Introduction
Adenocarcinoma (AC) is the most common histological
type of non-small cell lung cancer (NSCLC) (1). Overall, >80% of lung ACs are
diagnosed with mixed ACs, according to the 2004 World Health
Organization (WHO) classification (2). Therefore, a semiquantitative evaluation
system for calculating the components is necessary. A novel
classification based on a multidisciplinary approach to the
diagnosis of lung ACs was established by the International
Association for the Study of Lung Cancer, American Thoracic Society
and European Respiratory Society in 2011 (3). In the novel nomenclature system,
invasive ACs are classified by predominant components, such as
lepidic (formerly most mixed subtype tumors with non-mucinous BAC),
mucinous (formerly mucinous BAC), acinar, papillary, solid patterns
and micropapillary ACs (3). Although
multidisciplinary data from widely divergent clinical, radiologic,
molecular and pathological spectra accounts for the attributes of
lung AC, it remains unclear how to address the biological
properties of lung AC. Despite marked advances in the understanding
of this tumor in the past decades, the production of universally
accepted criteria for AC subtypes is required (4,5). Previous
studies have reported that the new classification is an independent
predictor of overall survival (6,7).
Epidermal growth factor receptor (EGFR)
mutations of exon 18 through exon 21 are reported to be associated
with the sensitivity to tyrosine kinase inhibitors (TKIs);
therefore, it is important to understand the nature of these
mutations. EGFR mutations are mainly categorized into two
groups, with ‘classical’ activating mutations including del19 and
L858R. Additional analyses are required on other variants with
unknown function (8). Tumor
development in human lung ACs is increased by the activation of the
EGFR signaling pathway. Therefore, target treatment with
gefitinib leads to specific inhibition through apoptosis of cancer
cells (9,10). A previous study on stage IB lung AC
identified that micropapillary-predominant AC is the most common AC
subtype with EGFR mutation, whereas solid-predominant AC has
a lower frequency of EGFR mutation (11).
Echinoderm microtubule associated protein like 4
(EML4)-anaplastic lymphoma receptor tyrosine kinase
(ALK), the major type of fusion gene resulting from
ALK rearrangement, has been reported to be a potent
oncogenic driver and a promising therapeutic target in ACs through
the administration of crizotinib (12–14).
Methods to detect ALK rearrangement include fluorescent
in situ hybridization (FISH), immunohistochemistry (IHC) and
reverse transcription-polymerase chain reaction (RT-PCR). FISH
analysis is the only approved diagnostic test for the detection of
the break-apart signal of ALK rearrangement. However, the
disadvantages of the FISH test are that particular apparatuses are
not always readily available in routine diagnostic laboratories,
and subtle intrachromosomal rearrangement may be challenging to
interpret; therefore, false-negative outcomes are inevitable. The
subtle changes may be challenging to interpret by FISH analysis,
and have led to false-negative results (15,16). IHC
has been considered as an alternative to FISH for the detection of
ALK rearrangement, and Ventana IHC for ALK fusion gene has
been approved by the European Union (17).
Crk-like (CRKL) is upregulated in malignant
tumors, including 49% of breast cancer, 55% of lung cancer, 67% of
skin cancer, 50% of ovarian carcinoma, and 63% of colon carcinoma
tumors (18). CRKL is a member
of the human Crk adapter protein family and has been found to be
amplified in lung cancer cells, with enhanced expression as a
result of the amplification. In addition, knockdown of CRKL
in lung cancer cell lines has led to a significant decrease in the
proliferation, progression, survival, motility and invasiveness of
lung cancer cells. All these data indicate that overexpression of
CRKL may result in the oncogenic phenotype of lung cancer (19). Although evidence favors CRKL
gene amplification in several human malignancies, including lung
cancer, whether the expression of CRKL is associated with
EGFR status in lung ACs remains to be elucidated.
AXL receptor tyrosine kinase (AXL) is
confirmed to be associated with the carcinogenesis of numerous
tumors. Elevated AXL expression and interaction with its ligand
growth arrest-specific 6 (Gas6) have been associated with cell
survival, proliferation, and migration in solid tumors (20,21). AXL
is increasingly upregulated during the multistep process of
esophageal carcinogenesis and is an adverse prognostic marker in
esophageal AC (22). A previous study
identified AXL activation as a novel mechanism of acquired
resistance to EGFR inhibitors in non-small cell lung cancer
(23). Overexpression of AXL is
consistently manifested in prostate cancer cell lines and human
prostate tumors. Blockage of AXL expression strongly inhibits the
proliferation, migration and invasion of tumor cells, and therefore
tumor growth (24).
The primary purpose of the present study is to
analyze the correlation between the original expression levels of
CRKL and AXL and status of the ALK and
EGFR genes and the prognosis of different AC histological
subtypes. Due to the presence of mixed AC components, it was
important to evaluate the role of CRKL and AXL
expression in AC subtypes combined with EGFR and ALK
status.
Materials and methods
Study design
The present study is a retrospective review of 108
treatment-naive patients with AC, with samples consisting of 91
samples of resected primary lung cancer and 15 samples of
metastatic nodules from advanced lung cancer from Beijing Chest
Hospital (Beijing, China) between 2006 and 2012. All sample
sections were evaluated by two pathologists to confirm the
diagnosis and predominance (>70%) of tumor tissues. All slides
were evaluated by pathologists based on the new classification
(25) using a multi-headed microscope
(objective, 40X; magnification, ×400) and the clinical stage was
defined according to the 7th Edition of the TNM Classification of
the Union for International Cancer Control (3,26). A mean
number of 4.5 slides (range, 2–11 slides) were reviewed. Patients
were examined at 3-month intervals for the first 2 years following
treatment and at 6-month intervals thereafter. The progression-free
survival (PFS) time was measured from the date of treatment to the
date of the first documented disease progression. The data were
collected from the medical record system of Beijing Chest Hospital.
The evaluation of disease progression included a physical
examination, computed tomography scan of the chest and abdomen,
brain magnetic resonance imaging, and bone scintigraphy. The last
follow-up date was January 1, 2014. In total, 27 patients were
censored from the current evaluation due to incomplete follow-up
data.
DNA extraction, polymerase chain
reaction amplification and direct sequencing for EGFR mutation
Genomic DNA was extracted from 50–100-mg tumor
tissues obtained from formalin-fixed and paraffin-embedded blocks.
The procedures followed a previously described protocol (27). PCR for exons 18–21 was performed using
100 ng template DNA in 50 µl volumes containing 0.75 U Hotstart Taq
DNA polymerase (Fermentas; Thermo Fisher Scientific, Inc.,
Pittsburgh, PA, USA), 5 µl PCR buffer, 0.8 µM dNTP (Fermentas;
Thermo Fisher Scientific, Inc.), 0.5 µM of each primer (Sangon
Biotech Co., Ltd., Shanghai, China), and various concentrations of
MgCl2, depending on varied markers. The nucleic acids
used for the mutations were based on NM_005228.3. The primers were
designed by Sangon Biotech Co., Ltd. as follows: Exon 18 forward,
5′-CAACCAAGCTCTCTTGAGGATC-3′ and reverse, 5′-CCCAGCCCAGAGGCCTGT-3′;
exon 19 forward, 5′-GCAGCATGTGGCACCATCTC-3′ and reverse,
5′-AGAGCCATGGACCCCCACAC-3′; exon 20 forward,
5′-CACACTGACGTGCCTCTCC-3′ and reverse, 5′-AGCAGGTACTGGGAGCCAAT-3′;
and exon 21 forward, 5′-TCTGTCCCTCACAGCAGGGTCT-3′ and reverse,
5′-GCTGGCTGACCTAAAGCCACC-3′. The amplification and sequencing of
exon fragments were performed as previously described (27). PCR products were sequenced in sense
and antisense directions. Only specimens in which a mutation was
identified in the two rounds were recorded as
mutation-positive.
IHC for CRKL, AXL gene and ALK
rearrangement
Tissue sections (4 µm) were prepared from tissue
microarray blocks, deparaffinized using xylene and rehydrated
through an ethanol series to water. Slides were incubated with the
rabbit polyclonal anti-CRKL (catalog no., ab151791; Abcam Inc.,
Cambridge, UK) and polyclonal goat anti-AXL (catalog no., AF154;
R&D Systems, Inc., Minneapolis, MN, USA) antibodies using a
MaxVision horseradish peroxidase-polymer system kit (catalog nos.
