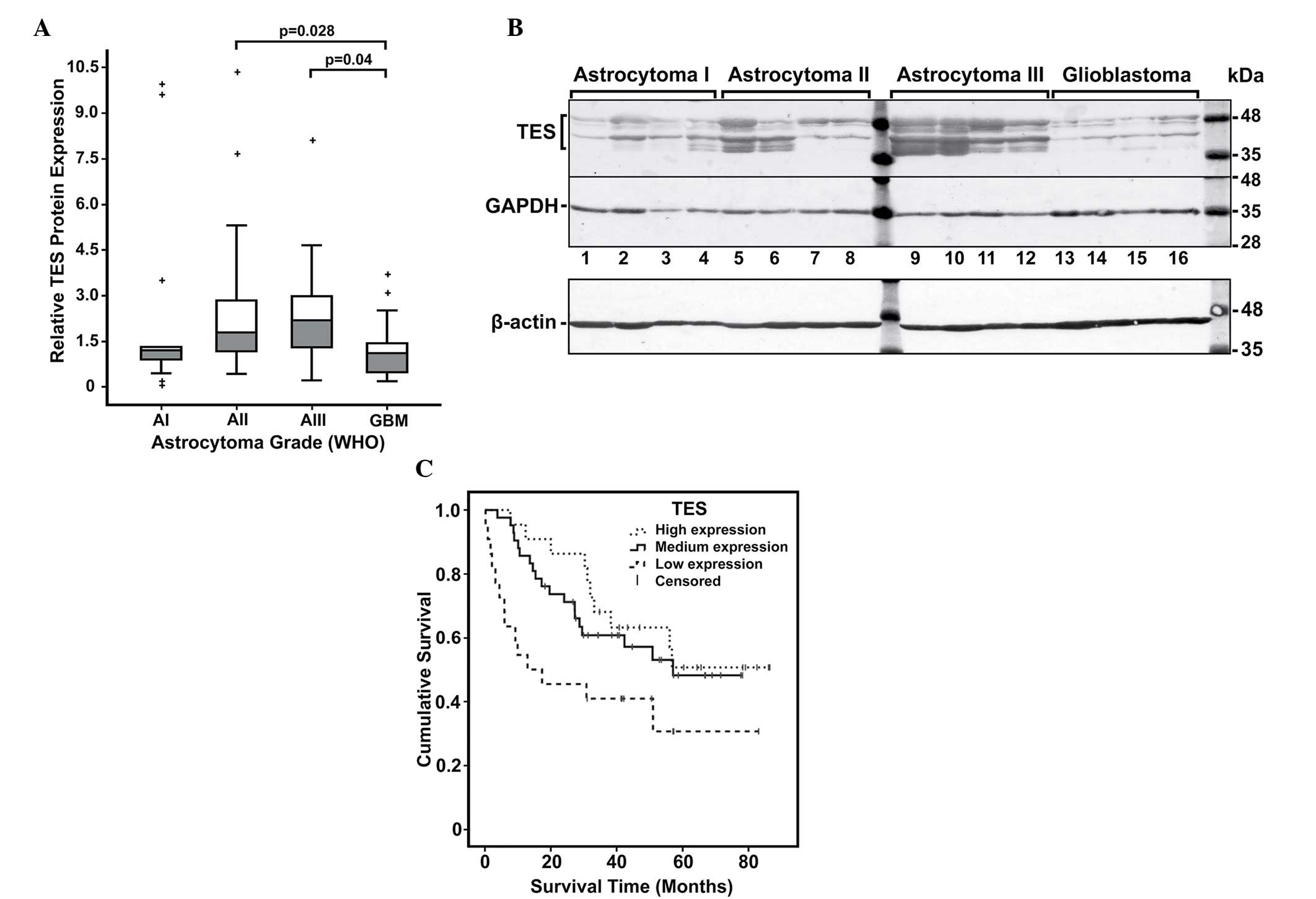 | Figure 2.Analysis of TES protein expression in
astrocytomas. (A) Relative TES protein expression in different
malignancy grade astrocytomas. Box plots of relative expression
measurements of TES obtained by western blot analysis of
astrocytoma samples. Pathological grades I, II, III and IV are
indicated as AI, AII, AIII and GBM, respectively. The line inside
each box represents the median; the lower and upper edges of the
boxes represent the 25th (1st quartile) and 75th (3rd quartile)
percentiles, respectively; and the upper and lower edges of the
boxes represent the Tukey's whiskers. The plus (+) symbols
represent the outliers (values greater than 1.5 interquartile
ranges below the 1st or above the 3rd quartile). (B) Representative
western blot results of TES protein expression in astrocytoma. The
highest molecular weight band represents the TES protein (~48 kDa),
while the other bands observed below may be possible degradation
products of TES. In addition, the ~42 kDa band that is steadily
visible in all samples could be a plausible isoform of TES. GADPH
in this example was re-probed on the same membrane, while β-actin
was detected on other membrane with the same set and arrangement of
samples. (C) Kaplan-Meier survival curves of astrocytoma patients
stratified by TES protein expression groups (high, medium and low)
(log-rank test, χ2=6.285, degrees of freedom =2, P=0.043). TES,
testin; GBM, glioblastoma; WHO, World Health Organization; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
