Introduction
Head and neck squamous cell carcinoma (HNSCC), the
sixth most common cancer worldwide, represents >500,000 cases of
all the cancer cases diagnosed every year (1,2). Previous
epidemiological studies have demonstrated that exposure to
carcinogens such as tobacco and alcohol, as well as infection by
oncogenic human papillomavirus 16 and 18, results in increased risk
of HNSCC development (2–4). Currently, the treatment of choice for
head and neck cancer is surgery, followed by postoperative chemo-
and/or radiotherapy (1,5,6). Despite
advances in conventional methods, the 5-year mortality rate of
patients with HNSCC has not improved (2). With progress in technologies and
molecular genetics, there is a growing potential of gene therapy as
a powerful tool for HNSCC treatment.
RNA interference (RNAi) is a natural physiological
process, which is supposed to protect the genome from viruses and
transposons (7). RNAi technology is
based on the inhibition of target transcript expression or
degradation by exogenous or endogenous homologous double-stranded
(ds)RNA, which has the ability to specifically and more effectively
silencing genes than single-stranded RNA individually does
(8,9).
The first phase of the RNAi mechanism is the hydrolysis of
exogenous dsRNAs to small interfering (si)RNAs by Dicer
endoribonuclease (10,11). Each strand of siRNA has 5′-phosphate
and 3′-hydroxyl termini, in addition to 2–3-nucleotides 3′
overhangs (11,12). In the next step, Dicer enzyme, in
cooperation with cofactors, mediates the binding of siRNAs to
RNA-induced silencing complex (RISC) (8–13). The
endogenous substrate of RNAi are small non-coding RNA molecules of
micro (mi)RNA (14). The long miRNA
precursor, termed primary (pri)-miRNA, is composed of several
hundred nucleotides and is cleaved by the Microprocessor complex
(15,16). The core of this complex are Drosha (a
class 2 ribonuclease III enzyme) and DiGeorge syndrome critical
region 8 protein (16). As a result
of the hydrolysis, precursor (pre)-miRNAs are formed, which are
hairpin-loop structures composed of ~70 nucleotides that are
subsequently transported from the nucleus to the cytosol, where
they undergo a maturation process mediated by Dicer nuclease
(16–22). Mature miRNAs are targeted to the RISC
effector complex (10,18–24).
RNAi is a potentially effective instrument in gene
therapy. However, it is required to establish efficient systems of
its in vitro and in vivo applications. These systems
should work specifically in a defined cell type or tissue, and
should also eliminate the risk of potential immunological response
(25). Currently, there are two main
systems of introduction of RNA molecules into organisms: Viral
(retroviruses, including lentiviruses), adenoviruses and
adeno-associated viruses) (26–33) and
non-viral (34,35). The aim of the present study is to
review the usefulness of the RNAi mechanism in head and neck
oncology.
Nanoparticle delivery of HIF1α siRNA
combined with PDT as a potential treatment strategy for head and
neck cancer
Hypoxia inducible factor 1 (HIF1) is a master
transcriptional regulator of the cellular and systemic hypoxia
response (36). HIF1 is a
heterodimer, and consists of two subunits (HIF1β and HIF1α)
(37). It belongs to the family of
basic helix-loop-helix transcription factors (37). Under normoxic conditions, HIF1α is
degraded rapidly with the participation of a proline hydroxylase,
which performs an oxygen-hydroxylation of proline residues 402 and
564 (37). Hydroxylated HIF1α is
subsequently recognized by Von Hippel-Lindau protein (pVHL), a
component of an E3 ubiquitin-protein ligase, and degraded in the
proteasome (37). Under low
concentration of oxygen, pVHL does not bind to HIF1α, and it is
translocated to the nucleus instead, where it forms a heterodimer
with the HIF1β subunit (37,38). This subunit (also known as aryl
hydrocarbon receptor nuclear translocator) specifically binds to
hypoxia-responsive elements of oxygen-regulated genes promoters
(37,38). The formation of HIF1 heterodimers
results in the transcriptional activation of several genes,
including vascular endothelial growth factor (VEGF), glucose
transporter 1 and carbonic anhydrase IX, which are involved in
self-renewal, survival and induction of angiogenesis and
metastases, which in turn contributes to increased cancer
progression and therapy resistance (39). Therefore, HIF1 plays a pivotal role in
tumorigenesis by determining the ability of self-renewal and
multipotency of cancer stem cells in a hypoxic environment
(36–40).