5030 and 5108; Maixin Bio, Fuzhou, China). Incubation with the
primary antibodies was performed overnight at 4°C and at a 1:200
dilution. The MaxVision horseradish peroxidase-polymer system kit
was used for immunostaining according to the manufacturer's
instructions. Detection was accomplished using diaminobenzidine
(DAB) (catalog no. CAS 7411-49-6; ImmunoCruz Staining System; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). The slides were then
counterstained in hematoxylin, and the stained tumor cells (≥1,000
cells), were scored by two independent observers. Cytoplasmic
staining was considered positive for both CRKL and AXL. The
immunoreactivity of carcinoma samples was semi-quantitatively
evaluated by two aspects: Percentage of positive cells and
staining. The staining strength was described as follows: 0, tumor
cells were not stained; 1, light-yellow stained cells; 2, yellow
stained cells; 3, brown stained cells. The observed area covered
all histological patterns. The raw scores, ranging from 0 to 300,
were calculated as follows: Percentage × staining strength. The
final scores for statistical analysis were the average of raw
scores from subtype components (Fig.
1A-D). Lower expression levels of AXL and CRKL were defined as
a staining score ≤100, otherwise the tissues were classified as
having a higher expression level.
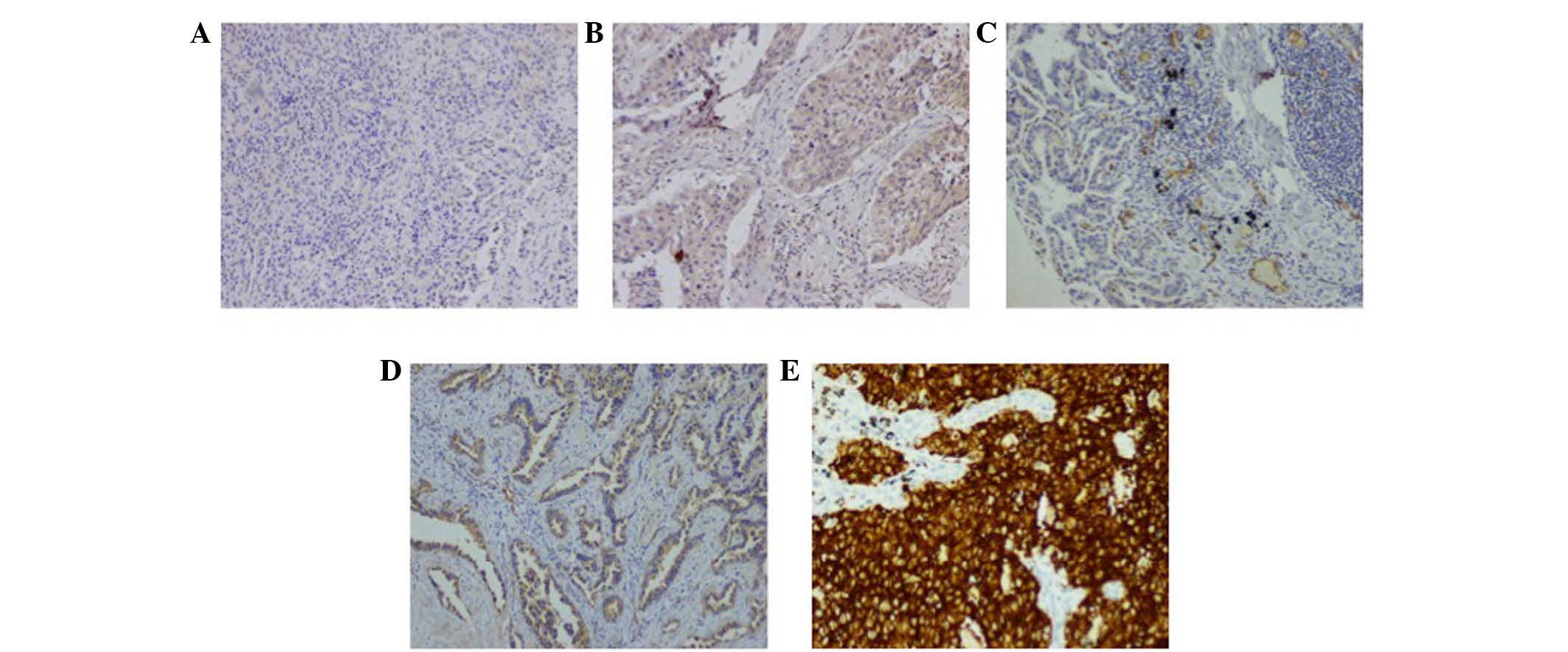 | Figure 1.IHC staining of CRKL, AXL and ALK
fusion protein in lung AC samples. (A and B) Representative acinar
and solid predominant AC tissues. CRKL antibodies showed (A) lower
expression level with weak cytoplasmic staining in acinar AC and
(B) higher level of protein expression in a solid AC (original
magnification, ×100). (C and D) AXL immunostaining also showed (C)
lower and (D) higher expression level, with weakly and moderately
positive reaction in two acinar ACs, respectively (original
magnification ×100). (E) ALK rearrangement positive staining by
Ventana IHC; brown staining granules full of cytoplasm could be
observed (original magnification, ×200). CRKL, Crk-like; AXL, AXL
receptor tyrosine kinase; ALK, anaplastic lymphoma receptor
tyrosine kinase; AC, adenocarcinoma; IHC, immunohistochemistry. |
IHC analysis for ALK rearrangement was
completed by using Ventana method on a Benchmark XT autostainer
(Roche Diagnostics, Indianapolis, IN, USA). This IHC method was an
automatic staining by utilizing a ready-to-use primary anti-ALK
rabbit monoclonal antibody (clone, D5F3; catalog no. 790–4794;
Ventana Medical Systems, Inc., Tucson, AZ, USA). The staining
procedure followed the Ventana ALK test protocol using an optiview
amplification kit and an optiview DAB IHC detection kit. Presence
or absence of ALK rearrangement was evaluated as positive or
negative following the manufacturer's protocol (Roche Diagnostics).
Neoplastic cells with diffuse dark brown cytoplasmic staining were
classified as ALK rearrangement-positive; any other colors
were classified as ALK rearrangement-negative (Fig. 1E).
Statistical analysis
CRKL and AXL expression was
categorized into lower and higher levels of expression according to
the aforementioned cut-off values. Association between CRKL
and AXL expression were analyzed with clinicopathological
factors by crosstab χ2 test or Fisher's exact test
(Table I). The impact of the
following factors on the progression-free survival (PFS) rates was
also evaluated: Gender; age; smoking status; clinical stage;
EGFR gene status; and ALK fusion gene status. These
clinicopathological factors were used in univariate and
multivariate analyses to determine whether they had a significant
effect on PFS (Table II). The
survival rates and pairwise comparisons were stratified by
clinicopathological characteristics and calculated using the
Kaplan-Meier method and log-rank test. All statistical tests were
two-sided and P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed on
SAS system for Windows, version 9.2 (SAS Institute Inc., Cary, NC,
USA). The survival curves were plotted using GraphPad Prism,
version 6 (GraphPad Software, Inc., La Jolla, CA, USA).
 | Table I.Association between CRKL and AXL
expression and clinicopathological characteristics. |
Table I.