 | Table II.Association between TES expression in
human glioma tissues and different clinicopathological
features. |
Table II.
Association between TES expression in
human glioma tissues and different clinicopathological
features.
|
|
| TES expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | Low, n (%) | Medium, n (%) | High, n (%) | P-value |
|---|
| Total patients | 86 | 22 (25.6) | 42 (48.8) | 22 (25.6) |
|
| Age (years) |
|
|
|
| 0.228 |
|
≤55 | 58 | 13 (22.4) | 27 (46.6) | 18 (31.0) |
|
|
>55 | 28 | 9
(32.1) | 15 (53.6) | 4
(14.3) |
|
| Gender |
|
|
|
| 0.472 |
|
Male | 40 | 8
(20.0) | 22 (55.0) | 10 (25.0) |
|
|
Female | 46 | 14 (30.4) | 20 (43.5) | 12 (26.1) |
|
| Pathological
grade |
|
|
|
| 0.012 |
| I | 13 | 3
(23.1) | 7
(54.8) | 3
(23.1) |
|
| II | 31 | 5
(16.1) | 17 (54.8) | 9
(29.1) |
|
|
III | 17 | 3
(17.6) | 7
(41.2) | 7
(41.2) |
|
| IV | 25 | 11 (44.0) | 11 (44.0) | 3
(12.0) |
|
Next, Kaplan-Meier analysis was performed using the
log-rank test to determine the association between TES expression
and clinical outcome of glioma patients (Fig. 2C). The analysis revealed that patients
with low TES protein expression level had significantly lower rate
of overall survival when compared with medium and high TES
expression patients (P=0.014; degrees of freedom =2; χ2=8.2)
(Fig. 2B). The survival analysis data
suggested an association between TES expression and astrocytoma
grade, and indicated that decreased level of TES could be
associated with tumour progression.