Chen et al (36) investigated the potential of silencing
HIF1α combined with Photosan-based photodynamic therapy (PDT) in
human oral (O)SCC. Anisamide-targeted lipid-calcium-phosphate
(LCP-AA) nanoparticles were used to deliver HIF1α siRNA to the
cytosol of SCC4 and SAS cell lines (derived from a squamous
carcinoma of human tongue with expression of sigma receptors)
(36). Cells were also subjected to
PDT. To investigate the efficiency of LCP delivery, double-stranded
HIF1α oligonucleotides (DNA) labeled with Texas Red dye were used.
The study revealed that LCP-AA was able to successfully and
efficiently deliver siRNA in a sigma receptor-mediated process
(36). To confirm these results, SCC4
tumor bearing nude mice were intravenously injected with
AA-targeted Texas Red labeled LCP-AA. After 4 h, the fluorescence
intensity in the tumor and organs was measured. The tumor region
exhibited the strongest signal, confirming the efficient delivery
of LCP-AA to SCC4 cells in vivo (36). The effect of HIF1α knockdown on the
viability of SCC4 cells, LCP toxicity and in vivo
therapeutic outcomes of the combined treatment were also evaluated.
HIF1α depletion by siRNA inhibited the proliferation of OSCC cells
and induced their apoptosis (36).
Immune response or toxicity of LCP were not observed (36). These studies demonstrate that systemic
administration of HIF1α siRNA by targeted LCP appears to enable the
stable and effective inhibition of OSCC proliferation (36). These results were also confirmed by
Ahn et al and Liang et al, who suppressed tumor
growth with HIF1α depletion via regulation of VEGF (5,6).
Suppression of ABCG2 inhibits the process of
LSCC tumor growth
ATP-binding cassette (ABC), subfamily G, member 2
(ABCG2, also known as breast cancer resistance protein) is a
655-amino acid protein of 72 kDa, which is a member of the ABC
transporter family (41–46). It was first cloned from
doxorubicin-resistant human MCF-7 breast cancer cells (41). Overexpression of ABCG2 is observed in
multiple tumor types, including leukemias and certain SCC (41). Increased expression of ABCG2 leads to
drug resistance by promoting the proliferation of tumor cells and
suppressing apoptosis (41–46).
Xie et al (41)
investigated the role of ABCG2 in laryngeal (L)SCC tumor growth and
its influence on the accumulation of mitoxantrone (MX) in cancer
cells. ABCG2 siRNA was introduced into two LSCC cell lines: Hep2
and Hep2T (Taxol-resistant). To evaluate the efficiency and effect
of silencing the ABCG2 gene in LSCC cells, a number of analyses,
including flow cytometry, apoptosis assay, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting, were performed. The results revealed that the
suppression of ABCG2 inhibits LSCC tumor growth by regulating cell
proliferation and apoptosis. It was also confirmed that ABCG2
knockdown increases MX accumulation in both Hep2 and Hep2T cell
lines, thus enhancing its anti-tumor effect (41). These findings suggest that ABCG2 is a
potential therapeutic target for LSCC.
Suppression of ITG3A increases
radiosensitivity and induces apoptosis in HNSCC cells
Integrins are heterodimeric surface receptors that
are responsible for cell adhesion to extracellular matrix (ECM)
proteins and are important in cell-cell adhesions, transmembrane
connections to the cytoskeleton and intracellular signaling
pathways (47–49). There are ≥24 distinct integrin
heterodimers formed by the combination of 18 α-subunits and 8
β-subunits, and each of them has a different specificity to ECM
proteins (38). A wide variety of
integrins contribute to tumor progression, including tumors derived
from epithelial cells (50,51). In addition, integrins may contribute
to migration, proliferation and survival of tumor cells (51). Furthermore, depending on the
environmental conditions, integrins have the ability to either
enhance cell survival or initiate apoptosis (51). Considering their features, integrins
are regarded as attractive targets for cancer therapy (47–51).
Steglich et al (48) investigated the influence of α3
integrin (ITG3A) depletion on the progression and radiosensitivity
of HNSCC cells. The expression of ITG3A in these cells was
evaluated by western blot analysis, which revealed that ITG3A is
overexpressed in HNSCC. Subsequently, the HNSCC cell lines UTSCC5,
UTSCC14, UTSCC15, Cal33 and HSC4 were transfected with siRNA
against ITG3A. It was demonstrated that ITG3A knockdown results in
reduced clonogenic cell survival, induced apoptosis and enhanced
radiosensitivity (48). Therefore,
ITG3A may be considered as a potential target in HNSCC
treatment.