Association between CRKL and AXL
expression and clinicopathological characteristics.
|
| CRKL
expression, n (%) |
| AXL expression, n (%) |
|
|---|
|
|
|
|
|
|
|---|
| Clinical | Lower level | Higher level | P-value | Lower level | Higher level | P-value |
|---|
| Gender |
|
| 0.53 |
|
| 0.7 |
|
Male | 16 (32.7) | 33 (67.3) |
| 21 (42.9) | 28 (57.1) |
|
|
Female | 15 (26.3) | 42 (73.7) |
| 27 (47.4) | 30 (52.6) |
|
| Adenocarcinoma
subtypes |
|
| 0.5 |
|
| 0.67 |
|
Acinar | 13 (40.6) | 19 (59.4) |
| 12 (37.5) | 20 (62.5) |
|
|
Micropapillary | 3
(27.3) | 8
(72.7) |
| 4
(36.4) | 7
(63.6) |
|
|
Papillary | 2
(16.7) | 10 (83.3) |
| 6
(50.0) | 6
(50.0) |
|
|
Solid | 12 (26.1) | 34 (73.9) |
| 23 (50.0) | 23 (50.0) |
|
|
Mucinous | 1
(20.0) | 4
(80.0) |
| 3
(60.0) | 2
(40.0) |
|
| Tumor size |
|
| 0.82 |
|
| 0.68 |
| ≤3
cm | 9
(27.3) | 24 (72.7) |
| 16 (48.5) | 17 (51.5) |
|
| >3
cm | 20 (30.3) | 46 (69.7) |
| 29 (43.9) | 37 (56.1) |
|
| EGFR
status |
|
| 0.83 |
|
| 0.019 |
|
Mutation | 17 (30.4) | 39 (69.6) |
| 19 (33.9) | 37 (66.1) |
|
| Wild
type | 14 (28.0) | 36 (72.0) |
| 29 (58.0) | 21 (42.0) |
|
| ALK
status |
|
| 0.77 |
|
| 0.29 |
| Fusion
gene | 4
(23.5) | 13 (76.5) |
| 10 (58.8) | 7
(41.2) |
|
|
Non-fusion gene | 27 (30.3) | 62 (69.7) |
| 38 (42.7) | 51 (57.3) |
|
| Smoking status |
|
| 0.36 |
|
| 1.0 |
| Never
smoker | 19 (26.0) | 54 (74.0) |
| 33 (45.2) | 40 (54.8) |
|
| Smoker
or ever smoker | 12 (36.4) | 21 (63.6) |
| 15 (45.5) | 18 (54.5) |
|
| Clinical stage |
|
| 0.68 |
|
| 0.28 |
|
I+II | 16 (32.7) | 33 (67.3) |
| 22 (44.9) | 27 (55.1) |
|
|
IIIA | 10 (24.4) | 31 (75.6) |
| 16 (39.0) | 25 (61.0) |
|
|
IIIB+IV | 5
(31.2) | 11 (68.8) |
| 10 (62.5) | 6
(37.5) |
|
 | Table II.Association of PFS and hazard ratios
with clinicopathological factors in ACs. |
Table II.
Association of PFS and hazard ratios
with clinicopathological factors in ACs.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | Total, n | Median PFS,
months | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Gender |
|
|
Male | 50 | 11.3 |
9.3–13.2 |
0.130 |
|
|
|
|
Female | 58 | 15.4 | 10.3–20.5 |
|
|
|
|
| Adenocarcinoma
subtypes |
|
| Acinar
predominant | 32 | 30.6 | 14.3–46.9 |
0.052 | 0.40 | 0.21–0.75 | 0.005a |
| MicroP
predominant | 11 | 13.0 | 10.6–15.4 |
| 0.61 | 0.28–1.32 | 0.210a |
|
Mucinous predominant | 5 | 10.5 |
9.4–11.6 |
| 0.96 | 0.37–2.52 | 0.940a |
|
Papillary predominant | 12 |
7.0 | 4.6–9.2 |
| 1.16 | 0.55–2.43 | 0.700a |
| Solid
predominant | 46 | 10.5 |
7.3–13.6 |
|
|
|
|
| CRKL expression
score |
|
|
≤100 | 31 | 13.6 |
8.3–18.8 |
0.790 | 0.78 | 0.47–1.31 | 0.350 |
|
>100 | 75 | 12.5 |
9.0–15.9 |
|
|
|
|
| AXL expression
score |
|
|
≤100 | 48 | 12.0 |
6.3–17.7 |
0.440 | 1.01 | 0.61–1.67 | 0.980 |
|
>100 | 58 | 13.5 | 11.2–15.8 |
|
|
|
|
| EGFR
statusb |
|
|
Mutation | 56 | 16.5 | 11.4–21.6 |
0.053c |
|
|
|
| Wild
type | 50 | 11.0 |
8.8–13.2 |
|
|
|
|
| Exon 18
mutation | 5 | 14.0 | 11.4–23.7 |
| 0.69 | 0.16–2.96 | 0.610d |
| Exon 19
mutation | 27 | 27.8 | 15.7–40.0 |
| 0.52 | 0.28–0.96 | 0.037d |
| Exon 21
mutation | 23 | 10.0 |
7.8–12.2 |
| 1.33 | 0.70–2.55 | 0.390d |
| ALK
status |
|
| Fusion
gene | 17 | 11.0 |
1.5–20.5 |
0.057 | 1.45 | 0.79–2.64 | 0.230 |
|
Non-fusion gene | 91 | 13.6 |
8.7–18.3 |
|
|
|
|
| Smoking status |
|
| Never
smoker | 74 | 14.5 |
9.0–20.0 |
0.062 |
|
|
|
| Smoker
or ever smoker | 34 | 10.5 |
8.2–12.8 |
|
|
|
|
| Clinical stage (vs.
I+II)e |
|
|
I+II | 49 | 27.3 | 18.0–36.5 | <0.001 | 1.97 | 1.38–2.83 | <0.001 |
|
IIIA | 42 | 10.0 |
5.2–14.7 |
|
|
|
|
|
IIIB+IV | 17 |
9.7 |
1.0–15.7 |
|
|
|
|
Results
Patient characteristics and
histopathological features
A total of 108 cases were originally involved in our
study; however, the results of 2 cases were not incomplete in
detecting the expression of AXL and CRKL. Therefore, 106 cases were
analyzed in the present study, whose clinicopathological features
are listed in Table I. Out of the 106
patients, AC was slightly more common in females (53.7%; 57/106).
The median age was 55 years in females (range, 23–73 years) and 62
years in males (range, 33–79 years). All samples were diagnosed as
invasive ACs and 82 (77.4%) of these samples were mixed ACs, which
were classified into five histological subtypes based on their
predominant components: 32 acinar ACs (30.19%), including 3 samples
with cribriform pattern; 12 papillary ACs (11.32%); 5 mucinous
carcinomas (4.72%); 11 micropapillary ACs (10.38%); and 46 solid
ACs (43.40%). In total, 32.4% of patients did not have a smoking
history. PFS comparisons among histological subtypes showed that
acinar-predominant AC had a marginally improved chemotherapeutic
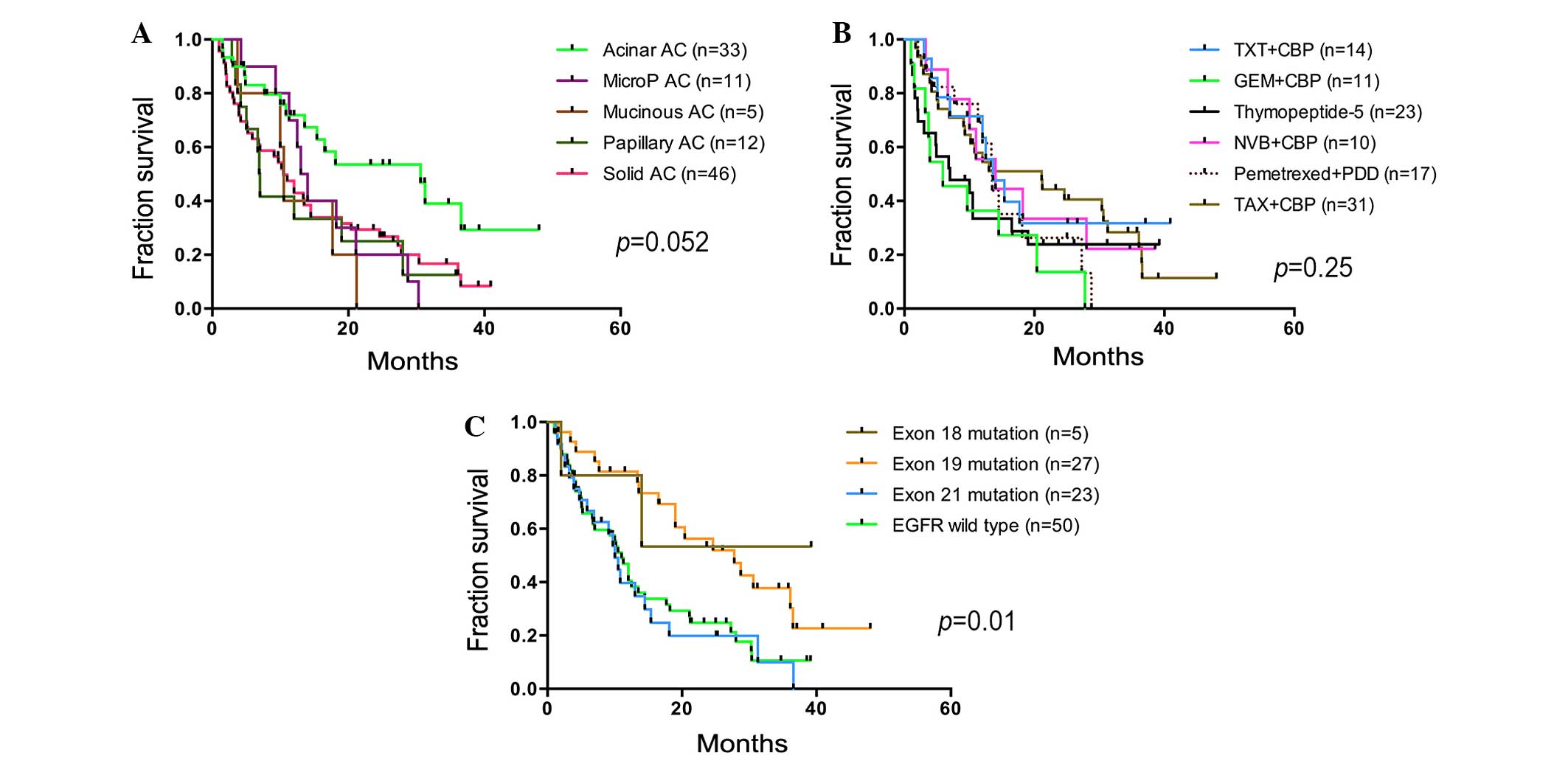
response than others (P=0.052; Fig.