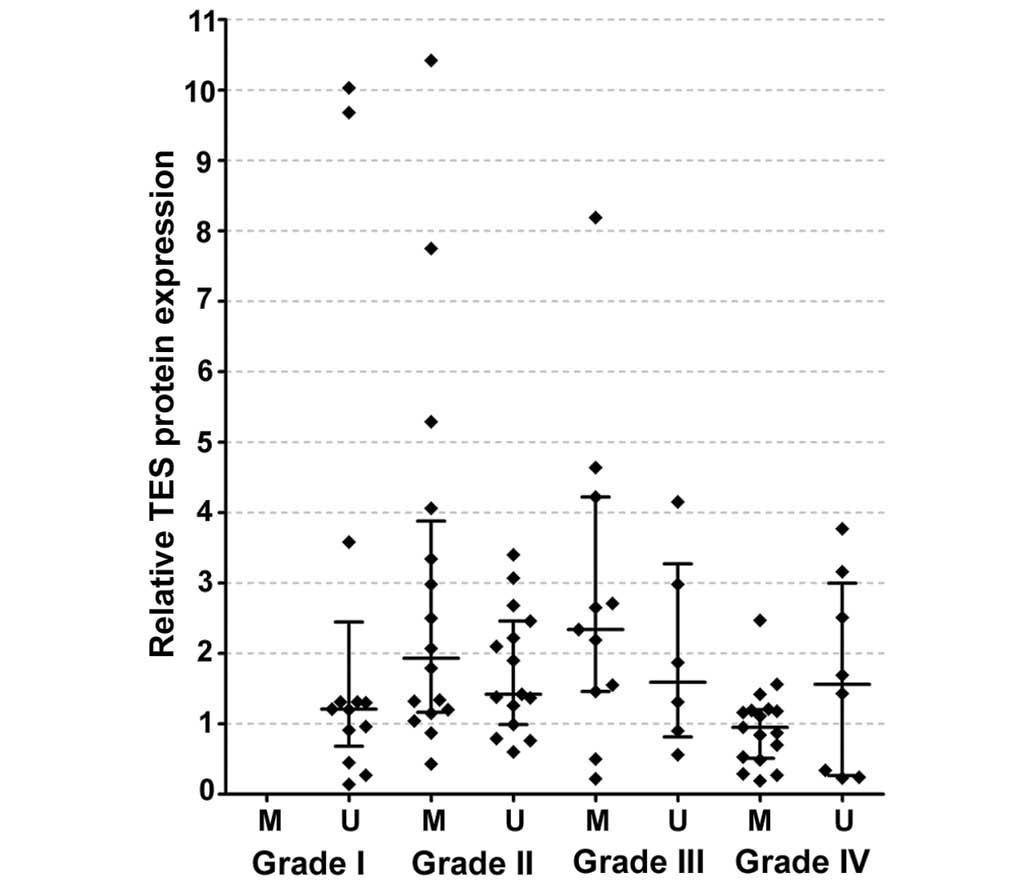
This hypothetical association between TES
promoter methylation and TES protein expression prompted us to
examine whether TES protein level differed in TES promoter
methylation groups of various grades. TES protein expression data
were divided into two groups according to gene promoter methylation
status: Methylated TES promoter and unmethylated TES
promoter in each WHO grade. No significant association between TES
protein expression and TES promoter methylation was observed
in any of the WHO groups analysed, which was most likely due to the
small number of cases (Fig. 3).
Furthermore, in addition to methylation, TES down-regulation could
be caused by other molecular mechanisms, including mutation, loss
of heterozygosity and microRNA regulation, which may affect the
correlation between gene expression and methylation.
Cox regression analysis was performed to assess the
prognostic independence of the measured variables. Molecular
variables such as TES gene promoter methylation and TES
protein expression, and clinical data such as astrocytoma
pathological grade, patients' age and gender, were selected to be
tested. Univariate Cox analysis revealed that TES protein
expression [P=0.020; hazard ratio (HR), 0.75], gene promoter
methylation (P=0.005; HR, 0.5), tumour pathological grade
(P<0.001) and patients' age (P<0.001) but not gender
(P=0.644) had a significant association as independent variables
with the patients' overall survival. All the variables that had a
strong impact on survival (P<0.05) were then assessed jointly in
a multivariate Cox analysis. However, the results from the
multivariate analysis did not confirm that TES methylation or TES
protein expression were independent prognostic factors (P=0.366 and
P=0.219, respectively), although it confirmed the prognostic
independence character of tumour pathological grade (P=0.008) and
patients' age (P=0.001). Therefore, future studies with larger
sample sizes should be conducted to confirm this tendency.