Genetic silencing and pharmacological
inhibition of Bmi1 as an instrument of TSCC gene therapy
Polycomb group proteins (PcG) are critical
transcriptional repressors that epigenetically modify chromatin and
contribute to cell fate, stem cell self-renewal and cancer
development (52,53). B lymphoma Mo-MLV insertion region 1
homolog (Bmi1) is the core member of the polycomb repressive
complex 1 (PRC1), one of two PRCs in PcG (53). Bmi1 is an epigenetic silencer of
multiple target genes, including p14Arf, p19Arf and p16Ink4a
(52,53).
Bmi1-mediated chromatin modifications are involved
in crucial cellular processes, including stem cell maintenance,
proliferation, senescence, apoptosis and epithelial-mesenchymal
transition (EMT) (52,53). Overexpression of Bmi1 is associated
with multiple human malignancies, including myeloid leukemia as
well as lung, breast and head and neck cancer (53). In addition, overexpression of Bmi1 is
associated with the therapeutic resistance, aggressive
clinicopathological behavior and poor prognosis of the above
cancers (52,53). Furthermore, it was demonstrated that
Bmi1 overexpression promotes malignant transformation, cancer cell
proliferation, EMT and metastatic spreading, while its suppression
inhibits cancer progression, thus inducing senescence and cell
apoptosis (52,53).
Li et al (53)
investigated the potential of Bmi1 as a target in the genetic and
pharmacological treatment of tongue (T)SCC. siRNAs against
different regions of Bmi1 were delivered to TSCC cell lines (HN4,
HN6, HN12, Tca8113, Cal27, SCC9 and SCC25) using Lipofectamine
2000. To evaluate the efficacy of Bmi1 suppression, cells were
cultured for 24 h and subsequently analyzed by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay, flow cytometry, RT-qPCR, cell migration and wound healing
assay. Simultaneously, an experiment on a mouse TSCC xenograft
model was conducted to determine the anticancer effects of histone
deacetylase inhibitors (HDACis). It was demonstrated that Bmi1
knockdown inhibits cell migration and proliferation and induces
cell senescence and apoptosis in TSCC. Furthermore, it was revealed
that HDACis such as sodium butyrate and trichostatin A inhibit Bmi1
expression in tongue cancer cells and exert an anticancer effect
(53). Therefore, the pharmacological
and genetic disruption of Bmi1 may represent a novel therapeutic
strategy against tongue cancers.
AURKA inhibition and paclitaxel as targeted
combination therapy for HNSCC
Aurora kinase A (AURKA) is a member of the conserved
serine/threonine protein kinase family, and a cell cycle-regulated
kinase involved in spindle formation and chromosome segregation
(54–60). It was observed that AURKA
overexpression induces oncogenic transformation accompanied by
centrosome amplification and aneuploidy in rodent cells (55,56).
Furthermore, the AURKA gene is amplified and overexpressed in
numerous human cancers, including breast, bladder, colorectal,
ovarian gastric and pancreatic cancer, causing resistance to
paclitaxel (Taxol) (54). A
correlation between AURKA mRNA overexpression, tumor progression
and shortened survival in patients with HNSCC was determined
(54–60).
Mazumdar et al (54) investigated the potential of AURKA as a
therapeutic target in HNSCC. AURKA-targeted siRNA was introduced
into HNSCC cell lines (Tu138, UMSCC1, Tu167, OSC19, Tu177 and
JMAR). Cells were cultured for 72 h and then harvested. AURKA
knockdown was confirmed by western blot analysis. MTT assay and
flow cytometry analysis were also performed to evaluate cell
proliferation and cell cycle disruption, respectively. It was
demonstrated that AURKA knockdown inhibited HNSCC cells
proliferation, significantly reducing the proportion of G1-phase
cells. Furthermore, AURKA depletion significantly increased the
cytotoxicity of paclitaxel (54). The
suppressive effect of AURKA depletion on tumor growth was also
confirmed by Tanaka et al (60), who demonstrated that AURKA plays a
pivotal role in the growth of human OSCC cells, and that AURKA
silencing appears to be a potentially useful therapeutic approach
for patients with OSCC.