2A). Overall, 44.4% of patients were diagnosed at early stage
(stage I and II), 39.8% of patients were diagnosed at stage IIIA,
and 15.7% of patients were diagnosed at stage IIIB and IV.
Conventional regimens of chemotherapy-combined use of carboplatin
or cisplatin with Taxol®, gemcitabine, navelbine,
docetaxel or pemetrexed were administered to 83 patients.
Thymopeptide-5 alone was administered to 23 patients.
EGFR mutation and ALK rearrangement in
lung ACs
Mutations of the EGFR kinase domain (exons
18–21) were successfully screened and 58 AC samples (53.7%) were
positive for EGFR mutations (Table
I), Two main types of EGFR mutation conferring TKI
sensitivity are deletion in exon 19 and the missense mutation L858R
in exon 21 (28). In the present
study, 27 and 24 AC samples harbored deletion in exon 19 (range,
K745-S753) and the L858R mutation, respectively. Deletion in exon
19 occurred in 12 acinar (12/27; 44.4%), 2 micropapillary (2/27;
7.4%), 3 papillary (3/27; 11.1%) and 10 solid (10/27; 37%) ACs.
L858R mutation was found in 12 acinar ACs (12/24; 50%), consisting
of one acinar with cribriform pattern, 1 micropapillary (1/24;
4.2%), 1 mucinous (1/24; 4.2%), 1 papillary (1/24; 4.2%) and 9
solid ACs (9/24; 37.5%). Deletion in exon 19 and L858R mutations
were mainly harbored in acinar and solid-type ACs. Other less
common mutations, such as G719A/S and S768I, were found in 6
patients.
Ventana IHC autostaining is a valid and convenient
method for testing ALK fusion protein detection and the
validity of Ventana IHC has been verified by FISH (Fig. 1E) (29).
In total, 17 ACs (17/106; 16.0%) were determined to be ALK
fusion positive by Ventana IHC. In the current study, ACs with
ALK rearrangement consisted of 2 micropapillary-predominant
(2/11; 18.2%), 1 mucinous-predominant (1/5; 20.0%), 1
papillary-predominant (1/12; 8.3%) and 11 solid-predominant (11/46;
23.9%) tumors, particularly with cytoplasmic mucin. All these
tissues stained positive for ALK rearrangement in a uniform
pattern, despite the different morphological subtypes (Fig. 1E). In addition, 2/3 ACs with
cribriform pattern were ALK rearrangement-positive.
EGFR mutation and ALK rearrangement did not coexist
in any AC subtype in the present study.
Effect of CRKL and AXL expression with
different EGFR and ALK statuses on the prognosis of AC
subtypes
Frequency crosstables were used to compare the
staining intensity of CRKL and AXL with
clinicopathological features (Table
I; Fig. 1A-D). CRKL and
AXL expression was not significantly associated with
clinicopathological features, such as gender, AC subtypes, tumor
size, ALK rearrangement and clinical stage (Table I). In total, 81 patients (81/106;
76.4%) experienced elapsed or progression during the follow-up
period and 25 patients were censored, since they did not experience
progressive disease or were lost to follow-up. The mean clinical
follow-up period was 14.9 months (range, 1–48 months). In total, 50
and 58 patients were diagnosed at an early and advanced stage of
disease, respectively. It was found that PFS time was not
associated with chemotherapeutic regimens (P=0.17; Fig. 2B). However, the prognosis was
significantly different between the two groups, with a median PFS
time of 24.6 months (95% CI, 15.6–33.6 months) in the early-stage
group and 10 months in the advanced group (95% CI, 5.8–14.1 months;
HR, 2.83; 95% CI, 1.76–4.5; P<0.0001). The median PFS time of
patients with acinar-predominant AC was 30.6 months, which was
significantly longer than the micropapillary (HR, 2.9; 95% CI,
1.1–7.6; P=0.03), mucinous (HR, 2.73; 95% CI, 1.1–18.2; P=0.04),
papillary (HR, 2.31; 95% CI, 1.1–7.3; P=0.016) and
solid-predominant subtypes of AC (HR, 2.16; 95% CI, 1.2–3.5;
P=0.009) (Table II). It is known
that clinical staging is an important factor that affects the
prognosis of patients with lung cancer. Subsequently, the PFS time
of different subtypes were stratified by clinical staging and
compared; it was found that only acinar AC had a longer PFS time
than papillary ACs at an early stage of disease, but prognostic
advantage was revealed at an advanced stage of disease (data not
shown; P=0.025). The PFS time (16.5 months) of the 56 patients with
EGFR mutation was increased compared with the PFS time of
patients with wild-type EGFR (11 months; P=0.052). The
mutation types were compared with the PFS of patients to elucidate
the effect of EGFR mutation types on prognosis and found
that they really played roles in prognosis of AC patients (P=0.01;
Fig. 2C). It was also found that
patients with exon 19 mutation (median PFS time, 27.8 months) had a
strikingly improved PFS time compared with patients with L858R
mutation (median PFS time, 10 months; HR, 2.5; 95% CI, 1.47–5.86;
P=0.003) and wild-type (median PFS time, 11 months; HR, 2.2; 95%
CI, 1.29–3.64; P=0.004) (Fig.
2C).
The median PFS time of 17 patients with the
ALK fusion gene was 11 months (95% CI, 1.5–20.5 months),
whereas the PFS time of the patients with wild-type ALK was
13 months (95% CI, 8.7–18.3 months). The prognosis of patients with
wild-type ALK was better than the prognosis of patients with
the ALK fusion gene (P=0.057).
No correlation was identified between CRKL
and AXL expression and the clinical factors, such as gender,
AC subtypes, tumor size, clinical stage, smoking history and
ALK status. Despite this finding, the diverse staining
resulted in the consideration of the components of ACs. Different
staining patterns were correlated with the components of AC. In
total, 82 AC lesions (77.4%) were composed of ≥2 variant
histological components, and the most frequent combination was
solid and acinar patterns (26/106; 24.5%). Other mixtures consisted
of solid, papillary, micropapillary and lepidic components of
varied proportions. In addition, 46.2 and 24.5% ACs was observed
with discrepant expression within components, respectively. In
addition, 56 ACs (52.8%) with EGFR mutation had an increased
level of AXL expression compared with ACs with wild-type
EGFR (P=0.019). In total, 31 and 48 cases were determined
with low expression of CRKL and AXL, respectively. A
low level of AXL expression was detected in 48/106 ACs
(45.3%) and exon 19 deletion was detected in 40.7% (11/27), L858R
in 30.4% (7/23) and wild-type EGFR in 58% (29/50) of ACs.