Discussion
Epigenetic changes, including aberrant DNA
methylation, are important in the pathogenesis of glial tumours
(4,14). Thus, the search for novel methylation
biomarkers or the validation of already identified methylation
biomarkers, which may facilitate glioma diagnosis, prognosis or
treatment decisions, remains a current issue (14). In the present study, the methylation
status of the TES gene, which has the potential to be used
in routine clinical setting as a biomarker for astrocytomas of
different malignancy grade, was analysed. Previous data have
suggested the possibility that down-regulation of TES is
associated with alterations in cell adhesion and motility, and
therefore, may lead to the development of tumours with an
aggressive phenotype (6). The
TES gene was noticed to be highly methylated in
glioblastomas (5,10,15), and
the tumour-associated methylation of TES was confirmed in
cultured glioblastomas and glioblastoma cell lines (10). These results indicate that TES
methylation may be involved in the loss of inter-networks in cells,
which allows them to migrate, leading to tumour infiltration of the
brain. Gunduz et al reported an association between
TES down-regulation and poor patients' outcome in head and
neck squamous cell carcinomas (16).
The down-regulation of TES was demonstrated to be correlated
with tumour differentiation and prognosis in gastric cancer
(13), and TES was densely methylated
in acute lymphoblastic leukemia (17). There is limited information about TES
methylation in astrocytomas. It has been reported that TES
appears to be frequently methylated in glioblastoma (58–69%)
(5,10,15).
In agreement with previous studies (5,10,15), the present study demonstrated a
similar TES methylation frequency (79.6%) in glioblastoma.
In addition, the current study revealed that TES gene
promoter was unmethlyated in grade I astrocytomas, whereas
substantial TES gene promoter methylation was identified in
grade II (45%) and grade III (65%)astrocytoma samples. Aligning the
methylation frequency of low-grade astrocytomas with glioblastoma,
it was noticed that TES gene promoter methylation in
astrocytomas occurs in the late stages of tumour development. Due
to TES gene promoter methylation, gene expression may be
lost, which may be associated with the increased tumour
invasiveness of gliomas.
The frequency of promoter methylation of the
TES gene based on the current MS-PCR results correlated with
tumour grade according to the WHO classification criteria, and also
with patients' age. Aggressive and invasive astrocytomas (WHO
grades IV and III) exhibited a higher frequency of methylated TES
promoter compared with low-grade tumours.
The results revealed that the overall survival of
patients with TES hypermethylation is significantly shorter
than those with hypomethylation. These observations suggest that
analyses based on the methylation status of the TES gene
promoter could potentially be useful as diagnostic or prognostic
tools in the case of tumours for which the histopathological
examination is uncertain. However, it remains to be determined
whether the increasing rate of TES methylation is associated
with the aging process or with the tumour characteristics, since it
is usually difficult to separate these two processes.
A previous study by Bai et al, where 37
different glioblastoma specimens were analysed, identified reduced
TES immunostaining (compared with normal human brain tissue) in a
subpopulation of glioblastoma cells (18). The role of TES protein
expression in low-grade astrocytomas according the data obtained in
the present study remains to be investigated. To the best of our
knowledge, the present study examined for the first time TES
expression at protein levels in astrocytoma tissues of different
malignancy grade. Using western blotting, a decreased protein level
of TES in glioblastomas was detected, compared with lower grade
astrocytomas, thus indicating that aberrant expression of TES may
be associated with malignant progression of astrocytomas. In
addition, TES down-regulation was distinctly associated with poor
prognosis in astrocytoma patients, particularly in those with high
WHO grade. The present results suggest that down-regulation of TES
predicts a worse outcome of glioblastoma patients, and that
promoter hypermethylation could be one of the reasons for TES
down-regulation, although mutation and loss of heterozygosity
cannot be excluded. Thus, these results indicate that promoter
hypermethylation may present a potential diagnostic tool in the
future.
Acknowledgements
The present study was funded by a grant (No.
LIG-11/2012) from the Research Council of Lithuania (Vilnius,
Lithuania).
References
|
1
|
Kostoro J, Chang SJ, Lai YC Clark, Wu CC,
Chai CY and Kwan AL: Overexpression of vascular adhesion protein-1
is associated with poor prognosis of astrocytomas. APMIS.