RNAi in clinical trials
The first clinical trial involving siRNA was
conducted in 2004 by Acuity Pharmaceuticals, Inc. (OPKO Health,
Inc., Miami, FL, USA) for the treatment of age-related macular
degeneration (AMD) (61). Naked siRNA
targeted to VEGF and VEGF receptor genes displayed therapeutic
potential in the inhibition of the excessive vascularization of the
eye that leads to AMD. The completed trials reported a good
tolerability of all siRNA doses and improved eyesight in patients
(61). The detailed data about
current RNAi trials are recorded in the ClinicalTrials.gov website (https://clinicaltrials.gov/), which is maintained by
the National Library of Medicine of the National Institutes of
Health (Bethesda, MD, USA), and provides access to information on
clinical studies on a wide range of diseases and conditions
(62). According to this database,
the Comprehensive Cancer Center of Wake Forest University (Elkin,
NC, USA) is currently conducting a phase I trial on the side
effects and best dose of siRNA-transfected peripheral blood
mononuclear cells (termed APN401) in treating patients with
melanoma, renal cancer, pancreatic cancer or other solid tumors
that cannot be removed by surgery (63). It has been hypothesized that these
modified immune cells will have a high anticancer activity
(63). The University of Texas MD
Anderson Cancer Center (Houston, TX, USA) is currently conducting a
phase I study on the safety and the highest tolerable dose of
siRNA-ephrin type-A receptor 2
(EphA2)-1,2-dioleoyl-sn-glycero-3-phosphocholine, which is designed
to shut down the activity of the genetic biomarker EphA2 to treat
patients with advanced solid tumors (64). Phase I trials are also being conducted
by Calando Pharmaceuticals, Inc. (Pasadena, CA, USA) to determine
the safety, toxicity and maximum tolerated dose of CALAA-01, which
is administered intravenously to patients with relapsed or
refractory cancer (65). The active
ingredient in CALAA-01 is a siRNA that inhibits tumor growth by
reducing the expression of the M2 subunit of ribonucleotide
reductase R2 (65). A phase I study
was conducted by Silenseed Ltd. (Modi'in, Israel) to investigate
the safety of siG12D Local Drug EluteR (LODER) in patients
diagnosed with pancreas adenocarcinoma (66). siG12D LODER is a miniature
biodegradable polymeric matrix that contains siRNA designed to shut
down the activity of the Kirsten rat sarcoma viral oncogene homolog
(KRAS) gene. It is considered that silencing KRAS will lead to the
apoptosis of cancer cells, and thereby slow down or even halt tumor
growth (66). Silenseed Ltd. is
actually conducting a phase II study on siG12D LODER to assess the
efficacy and local distribution of this drug in patients with
non-operable pancreas adenocarcinoma (67).
Summary
The discovery of RNAi has had a significant
influence on research and development. There is large evidence that
siRNA and miRNA molecules are strongly involved in the regulation
of different pathological processes, including cancer development.
Therefore, RNAi technology has become a valuable research tool for
better understanding the mechanisms of cancer pathogenesis.
Considering its ability to specific eliminate defective genes
products, siRNA is a theoretically perfect therapeutic instrument
for certain diseases, including cancer, viral infections and
diabetes (Fig. 1) (35). Its application in gene therapy has a
variety of advantages such as high efficiency and specificity,
which allow to reduce side effects, including immunological
response. Therefore, RNAi could be successfully used in the
treatment of numerous diseases whose molecular mechanisms of
pathogenesis are known.