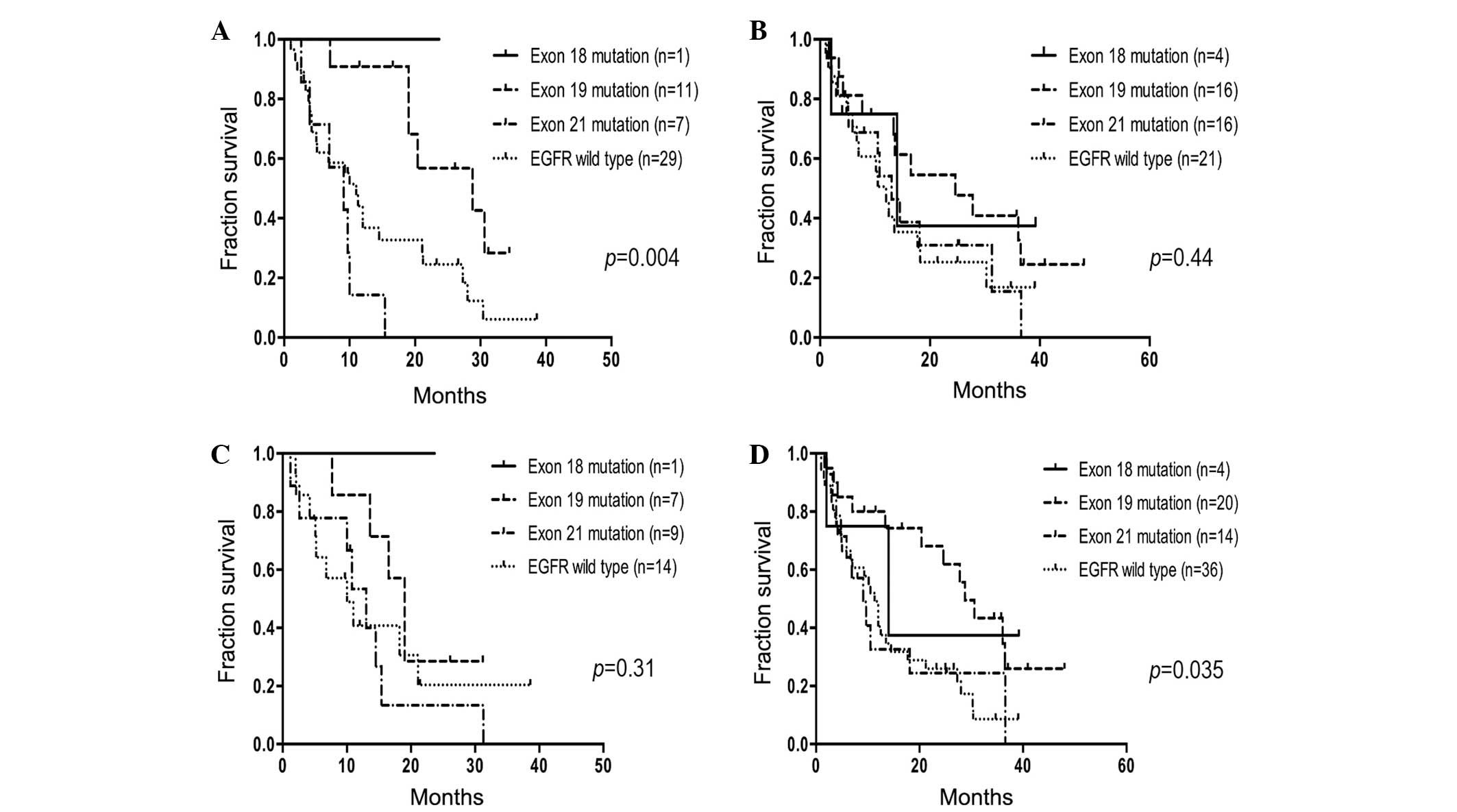
PFS comparison revealed that ACs with exon 19 deletion within the
group with low AXL expression had a longer PFS time (median
PFS time, 28.8 months) than those with the L858R mutation (median
PFS time, 9.1 months; HR, 6.04; 95% CI, 6.15–117.7; P<0.0001)
and wild-type EGFR (median PFS time, 11 months; HR, 2.88;
95% CI, 1.26–5.38; P=0.012) (Fig.
3A). There was no significant difference in PFS time among the
AC subtypes with high AXL expression (P>0.05; Fig. 3B). Among the AC subtypes with low
CRKL expression, the median PFS time of patients with exon
19 deletion (median PFS time, 19 months) was not significantly
longer than the median PFS time of patients with the L858R mutation
(median PFS time, 13 months) and wild-types EGFR (median PFS
time, 9.97 months) (P=0.29; Fig. 3C).
In contrast, for the 75 ACs with an increased level of CRKL
expression, the 20 patients (20/75; 26.7%) with exon 19 deletion
had a better PFS time (28.8 months) than the 14 patients (14/75;
18.7%) with the L858R mutation (9.1 months; HR, 2.79; 95% CI,
1.1–7.1; P=0.03) and all 50 patients (50/106; 47.2%) without
EGFR mutation (11.3 months; HR, 2.49; 95% CI, 1.33–4.67;
P=0.0046) (Fig. 3D).
Discussion
Overall, ~80% of lung ACs are categorized as mixed
subtype according to the 2004 WHO classification (2). It has been proposed that a
semiquantitative assessment of the percentages of various
histological components, such as acinar, papillary, micropapillary,
lepidic and solid, should be performed to classify tumors according
to the predominant components (30).
It is crucial to adopt a practical way to address tumors that are
comprised of a complex heterogeneous mixture of histological
subtypes, since 70–90% of surgically resected lung tumors are
diagnosed as invasive ACs (25). In
previous years, multiple independent research groups have
classified lung ACs according to the most predominant subtypes
(30–34). Prominent diverse structures in
morphology and heterogeneity in the biology of ACs have been
considered in an increasing number of studies following the
establishment of the new classification (3,35,36). The present study was commenced
subsequent to a review of all the tissue sections, and the
diagnoses were renewed based on the new classification. Studies on
the topic of micropapillary AC have reported that patients in an
early stage of disease have a poor prognosis (32,37). It
has recently been shown that micropapillary tumors also have a poor
prognosis, similar to that of ACs, with a predominantly solid
subtype (38). Patients with
papillary or acinar predominance or invasive mucinous AC show
similar overall survival (OS). Patients with solid predominance and
micropapillary predominance show the worst OS (6). In the present study, 106 AC patients
were administrated with conventional chemotherapy, and the PFS
times among different regimens were not significantly different
(P>0.05). However, the prognosis of different AC histological
mixtures was analyzed, and it was found that acinar-predominant AC
had a longer PFS time (30.6 months) than other AC subtypes, despite
the status of the EGFR, ALK, CRKL and
AXL genes. A previous study reports that patients with
micropapillary AC have a worse prognosis than patients with
mucinous, solid and colloid AC (38).
In the present cohort, the clinicopathological findings were
analyzed by univariate Kaplan-Meier test and Cox regression
analyses stratified with histological subtypes, EGFR and
ALK status. The multivariate model showed that AC subtypes
and EGFR mutation subtypes were independent factors that
affected PFS time in addition to clinical stage. Thereafter, AC
subtypes and EGFR status were compared with AXL and
CRKL expression. Patients with ACs with exon 19 deletion
(PFS time, 27.8 months) demonstrated an improved prognosis compared
with patients with ACs with L858R mutation (PFS time, 10 months)
and wild-type EGFR (PFS time, 11 months). A previous study
investigating 44 patients with lung cancer has reported that the
overall response rate to concurrent chemoradiotherapy is
significantly increased in the EGFR mutant group compared
with the wild-type EGFR group, and local regional relapse
occurs less frequently in patients with EGFR mutation
compared with patients with wild-type EGFR (39). It is well known that EGFR
mutation is most common in ACs in the eastern Asian population,
never-smokers and non-mucinous tumors (40–42). Lung
cancer-associated EGFR mutations are clustered within the
tyrosine kinase domain. In-frame exon 19 deletions occur just
downstream of a lysine residue at position 745 (K745), which is
critical for binding adenosine triphosphate. Absence of a few amino
acids located C-terminal to this lysine residue may affect the
configuration of the EGFR catalytic site (42). The L858R mutation occurs adjacent to
the highly conserved DFG motif in the activation loop region of the
kinase (43). Theoretically, these
mutations may all result in conformational changes that lead to
increased activity and TKI sensitivity (28,44). The
actual mechanism of mutant EGFR tumors with target therapy
has yet to be elucidated. The present results indicated that
activating EGFR mutations, particularly in-frame deletions
of exon 19, is more likely to be associated with clinical
significance and it is necessary to consider the EGFR status
of ACs. The EGFR status of ACs determined the response of
the tumors to conventional chemotherapy.