124:462–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claus EB, Walsh KM, Wiencke JK, Molinaro
AM, Wiemels JL, Schildkraut JM, Bondy ML, Berger M, Jenkins R and
Wrensch M: Survival and low-grade glioma: The emergence of genetic
information. Neurosurg Focus. 38:E62015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verma V and Mehta MP: Clinical
ramifications of "genomic staging" of low-grade gliomas. J
Neurooncol. Jul 11–2016.(Epub ahead of print). View Article : Google Scholar
|
|
4
|
Rasime K: Epigenetics of glioblastoma
multiforme. J Clinic Res Bioeth. 6:62015.
|
|
5
|
Laffaire J, Everhard S, Idbaih A, Crinière
E, Marie Y, de Reyniès A, Schiappa R, Mokhtari K, Hoang-Xuan K,
Sanson M, et al: Methylation profiling identifies 2 groups of
gliomas according to their tumorigenesis. Neuro Oncol. 13:84–98.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sarti M, Pinton S, Limoni C, Carbone GM,
Pagani O, Cavalli F and Catapano CV: Differential expression of
testin and survivin in breast cancer subtypes. Oncol Rep.
30:824–832. 2013.PubMed/NCBI
|
|
7
|
Tatarelli C, Linnenbach A, Mimori K and
Croce CM: Characterization of the human TESTIN gene localized in
the FRA7 G region at 7q31.2. Genomics. 68:1–12. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
The Human Protein Atlas:
v15.proteinatlas.org., . Testis derived transcript (3 LIM domains).
http://www.proteinatlas.org/ENSG00000135269-TES/geneJun
10–2016
|
|
9
|
Tobias ES, Hurlstone AF, MacKenzie E,
McFarlane R and Black DM: The TES gene at 7q31.1 is methylated in
tumours and encodes a novel growth-suppressing LIM domain protein.
Oncogene. 20:2844–2853. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mueller W, Nutt CL, Ehrich M,
Riemenschneider MJ, von Deimling A, van den Boom D and Louis DN:
Down-regulation of RUNX3 and TES by hypermethylation in
glioblastoma. Oncogene. 26:583–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller SA, Dykes DD and Polesky HF: A
simple salting out procedure for extracting DNA from human
nucleated cells. Nucleic Acids Res. 16:12151988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma HW, Weng D, Chen Y, Huang W, Pan K,
Wang H, Sun J, Wang Q, Zhou Z, Wang H and Xia J: Extensive analysis
of D7S486 in primary gastric cancer supports TESTIN as a candidate
tumor suppressor gene. Mol Cancer. 9:1902010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majchrzak-Celińska A, Paluszczak J,
Szalata M, Barciszewska AM, Nowak S, Kleszcz R, Sherba A and
Baer-Dubowska W: The methylation of a panel of genes differentiates
low-grade from high-grade gliomas. Tumour Biol. 36:3831–3841. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Martinez R, Martin-Subero JI, Rohde V,
Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G and
Esteller M: A microarray-based DNA methylation study of
glioblastoma multiforme. Epigenetics. 4:255–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gunduz E, Gunduz M, Beder L, Nagatsuka H,
Fukushima K, Sutcu R, Delibas N, Yamanaka N, Shimizu K and Nagai N:
Down-regulation of TESTIN and its association with cancer history
and a tendency toward poor survival in head and neck squamous cell
carcinoma. Arch Otolaryngol Head Neck Surg. 135:254–260. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weeks RJ, Kees UR, Song S and Morison IM:
Silencing of TESTIN by dense biallelic promoter methylation is the
most common molecular event in childhood acute lymphoblastic
leukaemia. Mol Cancer. 9:1632010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai Y, Zhang QG and Wang XH:
Down-regulation of TES by hypermethylation in glioblastoma reduces
cell apoptosis and predicts poor clinical outcome. Eur J Med Res.
19:662014. View Article : Google Scholar : PubMed/NCBI
|