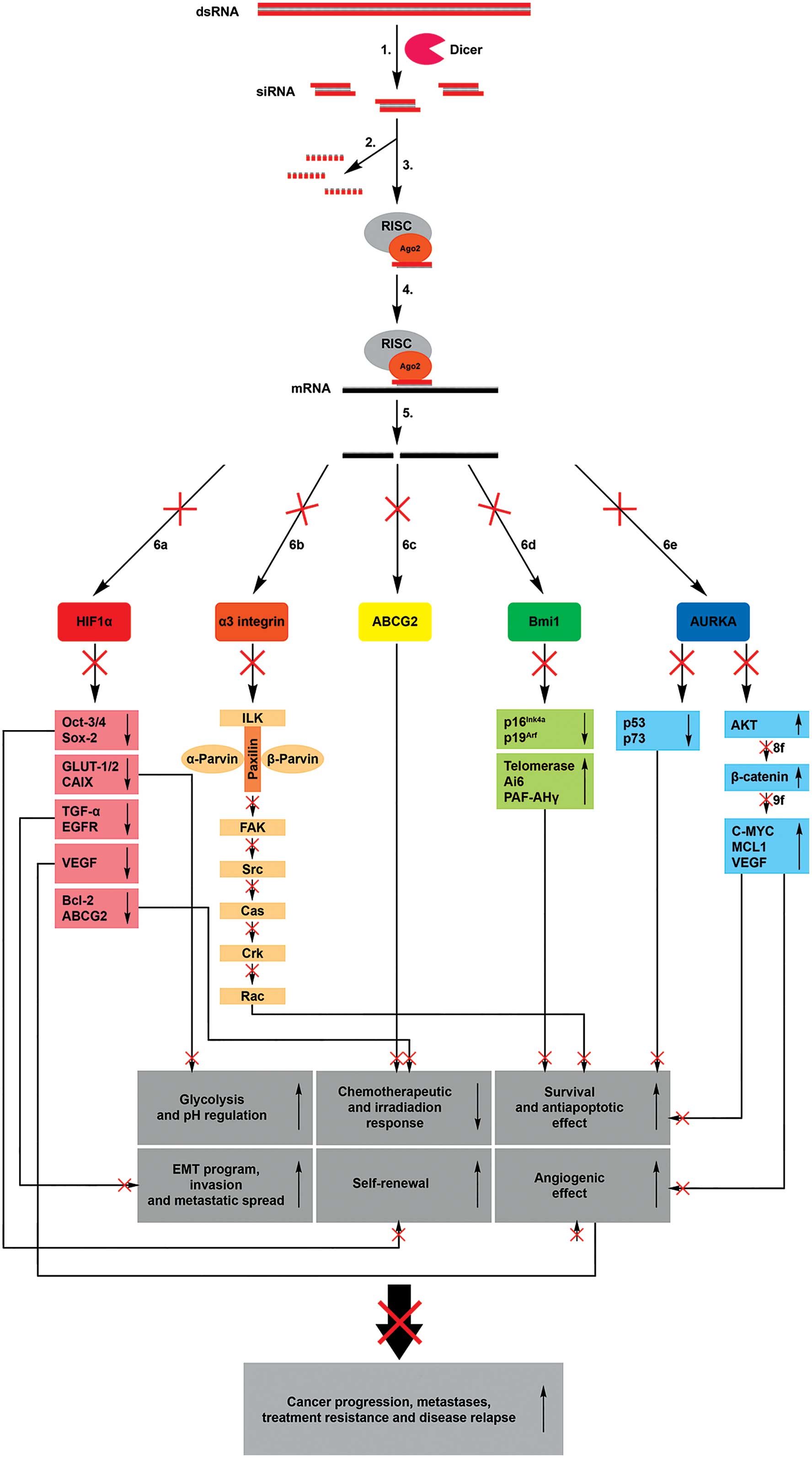 | Figure 1.Mechanisms of RNA interference.
Introducing exogenous dsRNA into a cell activates the ribonuclease
protein Dicer, which binds and cleaves dsRNA into short (21–24 bp)
fragments termed siRNAs (1). The
sense strand of siRNA is degraded (2), while the anti-sense strand binds to
active RISC (3). The RISC-siRNA
complex identifies the homologous target messenger RNA (4) and induces its cleavage (5), thus preventing the translation of the
target proteins hypoxia inducible factor 1α (6a), α3 integrin (6b),
ATP-binding cassette, subfamily G, member 2 (6c), B lymphoma Mo-MLV
insertion region 1 homolog (6d) and aurora kinase A (6e). This
process results in the transcriptional inhibition of numerous genes
involved in anaerobic glycolysis, pH regulation, survival,
antiapoptotic and angiogenic effects, metastases, self-renewal and
treatment resistance of cancer cells. ds, double-stranded; si,
small interfering; m, messenger; RISC, RNA-induced silencing
complex; HIF, hypoxia inducible factor; Ago, argonaute; ABCG2,
ATP-binding cassette, subfamily G, member 2; Bmi1, B lymphoma
Mo-MLV insertion region 1 homolog; AURKA, aurora kinase A; Oct,
organic cation transporter; GLUT, glucose transporter; TGF,
transforming growth factor; VEGF, vascular endothelial growth
factor; Bcl, B-cell lymphoma; ILK, integrin-linked kinase; FAK,
focal adhesion kinase; Cas, CRISPR-associated; Rac, Ras-related C3
botulinum toxin substrate; PAF-AH, platelet-activating
factor-acetylhydrolase; MCL1, myeloid cell keukemia; EMT,
epithelial-mesenchymal transition. |
Acknowledgements
The present work was supported by the National
Science Centre (grant no., 2015/17/N/NZ5/00686) and the Greater
Poland Cancer Centre research grant (grant no.,
9/11/2015/PRB/WCO/21).