ALK rearrangement results in fusion genes,
such as EML4-ALK, KIF5B-ALK and KLC1-ALK
(12,45,46).
Detection of ALK rearrangement by FISH or RT-PCR is
considered to be the standard procedure, but each method possesses
limitations. The FISH method, which is based on a break-apart
probe, has the limitation that it cannot determine the specific
form of translocation, whereas RT-PCR cannot quantify the tumor
cells with the ALK fusion gene. IHC using specific
antibodies corresponds well with detecting the activating
ALK fusion protein, and it may serve as a useful screening
method with quantitation and quality outcome (15,16,46,47).
The ALK fusion gene defines a distinct molecular subset of
NSCLC, in particular AC, which benefits from treatment with
ALK-inhibitors. Robust and reliable laboratory tests for
predictive biomarkers are critical to select appropriate patients
for targeted therapy. Patients with improved performance status and
EML4-ALK translocation have an increased overall survival
time compared with patients treated with conventional chemotherapy
(17,48). There is no significant difference in
clinical factors and survival outcome between the patients
harboring variant 1 and those harboring non-variant 1
EML4-ALK fusion genes (49).
The incidence of 17 ACs (16.0%) possessing ALK rearrangement
in the present study was substantially more frequent than that in
young male patients in Western countries (5.6%, 20/358) (15). No prognostic advantage of ALK
translocation was demonstrated in the present study and the
ALK fusion incidence was distributed regardless of
histological predilection, which is consistent with other reports
of EML4-ALK rearrangement (15,50,51).
Improved prognosis was also identified in the patients without
ALK rearrangement in the present study. We suspect that this
discrepancy with other reports may be caused by the constituents
resulting from the ‘acinar’, which is likely to lead to a better
outcome than other histological subtypes. Additional stratified
analysis is required to illuminate the prognostic difference of AC
subtypes.
AXL is a transmembrane receptor tyrosine
kinase whose overexpression has been reported in several human
cancers. In addition, Gas6-AXL signaling promotes cell
proliferation and survival, angiogenesis, and invasion and
metastasis through activation of the PI3K-AKT-mTOR and
RAS-RAF-MEK-MAPK pathways (52). EGFR-mutant lung cancer models
in vitro and in vivo with increased activation of
AXL have been shown to possess acquired resistance to
erlotinib without T790 M alteration. In lung AC, AXL
expression levels are associated with tumor advancement and the
survival of patients with adjuvant chemotherapy, thus rendering
AXL expression as a reliable biomarker and potential target
for treatment of lung AC (53). In
the present study, the role of EGFR activating mutation in
the prognosis of AC patients was confirmed. Additional stratified
pairwise comparisons were performed to elucidate the association
between AXL and EGFR activating mutation types. An
increased level of AXL expression was more evident in ACs
with EGFR mutation. Investigation of the role of AXL
in patient prognosis revealed an improved PFS time in patients with
low expression of AXL and exon 19 mutation, compared to patients
with higher expression of AXL and wild-type EGFR. The
AXL/Gas6 system remains an attractive therapeutic target
(54), and certain small molecules
with AXL inhibitory effects are already under development
(55).
CRKL expression is associated with enhanced
cancer cell proliferation and invasion (18,19).
Overexpression of CRKL is found in NSCLC and is associated
with poor tumor differentiation, AC, advanced p-TNM stage, high
proliferation index and poor overall survival. In addition,
overexpression of CRKL in cell lines promotes cell
proliferation by facilitating cell cycle progression (56). In the current study, the CRKL
protein level was not demonstrated to be significantly associated
with clinical features such as gender, smoking history, clinical
staging, EGFR status and ALK status. However,
overexpression or amplification of CRKL and activation of
AXL is reported associated with the resistance to TKI
(9,12,23,57). The
original CRKL expression level in AC subtypes with various
EGFR mutations remains limited; therefore, the comparison
was performed in the present study to investigate the effect of
CRKL expression concomitant with EGFR activated
mutation on the prognosis of AC patients. Patients with high
CRKL expression level and exon 19 mutation had an improved
prognosis compared with other patients with a low CRKL
expression level and other EGFR status. This indicates that
overexpression of CRKL may confer an improved response to
conventional chemotherapy in the ACs with exon 19 mutation. To
address the diverse expression of AXL and CRKL, the
understanding of the exceptional histological and biological
heterogeneity of lung carcinoma may be improved if the stem cell
theory of cancer development is considered. According to these
evolving views, neoplastic cells derive from abnormal progenitors
that retain the potential to give rise to a diverse progeny,
depending on the large variety of molecular abnormalities affecting
the neoplastic clones. Therefore, neoplastic cells produce a large
variety of carcinoma subtypes (12,58–63). The
unique features of these abnormal precursors may determine the
phenotype of neoplastic populations, organized in a hierarchy with
various properties and degrees of differentiation (64). Numerous studies have focused on the
pathological features of cases harboring EGFR mutations to
evaluate the predictive significance of morphological
characterization, but the available data are partly discordant
(65,66). In a recent study in which the
histological-genetic correlations of 100 ACs (94% mixed subtype)
were analyzed, the strongest molecular correlation was observed
between the EGFR mutation and the papillary subtype
(30), as previously suggested
(33). However, this contrasted with
studies claiming that EGFR mutations mainly occur in the
non-mucinous BAC and BAC AC mixed histological subtypes (67–69). In
addition, previous studies investigating the morphological features
of ACs that respond and do not respond to TKI therapies confirm
that certain histological differences exist (70–72).
However, there is morphological overlap, and WHO criteria may be
considered a confounding factor. Following consideration of these
limitations, the use of pure histology as a predictor for targeted
EGFR inhibitory therapy in lung ACs is highly controversial.
In accordance with the present results, the hypothesis that
EGFR mutation does not have a predilection to particular
histological subtypes was supported.
In conclusion, due to the incidence of EGFR
mutation in the Asian population, it is necessary to consider the
EGFR status as an underlying factor in limited therapeutic
options. The present findings are considered to contribute to the
understanding of CRKL and AXL expression as novel
biomarkers and therapeutic targets in lung AC.
Acknowledgements
The present study was supported by the Beijing
Foundation for Distinguished Scientists (grant no.
2009D003013000001; Beijing, China) and awards from the Beijing
Board of Health (Beijing, China; grant no. QN2009-035).
References
|
1
|
Curado MP, Edwards B, Shin HR, Storm H,
Ferlay J, Heanue M and Boyle P: Cancer Incidence in Five
Continents. IARC Scientific Publications; Lyon: 2007
|
|
2
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: World Health Organization Classification of
TumoursPathology & Genetics of Tumours of the Lung, Pleura,
Thymus and Heart. IARC Press; Lyon: 2004
|
|
3
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Pathology and Genetics: Tumours of the Lung,
Pleura, Thymus and Heart. IARC Press; Lyon, France: 2004,
View Article : Google Scholar
|
|
5
|
Travis WD, Colby TV, Corrin B, Shimosato Y
and Brambilla E: Histological Typing of Lung and Pleural Tumors.
Springer; Berlin: 1999, View Article : Google Scholar
|
|
6
|
Yanagawa N, Shiono S, Abiko M, Ogata SY,
Sato T and Tamura G: The correlation of the international
association for the study of lung cancer (IASLC)/American Thoracic
Society (ATS)/European Respiratory Society (ERS) classification
with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac
Surg. 98:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H
and Haga H: Validation of the IASLC/ATS/ERS lung adenocarcinoma
classification for prognosis and association with EGFR and KRAS
gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol.