References
|
1
|
Suh Y, Amelio I, Urbano T Guerrero and
Tavassoli M: Clinical update on cancer: Molecular oncology of head
and neck cancer. Cell Death Dis. 5:e10182014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas SM and Grandis JR: The current
state of head and neck cancer gene therapy. Hum Gene Ther.
20:1565–1575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maiti GP, Mondal P, Mukherjee N, Ghosh A,
Ghosh S, Dey S, Chakrabarty J, Roy A, Biswas J, Roychoudhury S and
Panda CK: Overexpression of EGFR in head and neck squamous cell
carcinoma is associated with inactivation of SH3GL2 and CDC25A
genes. PLoS One. 8:e634402013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martinez-Useros J and Garcia-Foncillas J:
The challenge of blocking a wider family members of EGFR against
head and neck squamous cell carcinomas. Oral Oncol. 51:423–430.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ahn SH, Choi JY, Kim DW, Lee DY, Jeon EH,
Jeong WJ and Paik JH: Targeting HIF1α peri-operatively increased
post-surgery survival in a tongue cancer animal model. Ann Surg
Oncol. 22:3041–3048. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liang J, Zhang Z, Liang L, Shen Y and
Ouyang K: HIF-1α regulated tongue squamous cell carcinoma cell
growth via regulating VEGF expression in a xenograft model. Ann
Transl Med. 2:922014.PubMed/NCBI
|
|
7
|
Hamilton AJ and Baulcombe D: A species of
small antisense RNA in posttranscriptional gene silencing in
plants. Science. 286:950–952. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Napoli C, Lemieux C and Jorgensen R:
Introduction of a chimeric chalcone synthase gene into petunia
results in reversible co-suppression of homologous genes in trans.
Plant Cell. 2:279–289. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Collins RE and Cheng X: Structural and
biochemical advances in mammalian RNAi. J Cell Biochem.
99:1251–1266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agrawal N, Dasaradhi PVN, Mohmmed A,
Malhotra P, Bhatnagar RK and Mukherjee SK: RNA interference:
Biology, mechanism, and applications. Microbiol Mol Biol Rev.
67:657–685. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J,
Lee J, Provost P, Rådmark O, Kim S and Kim VN: The nuclear RNase
III drosha initiates microRNA processing. Nature. 425:415–419.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239. 2003.
View Article : Google Scholar
|
|
14
|
Filipowicz W, Jaskiewicz L, Kolb FA and
Pillai RS: Post-transcriptional gene silencing by siRNAs and
miRNAs. Curr Opin Struct Biol. 15:331–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q and Paroo Z: Biochemical principles
of small RNA pathways. Annu Rev Biochem. 79:295–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ouellet DL, Perron MP, Gobeil LA, Plante P
and Provost P: MicroRNAs in gene regulation: When the smallest
governs it all. J Biomed Biotechnol. 2006:696162006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yeom KH, Lee Y, Han J, Suh MR and Kim VN:
Characterization of DGCR8/Pasha, the essential cofactor for Drosha
in primary miRNA processing. Nucleic Acids Res. 34:4622–4629. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khvorova A, Reynolds A and Jayasena SD:
Functional siRNAs and miRNAs exhibit strand bias. Cell.
115:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schwarz DS, Hutvágner G, Du T, Xu Z,
Aronin N and Zamore PD: Asymmetry in the assembly of the RNAi
enzyme complex. Cell. 115:199–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meister G, Landthaler M, Patkaniowska A,
Dorsett Y, Teng G and Tuschl T: Human Argonaute2 mediates RNA
cleavage targeted by miRNAs and siRNAs. Mol Cell. 15:185–197. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elbashir SM, Martinez J, Patkaniowska A,
Lendeckel W and Tuschl T: Functional anatomy of siRNAs for
mediating efficient RNAi in Drosophila melanogaster embryo lysate.
EMBO J. 20:6877–6888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eulalio A, Huntzinger E and Izaurralde E:
Getting to the root of miRNA-mediated gene silencing. Cell.
132:9–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregory RI, Chendrimada TP, Cooch N and
Shiekhattar R: Human RISC couples microRNA biogenesis and
posttranscriptional gene silencing. Cell. 123:631–640. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meister G: miRNAs get an early start on
translational silencing. Cell. 131:25–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pecot CV, Calin GA, Coleman RL,
Lopez-Berestein G and Sood AK: RNA interference in the clinic:
Challenges and future directions. Nat Rev Cancer. 11:59–67. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bouard D, Alazard-Dany D and Cosset FL:
Viral vectors: From virology to transgene expression. Br J
Pharmacol. 157:153–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kantor B, Bailey RM, Wimberly K, Kalburgi
SN and Gray SJ: Methods for gene transfer to the central nervous
system. Adv Genet. 87:125–197. 2014.PubMed/NCBI
|
|
28
|
Galanis E, Vile R and Russell SJ: Delivery
systems intended for in vivo gene therapy of cancer: Targeting and
replication competent viral vectors. Crit Rev Oncol Hemato.
38:177–192. 2001. View Article : Google Scholar
|
|
29
|
Morris KV and Rossi JJ:
Lentiviral-mediated delivery of siRNAs for antiviral therapy. Gene
Ther. 13:553–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wold WS and Toth K: Adenovirus vectors for
gene therapy, vaccination and cancer gene therapy. Curr Gene Ther.
13:421–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermens WT and Verhaagen J: Viral vectors,
tools for gene transfer in the nervous system. Prog Neurobiol.
55:399–432. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tomar RS, Matta H and Chaudhary PM: Use of
adeno-associated viral vector for delivery of small interfering
RNA. Oncogene. 22:5712–5715. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lentz TB, Gray SJ and Samulski RJ: Viral
vectors for gene delivery to the central nervous system. Neurobiol
Dis. 48:179–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ramamoorth M and Narvekar A: Non viral
vectors in gene therapy-an overview. J Clin Diagn Res. 9:GE01–GE06.
2015.PubMed/NCBI
|
|
35
|
Uprichard SL: The therapeutic potential of
RNA interference. FEBS Lett. 579:5996–6007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen WH, Lecaros RL, Tseng YC, Huang L and
Hsu YC: Nanoparticle delivery of HIF1α siRNA combined with
photodynamic therapy as a potential treatment strategy for
head-and-neck cancer. Cancer Lett. 359:65–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sasabe E, Tatemoto Y, Li D, Yamamoto T and
Osaki T: Mechanism of HIF-1alpha-dependent suppression of
hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer
Sci. 96:394–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sasabe E, Zhou X, Li D, Oku N, Yamamoto T
and Osaki T: The involvement of hypoxia-inducible factor-1alpha in
the susceptibility to gamma-rays and chemotherapeutic drugs of oral
squamous cell carcinoma cells. Int J Cancer. 120:268–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mimeault M and Batra SK: Hypoxia-inducing
factors as master regulators of stemness properties and altered
metabolism of cancer- and metastasis-initiating cells. J Cell Mol
Med. 17:30–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jing S, Wang J, Liu Q, Cheng Y, Yang C,
Wang Y, Cao F, Wen B, Jiao W and Guo Y: Relationship between
hypoxia inducible factor-1α and esophageal squamous cell carcinoma:
A meta analysis. Zhonghua Bing Li Xue Za Zhi. 43:593–599. 2014.(In
Chinese). PubMed/NCBI
|
|
41
|
Xie J, Jin B, Li DW, Shen B, Cong N, Zhang
TZ and Dong P: ABCG2 regulated by MAPK pathways is associated with
cancer progression in laryngeal squamous cell carcinoma. Am J
Cancer Res. 4:698–709. 2014.PubMed/NCBI
|
|
42
|
Doyle LA, Yang W, Abruzzo LV, Krogmann T,
Gao Y, Rishi AK and Ross DD: A multidrug resistance transporter
from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA.
95:15665–15670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen Z, Liu F, Ren Q, Zhao Q, Ren H, Lu S,
Zhang L and Han Z: Suppression of ABCG2 inhibits cancer cell
proliferation. Int J Cancer. 126:841–851. 2010.PubMed/NCBI
|
|
44
|
Noguchi K, Katayama K and Sugimoto Y:
Human ABC transporter ABCG2/BCRP expression in chemoresistance:
Basic and clinical perspectives for molecular cancer therapeutics.
Pharmgenomics Pers Med. 5:53–64. 2014.
|
|
45
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 15:1084–1103. 2012. View Article : Google Scholar
|
|
46
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): Its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Steglich A, Vehlow A, Eke I and Cordes N:
α integrin targeting for radiosensitization of three-dimensionally
grown human head and neck squamous cell carcinoma cells. Cancer
Lett. 357:542–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hehlgans S, Haase M and Cordes N:
Signalling via integrins: Implications for cell survival and
anticancer strategies. Biochim Biophys Acta. 1775:163–180.