8:52–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riely GJ, Politi KA, Miller VA and Pao W:
Update on epidermal growth factor receptor mutations in non-small
cell lung cancer. Clin Cancer Res. 12:7232–7241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai K, Arao T, Shimoyama T, Murofushi K,
Sekijima M, Kaji N, Tamura T, Saijo N and Nishio K: Dimerization
and the signal transduction pathway of a small in-frame deletion in
the epidermal growth factor receptor. FASEB J. 20:311–313.
2006.PubMed/NCBI
|
|
11
|
Sun Y, Yu X, Shi X, Hong W, Zhao J and Shi
L: Correlation of survival and EGFR mutation with predominant
histologic subtype according to the new lung adenocarcinoma
classification in stage IB patients. World J Surg Oncol.
12:1482014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwak EL, Bang YJ, Camidge DR, Shaw AT,
Solomon B, Maki RG, Ou SH, Dezube BJ, Jänne PA, Costa DB, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. N Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandhi L and Jänne PA: Crizotinib for
ALK-rearranged non-small cell lung cancer: A new targeted therapy
for a new target. Clin Cancer Res. 18:3737–3742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rodig SJ, Mino-Kenudson M, Dacic S, Yeap
BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, et
al: Unique clinicopathologic features characterize ALK-rearranged
lung adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mino-Kenudson M, Chirieac LR, Law K,
Hornick JL, Lindeman N, Mark EJ, Cohen DW, Johnson BE, Jänne PA,
Iafrate AJ and Rodig SJ: A novel, highly sensitive antibody allows
for the routine detection of ALK-rearranged lung adenocarcinomas by
standard immunohistochemistry. Clin Cancer Res. 16:1561–1571. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ying J, Guo L, Qiu T, Shan L, Ling Y, Liu
X and Lu N: Diagnostic value of a novel fully automated
immunochemistry assay for detection of ALK rearrangement in primary
lung adenocarcinoma. Ann Oncol. 24:2589–2593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
ten Hoeve J, Kaartinen V, Fioretos T,
Haataja L, Voncken JW, Heisterkamp N and Groffen J: Cellular
interactions of CRKL and SH2-SH3 adaptor protein. Cancer Res.
54:2563–2567. 1994.PubMed/NCBI
|
|
19
|
Senechal K, Halpern J and Sawyers CL: The
CRKL adaptor protein transforms fibroblasts and functions in
transformation by the BCR-ABL oncogene. J Biol Chem.
271:23255–23261. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hafizi S and Dahlbäck B: Signalling and
functional diversity within the Axl subfamily of receptor tyrosine
kinases. Cytokine Growth Factor Rev. 17:295–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goruppi S, Ruaro E, Varnum B and Schneider
C: Requirement of phosphatidylinositol 3-kinase-dependent pathway
and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3
fibroblasts. Mol Cell Biol. 17:4442–4453. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hector A, Montgomery EA, Karikari C, Canto
M, Dunbar KB, Wang JS, Feldmann G, Hong SM, Haffner MC, Meeker AK,
et al: The Axl receptor tyrosine kinase is an adverse prognostic
factor and a therapeutic target in esophageal adenocarcinoma.
Cancer Biol Ther. 10:1009–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Z, Lee JC, Lin L, Olivas V, Au V,
LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, et al:
Activation of the AXL kinase causes resistance to EGFR-targeted
therapy in lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paccez JD, Vasques GJ, Correa RG,
Vasconcellos JF, Duncan K, Gu X, Bhasin M, Libermann TA and Zerbini
LF: The receptor tyrosine kinase Axl is an essential regulator of
prostate cancer proliferation and tumor growth and represents a new
therapeutic target. Oncogene. 32:689–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th. Wiley-Blackwell;
Hoboken, NJ: 2009
|
|
27
|
Cai YR, Zhang HQ, Qu Y, Mu J, Zhao D, Zhou
LJ, Yan H, Ye JW and Liu Y: Expression of MET and SOX2 genes in
non-small cell lung carcinoma with EGFR mutation. Oncol Rep.
26:877–885. 2011.PubMed/NCBI
|
|
28
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Minca EC, Portier BP, Wang Z, Lanigan C,
Farver CF, Feng Y, Ma PC, Arrossi VA, Pennell NA and Tubbs RR: ALK
status testing in non-small cell lung carcinoma: Correlation
between ultrasensitive IHC and FISH. J Mol Diagn. 15:341–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Motoi N, Szoke J, Riely GJ, Seshan VE,
Kris MG, Rusch VW, Gerald WL and Travis WD: Lung adenocarcinoma:
Modification of the 2004 WHO mixed subtype to include the major
histologic subtype suggests correlations between papillary and
micropapillary adenocarcinoma subtypes, EGFR mutations and gene
expression analysis. Am J Surg Pathol. 32:810–827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sica G, Yoshizawa A, Sima CS, Azzoli CG,
Downey RJ, Rusch VW, Travis WD and Moreira AL: A grading system of
lung adenocarcinomas based on histologic pattern is predictive of
disease recurrence in stage I tumors. Am J Surg Pathol.
34:1155–1162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Oliveira Duarte Achcar R, Nikiforova MN
and Yousem SA: Micropapillary lung adenocarcinoma: EGFR, K-ras and
BRAF mutational profile. Am J Clin Pathol. 131:694–700. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim YH, Ishii G, Goto K, Nagai K, Tsuta K,
Shiono S, Nitadori J, Kodama T, Nishiwaki Y and Ochiai A: Dominant
papillary subtype is a significant predictor of the response to
gefitinib in adenocarcinoma of the lung. Clin Cancer Res.
10:7311–7317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ding L, Getz G, Wheeler DA, Mardis ER,
McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan
MB, et al: Somatic mutations affect key pathways in lung
adenocarcinoma. Nature. 455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Taniguchi K, Okami J, Kodama K,
Higashiyama M and Kato K: Intratumor heterogeneity of epidermal
growth factor receptor mutations in lung cancer and its correlation
to the response to gefitinib. Cancer Sci. 99:929–935. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Fujimoto J, Zhang J, Wedge DC,
Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, et al: Intratumor
heterogeneity in localized lung adenocarcinomas delineated by
multiregion sequencing. Science 346(6206). 256–259. 2014.
|
|
37
|
Tsutsumida H, Nomoto M, Goto M, Kitajima
S, Kubota I, Hirotsu Y, Wakimoto J, Hollingsworth MA and Yonezawa
S: A micropapillary pattern is predictive of a poor prognosis in
lung adenocarcinoma and reduced surfactant apoprotein A expression
in the micropapillary pattern is an excellent indicator of a poor
prognosis. Mod Pathol. 20:638–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshizawa A, Motoi N, Riely GJ, Sima CS,
Gerald WL, Kris MG, Park BJ, Rusch VW and Travis WD: Impact of
proposed IASLC/ATS/ERS classification of lung adenocarcinoma:
Prognostic subgroups and implications for further revision of
staging based on analysis of 514 stage I cases. Mod Pathol.