2007.PubMed/NCBI
|
|
50
|
Onodera Y, Nam JM and Sabe H:
Intracellular trafficking of integrins in cancer cells. Pharmacol
Ther. 140:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lukacs RU, Memarzadeh S, Wu H and Witte
ON: Bmi-1 is a crucial regulator of prostate stem cell self-renewal
and malignant transformation. Cell Stem Cell. 7:682–693. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li Z, Wang Y, Yuan C, Zhu Y, Qiu J, Zhang
W, Qi B, Wu H, Ye J, Jiang H, et al: Oncogenic roles of Bmi1 and
its therapeutic inhibition by histone deacetylase inhibitor in
tongue cancer. Lab Invest. 94:1431–1445. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mazumdar A, Henderson YC, El-Naggar AK,
Sen S and Clayman GL: Aurora kinase A inhibition and paclitaxel as
targeted combination therapy for head and neck squamous cell
carcinoma. Head Neck. 31:625–634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bischoff JR, Anderson L, Zhu Y, Mossie K,
Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, et
al: A homologue of Drosophila aurora kinase is oncogenic and
amplified in human colorectal cancers. EMBO J. 17:3052–3065. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Goepfert TM, Adigun YE, Zhong L, Gay J,
Medina D and Brinkley WR: Centrosome amplification and
overexpression of aurora A are early events in rat mammary
carcinogenesis. Cancer Res. 62:4115–4122. 2002.PubMed/NCBI
|
|
57
|
Kovarikova V, Burkus J, Rehak P, Brzakova
A, Solc P and Baran V: Aurora kinase A is essential for correct
chromosome segregation in mouse zygote. Zygote. 15:326–337. 2016.
View Article : Google Scholar
|
|
58
|
Katsha A, Belkhiri A, Goff L and El-Rifai
W: Aurora kinase A in gastrointestinal cancers: Time to target. Mol
Cancer. 20:1062015. View Article : Google Scholar
|
|
59
|
Sun JM, Yang LN, Xu H, Chang B, Wang HY
and Yang G: Inhibition of Aurora A promotes chemosensitivity via
inducing cell cycle arrest and apoptosis in cervical cancer cells.
Am J Cancer Res. 5:1133–1145. 2015.PubMed/NCBI
|
|
60
|
Tanaka H, Nakashiro K, Iwamoto K, Tokuzen
N, Fujita Y, Shirakawa R, Oka R, Goda H and Hamakawa H: Targeting
Aurora kinase A suppresses the growth of human oral squamous cell
carcinoma cells in vitro and in vivo. Oral Oncol. 49:551–559. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Whitehead KA, Langer R and Anderson DG:
Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug
Discov. 8:129–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: ClinicalTrials.gov Background. Available from.
https://clinicaltrials.gov/ct2/about-site/backgroundAccessed.
June 29–2015
|
|
63
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: APN401 in Treating Patients With Melanoma,
Kidney Cancer, Pancreatic Cancer, or Other Solid Tumors That Are
Metastatic or Cannot Be Removed By Surgery. Available from.
http://clinicaltrials.gov/show/NCT02166255Accessed.
June 29–2015
|
|
64
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: EphA2 Gene Targeting Using Neutral Liposomal
Small Interfering RNA Delivery. Available from. http://clinicaltrials.gov/show/NCT01591356Accessed.
June 29–2015
|
|
65
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: Safety Study of CALAA-01 to Treat Solid Tumor
Cancers. Available from. http://clinicaltrials.gov/show/NCT00689065Accessed.
June 29–2015.
|
|
66
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: Phase I-Escalating Dose Study of siG12D LODER
(Local Drug EluteR) in Patients With Locally Advanced
Adenocarcinoma of the Pancreas and a Single Dose Study of siG12D
LODER (Local Drug EluteR) in Patients With Non-operable
Adenocarcinoma of the Pancreas. Available from. http://clinicaltrials.gov/show/NCT01188785Accessed.
June 29–2015.
|
|
67
|
U.S. National Institutes of Health, .
ClinicalTrials.gov: A Phase II Study of siG12D LODER in Combination
With Chemotherapy in Patients With Unresectable Locally Advanced
Pancreatic Cancer. Available from. http://clinicaltrials.gov/show/NCT01676259Accessed.
June 29–2015
|