24:653–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akamatsu H, Kaira K, Murakami H, Serizawa
M, Koh Y, Ono A, Shukuya T, Tsuya A, Nakamura Y, Kenmotsu H, et al:
The impact of clinical outcomes according to EGFR mutation status
in patients with locally advanced lung adenocarcinoma who recieved
concurrent chemoradiotherapy. Am J Clin Oncol. 37:144–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pao W, Miller V, Zakowski M, Doherty J,
Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al:
EGF receptor gene mutations are common in lung cancers from ‘never
smokers’ and are associated with sensitivity of tumors to gefitinib
and erlotinib. Proc Natl Acad Sci USA. 101:13306–13311. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Finberg KE, Sequist LV, Joshi VA,
Muzikansky A, Miller JM, Han M, Beheshti J, Chirieac LR, Mark EJ
and Iafrate AJ: Mucinous differentiation correlates with absence of
EGFR mutation and presence of KRAS mutation in lung adenocarcinomas
with bronchioloalveolar features. J Mol Diagn. 9:320–326. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Honegger AM, Dull TJ, Felder S, Van
Obberghen E, Bellot F, Szapary D, Schmidt A, Ullrich A and
Schlessinger J: Point mutation at the ATP binding site of EGF
receptor abolishes protein-tyrosine kinase activity and alters
cellular routing. Cell. 51:199–209. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huse M and Kuriyan J: The conformational
plasticity of protein kinases. Cell. 109:275–282. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gazdar AF, Shigematsu H, Herz J and Minna
JD: Mutations and addiction to EGFR: The Achilles ‘heal’ of lung
cancers? Trends Mol Med. 10:481–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Togashi Y, Soda M, Sakata S, Sugawara E,
Hatano S, Asaka R, Nakajima T, Mano H and Takeuchi K: KLC1-ALK: A
novel fusion in lung cancer identified using a formalin-fixed
paraffin-embedded tissue only. PLoS One. 7:e313232012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Takeuchi K, Choi YL, Togashi Y, Soda M,
Hatano S, Inamura K, Takada S, Ueno T, Yamashita Y, Satoh Y, et al:
KIF5B-ALK, a novel fusion oncokinase identified by an
immunohistochemistry-based diagnostic system for ALK-positive lung
cancer. Clin Cancer Res. 15:3143–3149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Boland JM, Erdogan S, Vasmatzis G, Yang P,
Tillmans LS, Johnson MR, Wang X, Peterson LM, Halling KC, Oliveira
AM, et al: Anaplastic lymphoma kinase immunoreactivity correlates
with ALK gene rearrangement and transcriptional up-regulation in
non-small cell lung carcinomas. Hum Pathol. 40:1152–1158. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu SG, Kuo YW, Chang YL, Shih JY, Chen YH,
Tsai MF, Yu CJ, Yang CH and Yang PC: EML4-ALK translocation
predicts better outcome in lung adenocarcinoma patients with
wild-type EGFR. J Thorac Oncol. 7:98–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shaw AT, Yeap BY, Mino-Kenudson M,
Digumarthy SR, Costa DB, Heist RS, Solomon B, Stubbs H, Admane S,
McDermott U, et al: Clinical features and outcome of patients with
non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol.
27:4247–4253. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Takahashi T, Sonobe M, Kobayashi M,
Yoshizawa A, Menju T, Nakayama E, Mino N, Iwakiri S, Sato K,
Miyahara R, et al: Clinicopathologic features of non-small-cell
lung cancer with EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Verma A, Warner SL, Vankayalapati H,
Bearss DJ and Sharma S: Targeting Axl and Mer kinases in cancer.
Mol Cancer Ther. 10:1763–1773. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ishikawa M, Sonobe M, Nakayama E,
Kobayashi M, Kikuchi R, Kitamura J, Imamura N and Date H: Higher
expression of receptor tyrosine kinase Axl, and differential
expression of its ligand, Gas6, predict poor survival in lung
adenocarcinoma patients. Ann Surg Oncol. 20(Suppl 3): S467–S476.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Stommel JM, Kimmelman AC, Ying H,
Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE,
Ligon KL, Brennan C, et al: Coactivation of receptor tyrosine
kinases affects the response of tumor cells to targeted therapies.
Science. 318:287–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Eder JP, Shapiro GI, Appleman LJ, Zhu AX,
Miles D, Keer H, Cancilla B, Chu F, Hitchcock-Bryan S, Sherman L,
et al: A phase I study of foretinib, a multi-targeted inhibitor of
c-Met and vascular endothelial growth factor receptor 2. Clin
Cancer Res. 16:3507–3516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Dong QZ, Fu L, Stoecker M, Wang E
and Wang EH: Overexpression of CRKL correlates with poor prognosis
and cell proliferation in non-small cell lung cancer. Mol Carcinog.
52:890–899. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cheung HW, Du J, Boehm JS, He F, Weir BA,
Wang X, Butaney M, Sequist LV, Luo B, Engelman JA, et al:
Amplification of CRKL induces transformation and epidermal growth
factor receptor inhibitor resistance in human non-small cell lung
cancers. Cancer Discov. 1:608–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ladanyi M and Pao W: Lung adenocarcinoma:
Guiding EGFR-targeted therapy and beyond. Mod Pathol. 21(Suppl 2):
S16–S22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kwei KA, Kim YH, Girard L, Kao J,
Pacyna-Gengelbach M, Salari K, Lee J, Choi YL, Sato M, Wang P, et
al: Genomic profiling identifies TITF1 as a lineage-specific
oncogene amplified in lung cancer. Oncogene. 27:3635–3640. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun S, Schiller JH, Spinola M and Minna
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Marks JL, McLellan MD, Zakowski MF, Lash
AE, Kasai Y, Broderick S, Sarkaria IS, Pham D, Singh B, Miner TL,
et al: Mutational analysis of EGFR and related signaling pathway
genes in lung adenocarcinomas identifies a novel somatic kinase
domain mutation in FGFR4. PLoS One. 2:e4262007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kendall J, Liu Q, Bakleh A, Krasnitz A,
Nguyen KC, Lakshmi B, Gerald WL, Powers S and Mu D: Oncogenic
cooperation and coamplification of developmental transcription
factor genes in lung cancer. Proc Natl Acad Sci USA.
104:16663–16668. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eberhard DA, Johnson BE, Amler LC, Goddard
AD, Heldens SL, Herbst RS, Ince WL, Jänne PA, Januario T, Johnson
DH, et al: Mutations in the epidermal growth factor receptor and in
KRAS are predictive and prognostic indicators in patients with
non-small-cell lung cancer treated with chemotherapy alone and in
combination with erlotinib. J Clin Oncol. 23:5900–5909. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sell S: Stem cell origin of cancer and
differentiation therapy. Crit Rev Oncol Hematol. 51:1–28. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ninomiya H, Hiramatsu M, Inamura K, Nomura
K, Okui M, Miyoshi T, Okumura S, Satoh Y, Nakagawa K, Nishio M, et
al: Correlation between morphology and EGFR mutations in lung
adenocarcinomas. Significance of the micropapillary pattern and the
hobnail cell type. Lung Cancer. 63:235–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Tsuta K, Kawago M, Yoshida A, Sekine S,
Asamura H, Furuta K and Kushima R: Primary lung adenocarcinoma with
morule-like components: A unique histologic hallmark of aggressive
behavior and EGFR mutation. Lung Cancer. 85:12–18. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Blons H, Côté JF, Le Corre D, Riquet M,
Fabre-Guilevin E, Laurent-Puig P and Danel C: Epidermal growth
factor receptor mutation in lung cancer are linked to
bronchioloalveolar differentiation. Am J Surg Pathol. 30:1309–1315.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sakuma Y, Matsukuma S, Yoshihara M,
Nakamura Y, Noda K, Nakayama H, Kameda Y, Tsuchiya E and Miyagi Y:
Distinctive evaluation of nonmucinous and mucinous subtypes of
bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation
analyses for Japanese lung adenocarcinomas: Confirmation of the
correlations with histologic subtypes and gene mutations. Am J Clin
Pathol. 128:100–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Miller VA, Riely GJ, Zakowski MF, Li AR,
Patel JD, Heelan RT, Kris MG, Sandler AB, Carbone DP, Tsao A, et
al: Molecular characteristics of bronchioloalveolar carcinoma and
adenocarcinoma, bronchioloalveolar carcinoma subtype, predict
response to erlotinib. J Clin Oncol. 26:1472–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shim HS, Lee DH, Park EJ and Kim SH:
Histopathologic characteristics of lung adenocarcinomas with
epidermal growth factor receptor mutations in the International
Association for the Study of Lung Cancer/American Thoracic
Society/European Respiratory Society lung adenocarcinoma
classification. Arch Pathol Lab Med. 135:1329–1334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wu SG, Chang YL, Hsu YC, Wu JY, Yang CH,
Yu CJ, Tsai MF, Shih JY and Yang PC: Good response to gefitinib in
lung adenocarcinoma of complex epidermal growth factor receptor
(EGFR) mutations with the classical mutation pattern. Oncologist.
13:1276–1284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yoshida T, Ishii G, Goto K, Yoh K, Niho S,
Umemura S, Matsumoto S, Ohmatsu H, Nagai K, Ohe Y and Ochiai A:
Solid predominant histology predicts EGFR tyrosine kinase inhibitor
response in patients with EGFR mutation-positive lung
adenocarcinoma. J Cancer Res Clin Oncol. 139:1691–1700. 2013.
View Article : Google Scholar : PubMed/NCBI
|